Abstract
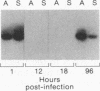
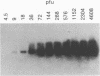
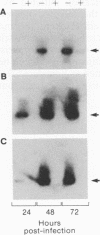
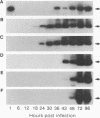
Rabies virus pathogenesis was studied in a mouse model by inoculation of the masseter muscle. At different intervals, the masseter muscle, trigeminal ganglia, and brain were analyzed for virus-specific RNA with a polymerase chain reaction assay, which revealed that as early as 18 h postinfection (p.i.), virus-specific RNA was present in the trigeminal ganglia, and at 24 h p.i., viral RNA was identified in the brain stem. Analysis of the masseter muscle demonstrated virus at 1 h p.i. but no virus-specific RNA between 6 and 30 h p.i., indicating that virus invaded the nerve ending directly, without prior replication in the muscle. At 36 h p.i., viral RNA was detected again in the masseter muscle. Selective amplification of plus- and minus-strand RNA isolated from the masseter muscle at 96 h p.i. revealed that the majority of the rabies virus-specific RNA was in the positive sense, suggesting virus replication in muscle tissue during late stages of infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlton K. M., Casey G. A. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest. 1979 Jul;41(1):36–44. [PubMed] [Google Scholar]
- Coulon P., Derbin C., Kucera P., Lafay F., Prehaud C., Flamand A. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J Virol. 1989 Aug;63(8):3550–3554. doi: 10.1128/jvi.63.8.3550-3554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu M., Shaddock J. H. Peripheral distribution of virus in dogs inoculated with two strains of rabies virus. Am J Vet Res. 1984 Apr;45(4):724–729. [PubMed] [Google Scholar]
- Johnson R. T. Experimental rabies. Studies of cellular vulnerability and pathogenesis using fluorescent antibody staining. J Neuropathol Exp Neurol. 1965 Oct;24(4):662–674. [PubMed] [Google Scholar]
- Kucera P., Dolivo M., Coulon P., Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985 Jul;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Schumacher C. L., Dietzschold B., Ertl H. C., Niu H. S., Rupprecht C. E., Koprowski H. Use of mouse anti-rabies monoclonal antibodies in postexposure treatment of rabies. J Clin Invest. 1989 Sep;84(3):971–975. doi: 10.1172/JCI114260. [DOI] [PMC free article] [PubMed] [Google Scholar]