Abstract
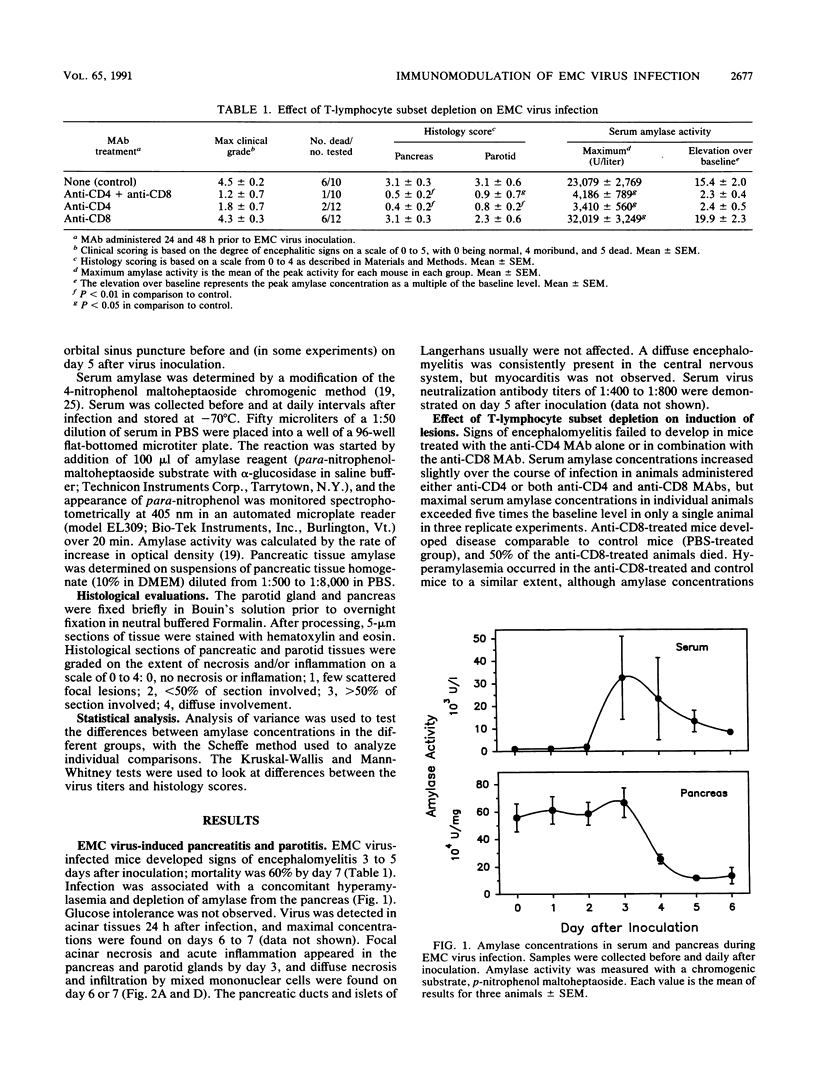
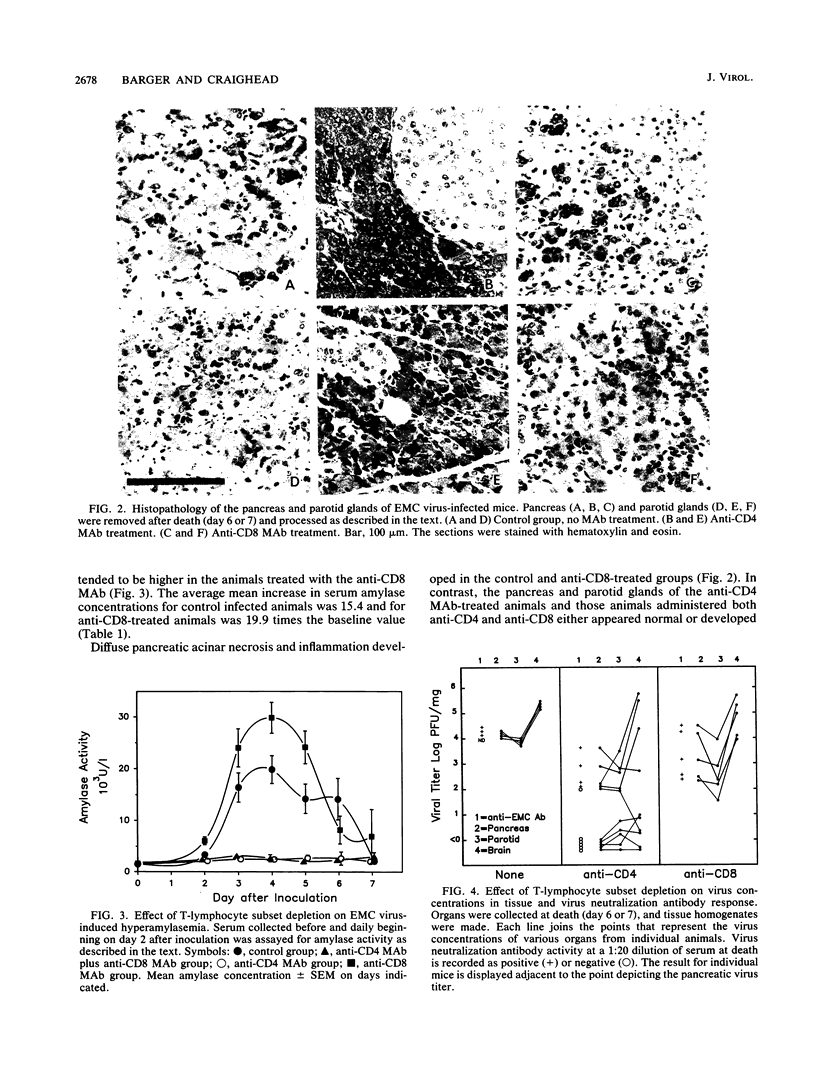
The E variant of encephalomyocarditis (EMC) virus causes an encephalomyelitis and coagulative necrosis of the pancreas and parotid glands in some but not all strains of inbred and outbred mice. In other models of disease caused by picornaviruses, depletion of specific lymphocyte subsets abrogates the development of tissue lesions. In this study, severe encephalomyelitis and acinar pancreatitis and parotitis developed in adult male A/J mice infected with 100 PFU of EMC virus. Depletion of the CD4+ subset of T lymphocytes in vivo with a monoclonal antibody (MAb) prior to EMC virus inoculation protects mice from developing encephalomyelitis, pancreatitis, and parotitis. This effect is also seen when animals are treated with anti-CD4 and anti-CD8 in combination, but the anti-CD8 MAb alone does not ameliorate the disease. Overall, concentrations of virus in tissues from anti-CD4-treated animals are lower than in immunologically intact control mice. Small-plaque variants of virus were also recovered from the tissues in some animals in this group. CD4+ lymphocytes are involved in the expression of EMC virus-induced pancreatitis and parotitis in A/J mice. This specific subset of T cells would appear to influence EMC viral tropism or replication in various organs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu P. G., Huber S. A., Craighead J. E. Contrasting features of T-lymphocyte-mediated diabetes in encephalomyocarditis virus-infected Balb/cBy and Balb/cCum mice. Am J Pathol. 1986 Aug;124(2):193–198. [PMC free article] [PubMed] [Google Scholar]
- Blay R., Simpson K., Leslie K., Huber S. Coxsackievirus-induced disease. CD4+ cells initiate both myocarditis and pancreatitis in DBA/2 mice. Am J Pathol. 1989 Nov;135(5):899–907. [PMC free article] [PubMed] [Google Scholar]
- Boucher D. W., Hayashi K., Rosenthal J., Notkins A. L. Virus-induced diabetes mellitus. III. Influence of the sex and strain of the host. J Infect Dis. 1975 Apr;131(4):462–466. doi: 10.1093/infdis/131.4.462. [DOI] [PubMed] [Google Scholar]
- CRAIGHEAD J. E. SOME PROPERTIES OF THE ENCEPHALOMYOCARDITIS, COLUMBIA SK AND MENGO VIRUSES. Proc Soc Exp Biol Med. 1965 Jun;119:408–412. doi: 10.3181/00379727-119-30196. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Wong G. H., Schrader J. W., Harrison L. C. Interferon-gamma enhances the expression of the major histocompatibility class I antigens on mouse pancreatic beta cells. Diabetes. 1985 Nov;34(11):1205–1209. doi: 10.2337/diab.34.11.1205. [DOI] [PubMed] [Google Scholar]
- Cohen J. I. Toward understanding the molecular basis for attenuation of picornaviruses. Adv Virus Res. 1989;36:153–180. doi: 10.1016/s0065-3527(08)60584-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. H., Naviaux R. K., vanden Brink K. M., Jordan G. W. Comparison of the nucleotide sequences of diabetogenic and nondiabetogenic encephalomyocarditis virus. Virology. 1988 Oct;166(2):603–607. doi: 10.1016/0042-6822(88)90534-x. [DOI] [PubMed] [Google Scholar]
- Cook S. H., Loria R. M., Madge G. E. Host factors in Coxsackievirus B4-induced pancreopathy. Lab Invest. 1982 Apr;46(4):377–382. [PubMed] [Google Scholar]
- Craighead J. E. Necrosis of the pancreas, parotid and lachrymal glands associated with encephalomyocarditis virus infection. Nature. 1965 Sep 18;207(5003):1268–1269. doi: 10.1038/2071268a0. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pathogenicity of the M and E variants of the encephalomyocarditis (EMC) virus. I. Myocardiotropic and neurotropic properties. Am J Pathol. 1966 Feb;48(2):333–345. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. Pathogenicity of the M and E variants of the encephalomyocarditis (EMC) virus. II. Lesions of the pancreas, parotid and lacrimal glands. Am J Pathol. 1966 Mar;48(3):375–386. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Prog Med Virol. 1975;19:161–214. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. B., Grota L. J., Glasgow L. A. Differential susceptibility of male and female mice to encephalomyocarditis virus: effects of castration, adrenalectomy, and the administration of sex hormones. Infect Immun. 1972 May;5(5):637–644. doi: 10.1128/iai.5.5.637-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines K. L., Kayes S. G., Wilson G. L. Altered pathogenesis in encephalomyocarditis virus (D variant)-infected diabetes-susceptible and resistant strains of mice. Diabetologia. 1986 May;29(5):313–320. doi: 10.1007/BF00452069. [DOI] [PubMed] [Google Scholar]
- Gould C. L., Trombley M. L., Bigley N. J., McMannama K. G., Giron D. J. Replication of diabetogenic and nondiabetogenic variants of encephalomyocarditis (EMC) virus in ICR Swiss mice. Proc Soc Exp Biol Med. 1984 Apr;175(4):449–453. doi: 10.3181/00379727-175-41819. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Harrison A. K., Bauer S. P., Murphy F. A. Viral pancreatitis: ultrastructural pathological effects of Coxsackievirus B3 infection in newborn mouse pancreas. Exp Mol Pathol. 1972 Oct;17(2):206–219. doi: 10.1016/0014-4800(72)90070-6. [DOI] [PubMed] [Google Scholar]
- Haynes M. K., Huber S. A., Craighead J. E. Helper-inducer T-lymphocytes mediate diabetes in EMC-infected BALB/c ByJ mice. Diabetes. 1987 Jul;36(7):877–881. doi: 10.2337/diab.36.7.877. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Babu P. G., Craighead J. E. Genetic influences on the immunologic pathogenesis of encephalomyocarditis (EMC) virus-induced diabetes mellitus. Diabetes. 1985 Nov;34(11):1186–1190. doi: 10.2337/diab.34.11.1186. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Hägele E. O., Schaich E., Rauscher E., Lehmann P., Bürk H., Wahlefeld A. W. Mechanism of action of human pancreatic and salivary alpha-amylase on alpha-4-nitrophenyl maltoheptaoside substrate. Clin Chem. 1982 Nov;28(11):2201–2205. [PubMed] [Google Scholar]
- JANSEN A. P., WYDEVELD P. G. Alpha-(p-nitrophenyl)maltoside as a substrate for the assay of amylase. Nature. 1958 Aug 23;182(4634):525–526. doi: 10.1038/182525b0. [DOI] [PubMed] [Google Scholar]
- Kromann H., Lernmark A., Vestergaard B. F., Egeberg J., Nerup J. The influence of the major histocompatibility complex (H-2) on experimental diabetes in mice. Diabetologia. 1979 Feb;16(2):107–114. doi: 10.1007/BF01225459. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Christianson G. J., Serreze D. V., Ting A. T., Worthen S. M. MHC antigen induction by interferon gamma on cultured mouse pancreatic beta cells and macrophages. Genetic analysis of strain differences and discovery of an "occult" class I-like antigen in NOD/Lt mice. J Exp Med. 1989 Oct 1;170(4):1243–1262. doi: 10.1084/jem.170.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynski D. Interleukin-1 alpha inhibits the effects of gamma-interferon and tumor necrosis factor alpha on the expression of the major histocompatibility antigens by the rat endothelium. Am J Pathol. 1990 Jan;136(1):229–237. [PMC free article] [PubMed] [Google Scholar]
- Lodge P. A., Herzum M., Olszewski J., Huber S. A. Coxsackievirus B-3 myocarditis. Acute and chronic forms of the disease caused by different immunopathogenic mechanisms. Am J Pathol. 1987 Sep;128(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- Loria R. M., Montgomery L. B., Corey L. A., Chinchilli V. M. Influence of diabetes mellitus heredity on susceptibility to coxsackievirus B4. Arch Virol. 1984;81(3-4):251–262. doi: 10.1007/BF01309997. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Onodera T., Yoon J. W., Brown K. S., Notkina A. L. Evidence for a single locus controlling susceptibility to virus-induced diabetes mellitus. Nature. 1978 Aug 17;274(5672):693–696. doi: 10.1038/274693a0. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Cooper S. M., Sriram S. In vivo immunomodulation by monoclonal anti-L3T4. 1. Effects on humoral and cell-mediated immune response. Cell Immunol. 1987 Apr 15;106(1):163–173. doi: 10.1016/0008-8749(87)90159-6. [DOI] [PubMed] [Google Scholar]
- Ross M. E., Hayashi K., Notkins A. L. Virus-induced pancreatic disease: alterations in concentration of glucose and amylase in blood. J Infect Dis. 1974 Jun;129(6):669–676. doi: 10.1093/infdis/129.6.669. [DOI] [PubMed] [Google Scholar]
- Ross M. E., Onodera T., Hayashi K., Notkins A. L. Virus-induced diabetes mellitus. V. Biological differences between the M variant and other strains of encephalomyocarditis virus. Infect Immun. 1975 Nov;12(5):1224–1226. doi: 10.1128/iai.12.5.1224-1226.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Webb S. R., Loria R. M., Madge G. E., Kibrick S. Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J Exp Med. 1976 May 1;143(5):1239–1248. doi: 10.1084/jem.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Madge G. E. The role of host genetics in the pathogenesis of coxsackievirus infection in the pancreas of mice. J Infect Dis. 1980 Jan;141(1):47–54. doi: 10.1093/infdis/141.1.47. [DOI] [PubMed] [Google Scholar]
- Wellmann K. F., Volk B. W., Brancato P., Amsterdam D. Encephalomyocarditis virus (E variant) in mice. Pancreatic changes. Arch Pathol. 1973 May;95(5):304–311. [PubMed] [Google Scholar]
- Wuthrich R. P., Jevnikar A. M., Takei F., Glimcher L. H., Kelley V. E. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990 Feb;136(2):441–450. [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980 Oct 1;152(4):878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., Notkins A. L. Virus-induced diabetes mellitus. VI. Genetically determined host differences in the replicating of encephalomyocarditis virus in pancreatic beta cells. J Exp Med. 1976 May 1;143(5):1170–1185. doi: 10.1084/jem.143.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Onodera T., Notkins A. L. Virus-induced diabetes mellitus: VIII. Passage of encephalomyocarditis virus and severity of diabetes in susceptible and resistant strains of mice. J Gen Virol. 1977 Nov;37(2):225–232. doi: 10.1099/0022-1317-37-2-225. [DOI] [PubMed] [Google Scholar]




