Abstract
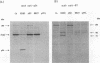
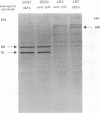
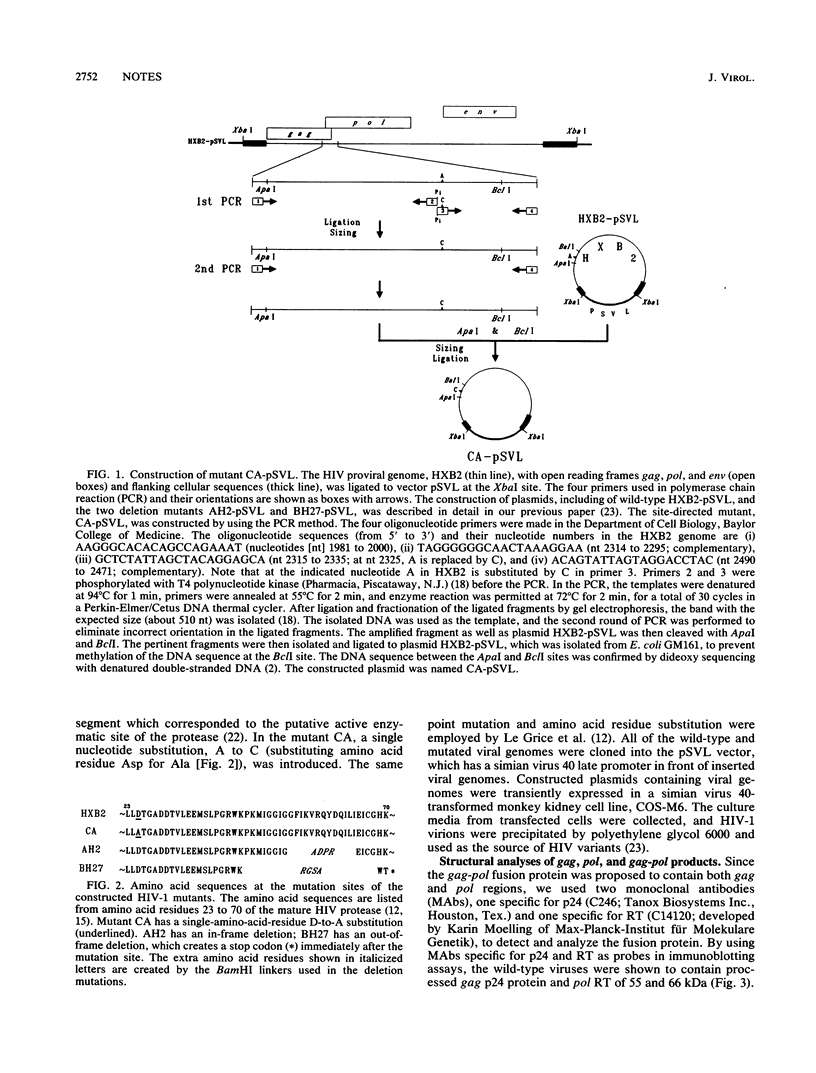
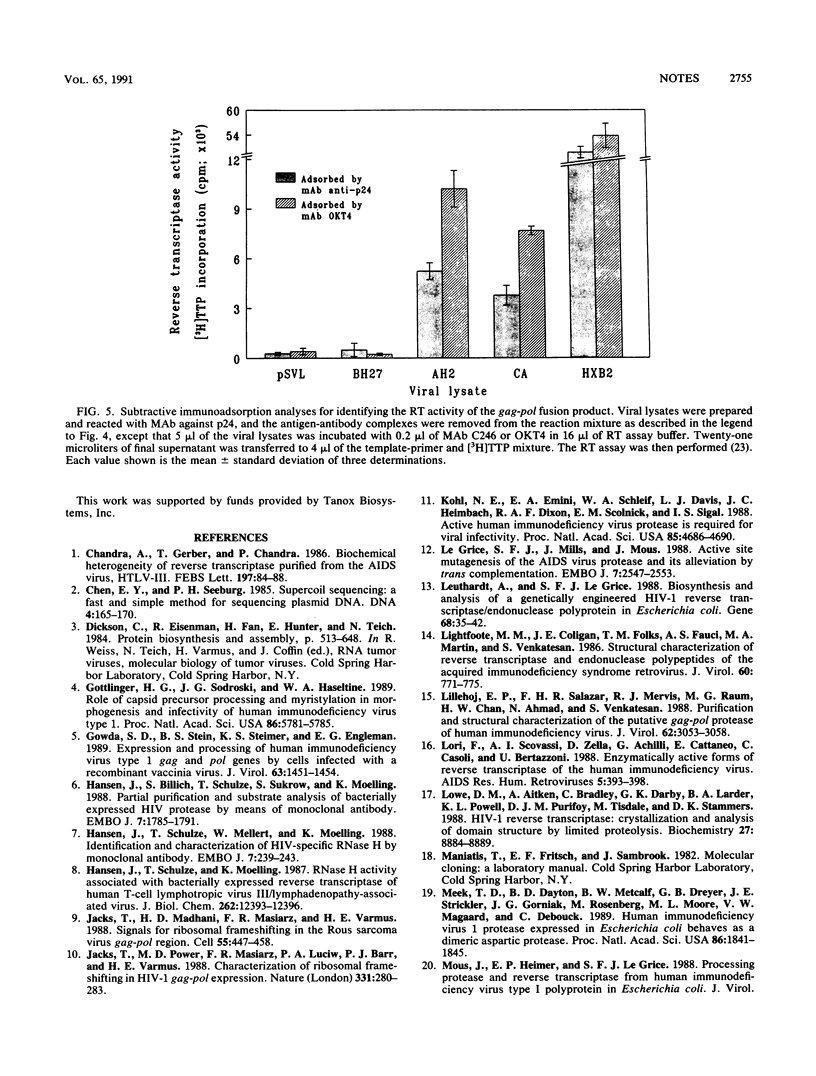
Three human immunodeficiency virus type 1 (HIV-1) mutants were constructed with mutations in their protease genes: AH2-pSVL, with an in-phase deletion; BH27-pSVL, with an out-of-phase deletion creating a stop codon immediately after the deletion site; and CA-pSVL, with a point mutation creating an Asp-to-Ala substitution at the putative protease active site. The wild-type, HXB2-pSVL, and the mutated viral genomes were used to transfect COS-M6 cells and to produce virions. Immunoblotting assays with a monoclonal antibody (MAb) specific for p24 showed that all three mutant contained a gag precursor, Pr56gag, with AH2 and CA expressing an extra band of about 160 kDa. Similar assays with a MAb specific for HIV-1 reverse transcriptase (RT) also revealed a 160-kDa protein from AH2 and CA virions and two mature p66 and p51 RT subunits from HXB2 virions. In addition, HXB2, AH2, and CA but not BH27 virions exhibited RT activity. The same protein in the 160-kDa band seemed to possess both p24 and RT components, since the MAb against p24 was able to immunoadsorb RT antigen and enzymatic activity. These results indicate that the HIV-1 gag-pol fusion protein produced in mammalian cells expressed significant RT activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandra A., Gerber T., Chandra P. Biochemical heterogeneity of reverse transcriptase purified from the AIDS virus, HTLV-III. FEBS Lett. 1986 Mar 3;197(1-2):84–88. doi: 10.1016/0014-5793(86)80303-9. [DOI] [PubMed] [Google Scholar]
- Chatton B., Walter P., Ebel J. P., Lacroute F., Fasiolo F. The yeast VAS1 gene encodes both mitochondrial and cytoplasmic valyl-tRNA synthetases. J Biol Chem. 1988 Jan 5;263(1):52–57. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Steimer K. S., Engleman E. G. Expression and processing of human immunodeficiency virus type 1 gag and pol genes by cells infected with a recombinant vaccinia virus. J Virol. 1989 Mar;63(3):1451–1454. doi: 10.1128/jvi.63.3.1451-1454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Billich S., Schulze T., Sukrow S., Moelling K. Partial purification and substrate analysis of bacterially expressed HIV protease by means of monoclonal antibody. EMBO J. 1988 Jun;7(6):1785–1791. doi: 10.1002/j.1460-2075.1988.tb03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Schulze T., Mellert W., Moelling K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. EMBO J. 1988 Jan;7(1):239–243. doi: 10.1002/j.1460-2075.1988.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Schulze T., Moelling K. RNase H activity associated with bacterially expressed reverse transcriptase of human T-cell lymphotropic virus III/lymphadenopathy-associated virus. J Biol Chem. 1987 Sep 15;262(26):12393–12396. [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Mills J., Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988 Aug;7(8):2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthardt A., Le Grice S. F. Biosynthesis and analysis of a genetically engineered HIV-1 reverse transcriptase/endonuclease polyprotein in Escherichia coli. Gene. 1988 Aug 15;68(1):35–42. doi: 10.1016/0378-1119(88)90596-3. [DOI] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. P., Salazar F. H., Mervis R. J., Raum M. G., Chan H. W., Ahmad N., Venkatesan S. Purification and structural characterization of the putative gag-pol protease of human immunodeficiency virus. J Virol. 1988 Aug;62(8):3053–3058. doi: 10.1128/jvi.62.8.3053-3058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori F., Scovassi A. I., Zella D., Achilli G., Cattaneo E., Casoli C., Bertazzoni U. Enzymatically active forms of reverse transcriptase of the human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988 Oct;4(5):393–398. doi: 10.1089/aid.1988.4.393. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Aitken A., Bradley C., Darby G. K., Larder B. A., Powell K. L., Purifoy D. J., Tisdale M., Stammers D. K. HIV-1 reverse transcriptase: crystallization and analysis of domain structure by limited proteolysis. Biochemistry. 1988 Dec 13;27(25):8884–8889. doi: 10.1021/bi00425a002. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Dayton B. D., Metcalf B. W., Dreyer G. B., Strickler J. E., Gorniak J. G., Rosenberg M., Moore M. L., Magaard V. W., Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Tanese N., Prasad V. R., Goff S. P. Structural requirements for bacterial expression of stable, enzymatically active fusion proteins containing the human immunodeficiency virus reverse transcriptase. DNA. 1988 Jul-Aug;7(6):407–416. doi: 10.1089/dna.1.1988.7.407. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., Rahman R., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Immunological and chemical analysis of P6, the carboxyl-terminal fragment of HIV P15. AIDS Res Hum Retroviruses. 1987 Fall;3(3):253–264. doi: 10.1089/aid.1987.3.253. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science. 1986 Mar 14;231(4743):1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]