Abstract
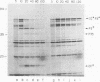
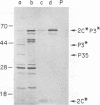
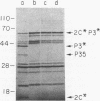
Although the genome organization and overall structure of hepatitis A virus are similar to those of other picornaviruses, nothing is known about the protein-processing pathways used by this virus to generate its capsid and nonstructural proteins from the polyprotein precursor. RNA transcripts of cloned hepatitis A virus cDNAs representing parts of the P2 and P3 regions of the genome were translated in rabbit reticulocyte lysates in vitro, and the translation products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before and after immunoprecipitation with specific antisera. Pulse-chase experiments demonstrated rapid cleavage at the P2-P3 junction, followed by further but incomplete processing at the 3C-3D junction. Mutation of the 3C coding sequence eliminated all cleavages. Efforts to demonstrate intermolecular cutting of the P2-P3 cleavage site by active 3C or 3CD sequences were unsuccessful; thus, it is likely that this cleavage occurs by intramolecular reaction, in cis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cho M. W., Ehrenfeld E. Rapid completion of the replication cycle of hepatitis A virus subsequent to reversal of guanidine inhibition. Virology. 1991 Feb;180(2):770–780. doi: 10.1016/0042-6822(91)90090-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. C., Wimmer E., von der Helm K., Deinhardt F. The genomic map of hepatitis A virus: an alternate analysis. Microb Pathog. 1986 Apr;1(2):217–219. doi: 10.1016/0882-4010(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Harmon S. A., Richards O. C., Summers D. F., Ehrenfeld E. The 5'-terminal nucleotides of hepatitis A virus RNA, but not poliovirus RNA, are required for infectivity. J Virol. 1991 May;65(5):2757–2760. doi: 10.1128/jvi.65.5.2757-2760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Summers D. F., Ehrenfeld E. Detection of hepatitis A virus RNA and capsid antigen in individual cells. Virus Res. 1989 Apr;12(4):361–369. doi: 10.1016/0168-1702(89)90093-2. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Towatari T., Ray J., Korant B. D., Petteway S. R., Jr Expression and site-specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Picornavirus protein processing--enzymes, substrates, and genetic regulation. Curr Top Microbiol Immunol. 1990;161:49–87. [PubMed] [Google Scholar]
- Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976 Sep 5;106(1):37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Martin-Gallardo A., Hughes J. V., Young A., Mitra S. W. Molecular cloning and partial sequencing of hepatitis A viral cDNA. J Virol. 1985 May;54(2):247–255. doi: 10.1128/jvi.54.2.247-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Baker J. C., Palmenberg A. C. Proteolytic cleavage of encephalomyocarditis virus capsid region substrates by precursors to the 3C enzyme. J Virol. 1989 Mar;63(3):1054–1058. doi: 10.1128/jvi.63.3.1054-1058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]