Abstract
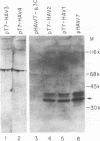
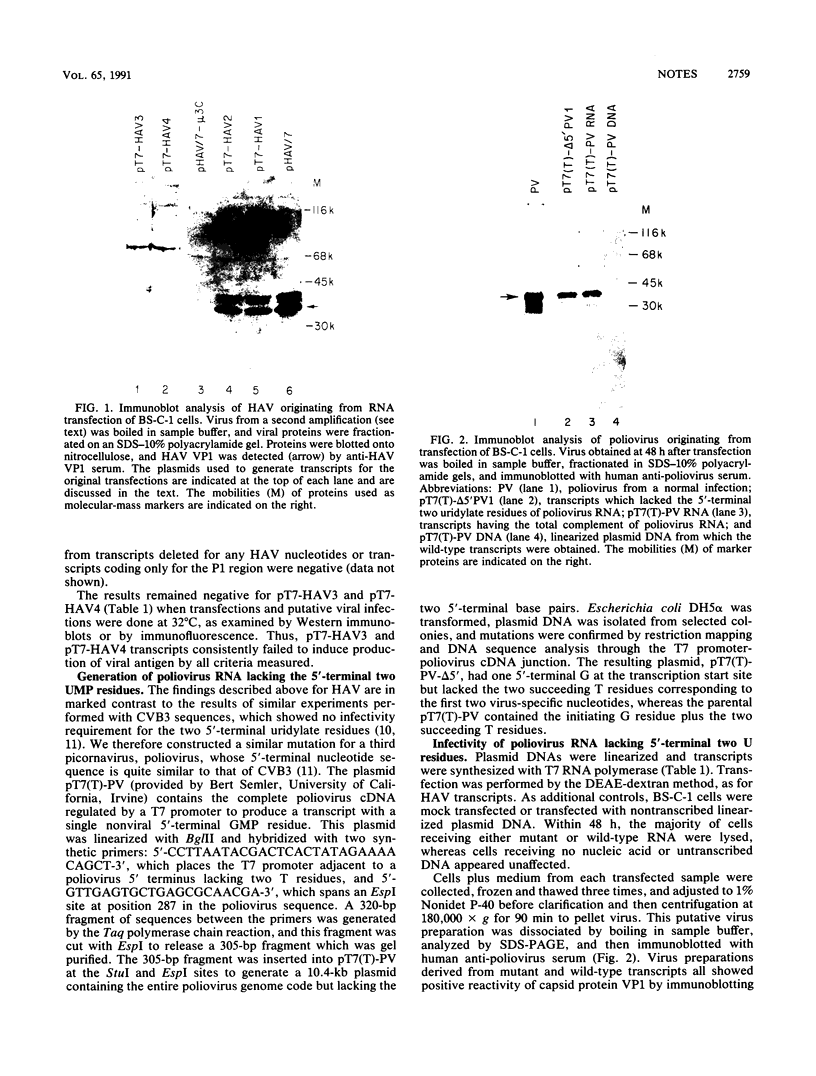
A series of plasmids containing hepatitis A virus (HAV) cDNA was constructed such that positive-strand HAV RNA could be transcribed with T7 RNA polymerase. The plasmids differed in the number of 5'-terminal nucleotides representing the junctions between vectors and HAV sequences that were present in the transcripts. When these transcripts were used to transfect cultured BS-C-1 cells, it was found that only those transcripts that contained all of the 5'-terminal HAV nucleotides, in addition to one or more nucleotides from the vector, were capable of initiating an infectious cycle leading to production of progeny virus. Transcripts that contained one 5'-terminal nucleotide from the vector sequence but were missing two uridylate residues corresponding to the first two nucleotides of HAV sequences, or were missing U and C residues corresponding to nucleotides 2 and 3 of the HAV sequence, were not infectious. A similar plasmid containing poliovirus cDNA was engineered to produce transcripts similarly lacking the first two uridylate residues of the poliovirus RNA sequence. These transcripts were infectious.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsaadi S., Hassard S., Stanway G. Sequences in the 5' non-coding region of human rhinovirus 14 RNA that affect in vitro translation. J Gen Virol. 1989 Oct;70(Pt 10):2799–2804. doi: 10.1099/0022-1317-70-10-2799. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Feinstone S. M., Rosenblum B., Purcell R. H. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J Virol. 1987 Oct;61(10):3035–3039. doi: 10.1128/jvi.61.10.3035-3039.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichot O., Girard M. An improved method for sequencing of RNA templates. Nucleic Acids Res. 1990 Oct 25;18(20):6162–6162. doi: 10.1093/nar/18.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust I. D., Coulepis A. G., Feinstone S. M., Locarnini S. A., Moritsugu Y., Najera R., Siegl G. Taxonomic classification of hepatitis A virus. Intervirology. 1983;20(1):1–7. doi: 10.1159/000149367. [DOI] [PubMed] [Google Scholar]
- Harmon S. A., Johnston J. M., Ziegelhoffer T., Richards O. C., Summers D. F., Ehrenfeld E. Expression of hepatitis A virus capsid sequences in insect cells. Virus Res. 1988 May;10(2-3):273–280. doi: 10.1016/0168-1702(88)90022-6. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Luz N., Beck E. Functional analysis of the internal translation initiation site of foot-and-mouth disease virus. J Virol. 1990 Oct;64(10):4625–4631. doi: 10.1128/jvi.64.10.4625-4631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Classification of hepatitis A virus as enterovirus type 72 and of hepatitis B virus as hepadnavirus type 1. Intervirology. 1982;18(3):105–106. doi: 10.1159/000149313. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Chernov B. K., Dmitrieva T. M., Agol V. I. Conservation of the secondary structure elements of the 5'-untranslated region of cardio- and aphthovirus RNAs. Nucleic Acids Res. 1989 Jul 25;17(14):5701–5711. doi: 10.1093/nar/17.14.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Romanova L. I., Sinyakov A. N., Maslova S. V., Agol V. I. Conserved structural domains in the 5'-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989 Feb;168(2):201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Meriam C. Poliovirus temperature-sensitive mutant containing a single nucleotide deletion in the 5'-noncoding region of the viral RNA. Virology. 1986 Dec;155(2):498–507. doi: 10.1016/0042-6822(86)90211-4. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Welsh J. D., Maizel J. V., Jr Comparative sequence analysis of the 5' noncoding region of the enteroviruses and rhinoviruses. Virology. 1988 Jul;165(1):42–50. doi: 10.1016/0042-6822(88)90656-3. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Racaniello V. R., Dunn G., Cooper J., Minor P. D., Almond J. W. New model for the secondary structure of the 5' non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989 May 20;207(2):379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- Takeda N., Kuhn R. J., Yang C. F., Takegami T., Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986 Oct;60(1):43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]