Abstract
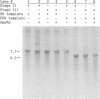
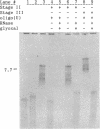
RNA template- and primer-dependent preparations of RNA polymerase were purified from encephalomyocarditis virus-infected Krebs-2 cells, using a three-step chromatographic procedure. The RNA duplex-unwinding activity of these preparations was investigated by two assays, using a partially double-stranded RNA template (encephalomyocarditis virus RNA annealed with a long segment of antisense transcript). Less purified preparations of the polymerase appeared to be able to efficiently displace, in an ATP-dependent and RNA elongation-dependent reaction, the antisense segment from the template. However, upon a more extensive purification, the unwinding activity of the RNA polymerase preparations was lost.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. H., Baltimore D. In vitro copying of viral positive strand RNA by poliovirus replicase. Characterization of the reaction and its products. J Biol Chem. 1982 Oct 25;257(20):12359–12366. [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Agol V. I. Selective inhibition of the synthesis of single-stranded RNA of encephalomyocarditis virus by 2-(alpha-hydroxybenzyl)-benzimidazole in cell-free systems. Arch Gesamte Virusforsch. 1974;45(1-2):17–26. doi: 10.1007/BF01240538. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Eremeeva T. P., Alatortseva G. I., Agol V. I. On the mechanism of single-stranded RNA synthesis by encephalomyocarditis virus replication complexes: preferential inhibition by adenylyl (beta, gamma-methylene)-diphosphonate. FEBS Lett. 1980 Jun 16;115(1):19–22. doi: 10.1016/0014-5793(80)80717-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Shcheglova M. V., Agol V. I. Inhibition of activity of encephalomyocarditis virus-induced RNA polymerase by antibodies against cellular components. Virology. 1979 Jan 30;92(2):271–277. doi: 10.1016/0042-6822(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Van Dyke T. A. Isolation of a soluble and template-dependent poliovirus RNA polymerase that copies virion RNA in vitro. J Virol. 1979 Oct;32(1):155–161. doi: 10.1128/jvi.32.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Wolf Y. I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990 Mar 12;262(1):145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- Hey T. D., Richards O. C., Ehrenfeld E. Synthesis of plus- and minus-strand RNA from poliovirion RNA template in vitro. J Virol. 1986 Jun;58(3):790–796. doi: 10.1128/jvi.58.3.790-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski J. M., Ransone L. J., Dasgupta A. Primer-dependent synthesis of covalently linked dimeric RNA molecules by poliovirus replicase. J Virol. 1987 Oct;61(10):2997–3003. doi: 10.1128/jvi.61.10.2997-3003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Rickles R. J., Flanegan J. B. Genome-length copies of poliovirion RNA are synthesized in vitro by the poliovirus RNA-dependent RNA polymerase. J Biol Chem. 1982 Apr 25;257(8):4610–4617. [PubMed] [Google Scholar]
- Wimmer E., Kuhn R. J., Pincus S., Yang C. F., Toyoda H., Nicklin M. J., Takeda N. Molecular events leading to picornavirus genome replication. J Cell Sci Suppl. 1987;7:251–276. doi: 10.1242/jcs.1987.supplement_7.18. [DOI] [PubMed] [Google Scholar]
- Young D. C., Tuschall D. M., Flanegan J. B. Poliovirus RNA-dependent RNA polymerase and host cell protein synthesize product RNA twice the size of poliovirion RNA in vitro. J Virol. 1985 May;54(2):256–264. doi: 10.1128/jvi.54.2.256-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]