Abstract
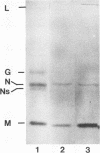
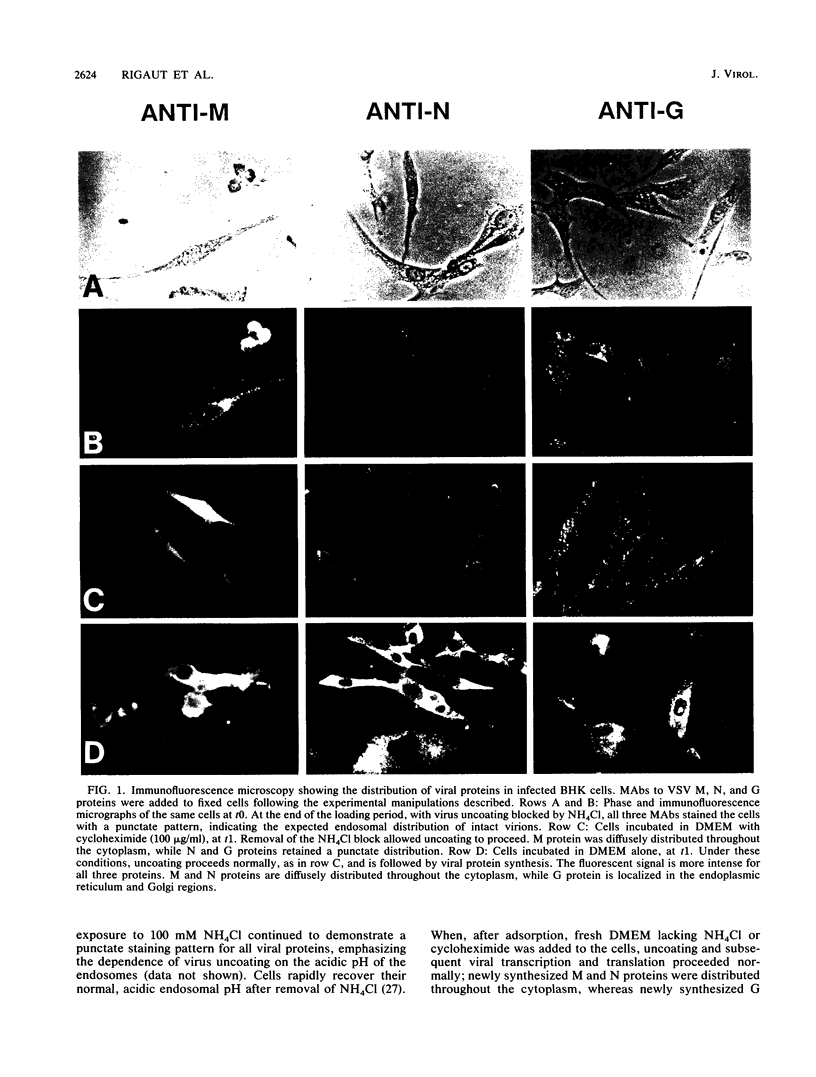
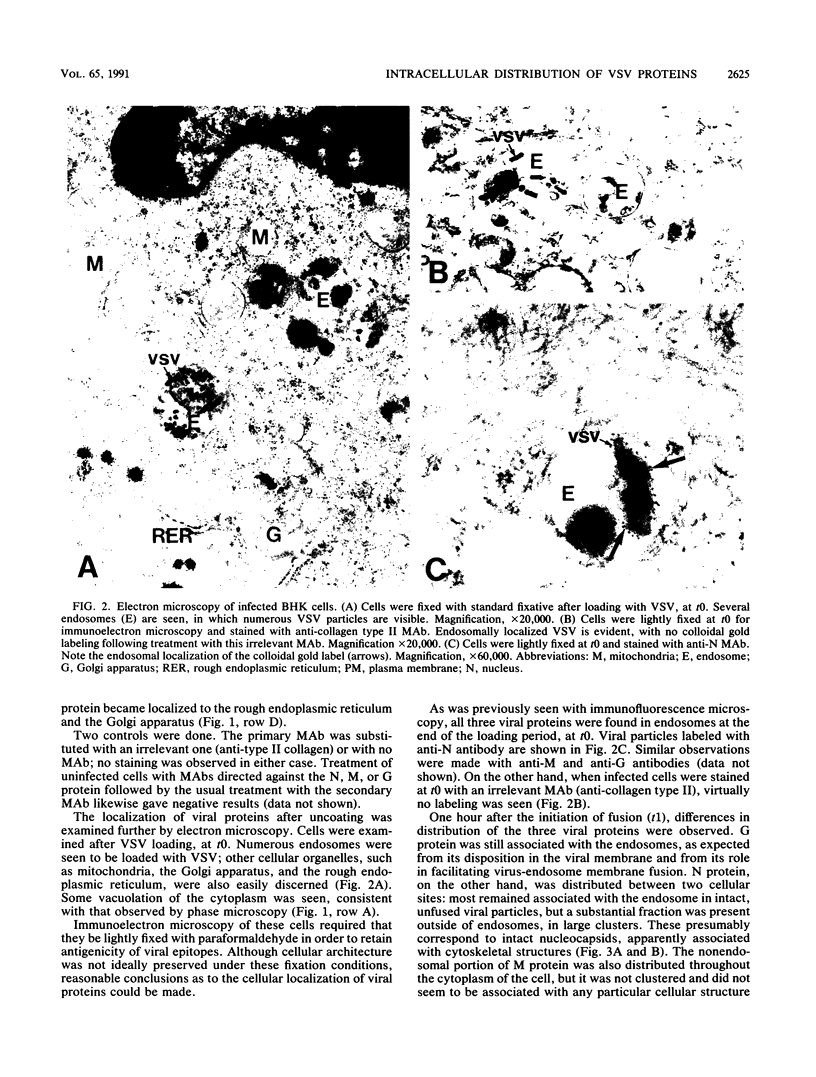
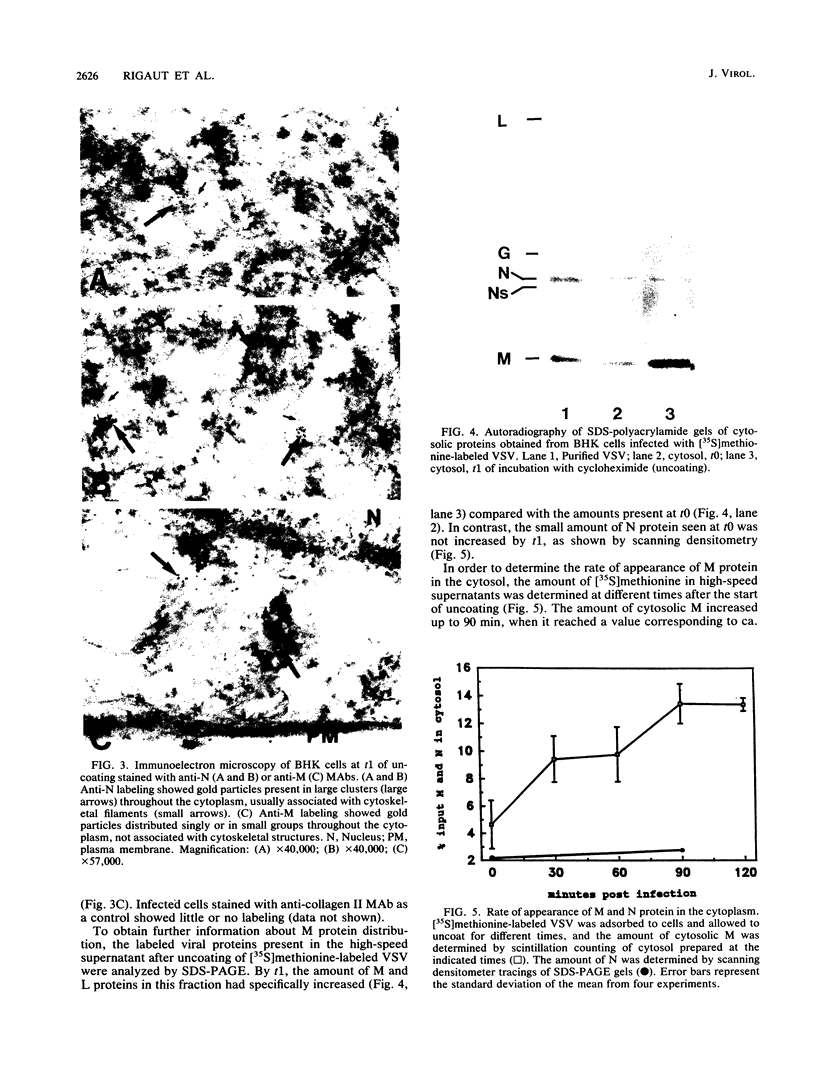
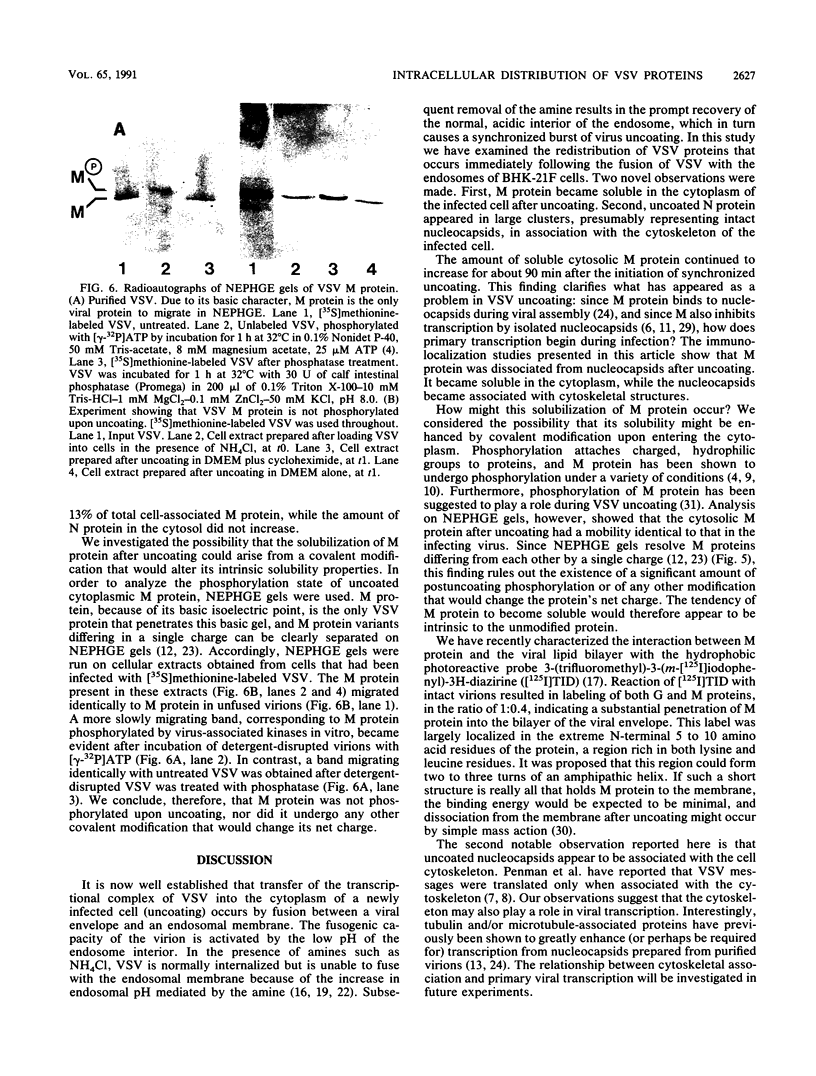
We have examined the fate of input viral proteins following the uncoating of vesicular stomatitis virus (VSV) by immunofluorescence microscopy, immunoelectron microscopy, and cell fractionation. VSV was adsorbed to BHK cells and allowed to become internalized in the presence of 100 mM NH4Cl; the NH4Cl was then removed to initiate synchronized uncoating. The three major structural proteins of VSV, the matrix protein (M), the nucleocapsid protein (N), and the glycoprotein (G), were each distributed uniquely after uncoating. Immunofluorescence microscopy showed that both G and N proteins retained a punctate distribution, whereas M protein was diffusely distributed throughout the cytoplasm, suggesting that it had become soluble. Immunoelectron microscopy showed that N protein was found in clusters (presumably in intact nucleocapsids) associated with the cell cytoskeleton and in unfused virions in endosomes and lysosomes. M protein was found diffusely distributed throughout the cytoplasm and also in endosomes and lysosomes. G protein was found only in association with endosomes and lysosomes after uncoating. Electrophoretic analysis of the high-speed cytosol fraction from infected cells showed that it contained chiefly M protein. The amount of M protein in the cytosol increased continuously during 90 min of uncoating, confirming its solubilization during uncoating. M protein was not covalently modified by phosphorylation upon uncoating, as evidenced by its mobility on nonequilibrium pH gradient gel electrophoresis. We suggest that those nucleocapsids associating with the cytoskeleton after uncoating may represent the sites of primary viral transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. The transcription complex of vesicular stomatitis virus. Cell. 1987 Feb 13;48(3):363–364. doi: 10.1016/0092-8674(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Keller D. S., Balch W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987 Aug 14;50(4):523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Beckes J. D., Childers L. C., Perrault J. Phosphorylation of vesicular stomatitis virus M protein: evidence for a second virion-associated protein serine kinase activity. Virology. 1989 Mar;169(1):161–171. doi: 10.1016/0042-6822(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Cervera M. M., Penman S. Formation of vesicular stomatitis virus nucleocapsid from cytoskeletal framework-bound N protein: possible model for structure assembly. Mol Cell Biol. 1984 Oct;4(10):2231–2234. doi: 10.1128/mcb.4.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Guerina N. G., Guo H. Y., Huang A. S. Host-dependent phosphorylation and kinase activity associated with vesicular stomatitis virus. J Biol Chem. 1982 Mar 25;257(6):3313–3319. [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna Y., Lenard J. Sequence alterations in temperature-sensitive M-protein mutants (complementation group III) of vesicular stomatitis virus. J Virol. 1985 Dec;56(3):655–659. doi: 10.1128/jvi.56.3.655-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill V. M., Harmon S. A., Summers D. F. Stimulation of vesicular stomatitis virus in vitro RNA synthesis by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5410–5413. doi: 10.1073/pnas.83.15.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Antigenic determinants of vesicular stomatitis virus: analysis with antigenic variants. J Immunol. 1983 Jan;130(1):394–398. [PubMed] [Google Scholar]
- Lenard J., Vanderoef R. Localization of the membrane-associated region of vesicular stomatitis virus M protein at the N terminus, using the hydrophobic, photoreactive probe 125I-TID. J Virol. 1990 Jul;64(7):3486–3491. doi: 10.1128/jvi.64.7.3486-3491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3605–3609. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Inhibition of vesicular stomatitis virus infection by spike glycoprotein. Evidence for an intracellular, G protein-requiring step. J Cell Biol. 1980 Feb;84(2):430–437. doi: 10.1083/jcb.84.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Vanderoef R., Lenard J. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J Virol. 1987 Feb;61(2):256–263. doi: 10.1128/jvi.61.2.256-263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. M., Groppi V. E., Jr, Browning E. T. Resolution of basic cellular proteins including histone variants by two-dimensional gel electrophoresis: evaluation of lysine to arginine ratios and phosphorylation. Anal Biochem. 1980 Mar 15;103(1):157–165. doi: 10.1016/0003-2697(80)90250-x. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Witt D. J., Naeve C. W., Summers D. F. Phosphorylation of vesicular stomatitis virus proteins as a possible contributing factor in virion uncoating. J Gen Virol. 1981 Oct;56(Pt 2):383–391. doi: 10.1099/0022-1317-56-2-383. [DOI] [PubMed] [Google Scholar]