Few methods in neuroscience have attracted wider interest, among scientists and the general public, than those demonstrating patterns of brain activity in relation to behavior. First measuring 2-deoxyglucose (2DG) uptake in animals (1), then positron emission tomography (PET) in humans (2), and most recently functional MRI (fMRI) (3, 4) have provided dramatic views of what appears to be “the brain in action”: sensing, moving, feeling, reading, speaking, and even thinking. Although these patterns have been intriguing, what they actually mean in terms of brain mechanisms has been unclear. That they reflect local energy demands of active nerve cells, dependent on oxygen supplied by the local vasculature, is agreed for all methods. But what kinds of cellular activity are the patterns actually indicating? Are they due to neurons or glia; axons or synapses; action potentials or synaptic potentials; excitatory or inhibitory synaptic potentials? And how is the microvasculature matched to the demands of this activity? These questions are rapidly defining a new frontier in brain scanning (5).
Answers to these questions require animal experiments that can image local vasculature in relation to local active cells. A recent method for accomplishing this is two-photon microscopy, which enables observations of functional changes in cellular structures down to a micrometer or less in diameter. This approach has permitted imaging of red blood cell (RBC) flow in capillaries within the cerebral cortex of rats (6). Chaigneau et al. (7) have taken this a step further and, in this issue of PNAS, report the tracking of individual red blood cells within individual capillaries in relation to local areas of high synapse density and of thin unmyelinated axons. The results give intriguing insights into red blood cell movements in brain capillaries and raise some new questions about the energetics of brain cells and their processes.
The clarity of the new results stems in part from use of an ideal model system, the olfactory bulb. This structure is a kind of olfactory retina, with a rigid layered organization (8). The axons from receptor cells in the nose form a dense nerve layer on the bulbar surface before terminating just below in a layer of modules called olfactory glomeruli. Thousands of sensory axons converge on a single glomerulus, giving it one of the highest densities of synaptic terminals in the brain. Chaigneau et al. proposed to take advantage of this by making two-photon microscopic observations on blood flow in the local capillaries supplying an individual glomerulus during synaptic activation aroused by odor stimulation.
Intravascular injection of fluorescein dextran highlighted the network of precapillaries and capillaries that penetrate the glomerular layer, and intranasal injections of Oregon green highlighted the incoming axons and their terminals in the glomeruli. Individual RBCs in individual capillaries (inner diameters <6 μm) were observed. Odor stimulation caused an increase in instantaneous RBC velocity and flow in most cases. Functional recruitment of RBCs had been shown in the cortex (6); this study showed the effect in a single capillary, where it could be seen that when RBC velocity increases the RBCs elongate in shape. The basic dynamics of a capillary were in line with expectations. The responses appeared to be glomerulus and odor-specific: in a given intraglomerular capillary, one odor would cause increases in flow and another would cause decreases; two capillaries to the same glomerulus showed similar changes.
Contrary to expectations, the capillary network was relatively uniform through the glomerular and interglomerular spaces; there was no modularization of the microvasculature matching the modularization of glomerular synapses. This finding implied that diversion of blood flow to an active glomerulus must occur exclusively through local capillary regulation that adjusts flow within the glomerulus to the level of glomerular activity throughout the glomerulus. It will be interesting to test this mechanism in other parts of the brain with modular organization, such as the barrels of rodent somatosensory cortex (whose microvasculature has been characterized in ref. 9) or the cell islands/patches of the neostriatum.
The mechanism for this local control was not clear. The answer may be related to another unexpected result: no capillaries were observed within the olfactory nerve layer on the surface of the bulb. This finding is surprising because the nerve layer is filled with fine unmyelinated axons only 0.2 μm in diameter, and it is known from classic physiological studies of unmyelinated axons that their high surface-to-volume ratios give them very high energy demands in generating impulse activity (10). Both 2DG (11, 12) and fMRI (13) show not only high energy demands in the activated glomerular layer, but also high energy demands in the nerve layer adjacent to active glomeruli. Thus, it was anticipated that local microvasculature would be present in the olfactory nerve layer to provide the oxygen and glucose to meet these needs.
The authors combine these two unexpected findings to suggest that the local control of microvasculature may be at the level of venules draining blood from the glomerular layer; in such cases, the fMRI signal in the nerve layer “must relate to an increase in deoxyhemoglobin concentration in the veins [passing through the nerve layer] downstream of activated glomeruli and not to a local increase in metabolism triggered by the activation of olfactory nerve... bundles.” They were not able to identify venules to test this theory.
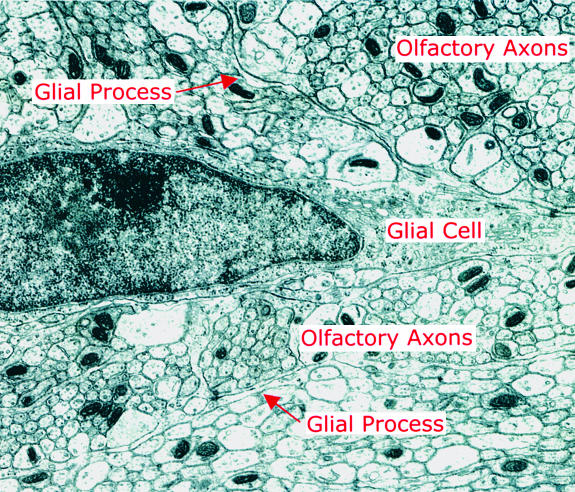
The current model for the relations between blood supply and active neurons has glial cells placed strategically between (14, 15). High-resolution 2DG shows active uptake in presumed glia cells in the nerve layer (16), supporting the idea that the glia may play a critical role here. The axons are known to be packed membrane-to-membrane in bundles surrounded by glia (Fig. 1). The outer axons within the bundle are obviously closely related to their glial wrapping (see the electron micrograph in Fig. 1), but how do the axons deep within the bundle get their oxygen and glucose supply? This problem arose with the first 2DG observations of activity in the nerve layer. What are the mechanisms by which a membrane pump, activated by an action potential, is able to signal the energy it needs to a mitochondrion that may be many microns distant and the mechanisms by which the mitochondrion signals its need for oxygen and glucose to the plasma membrane? These problems apply, as well, to the relation between a single capillary and the synapses within a glomerulus, which has a diameter (in the rat) of up to 100 μm. Perhaps sparsely active axons and terminals can draw on the surrounding extracellular milieu or neighboring inactive processes for immediate energy substrates.
Fig. 1.
Electron micrograph of the olfactory nerve layer of the rat olfactory bulb, showing unmyelinated olfactory axons arranged in bundles, demarcated by glial processes (arrows). No blood vessels are visible in this field. The electron micrograph was courtesy of Charles A. Greer, and the figure was prepared by C. Iwema.
Local control of microvasculature may be at the level of venules draining blood from the glomerular layer.
The study of Chaigneau et al. thus contributes to a broadening of interest in studies of cerebral metabolism, from the energy demands of synaptic activity to include unmyelinated axons. Unmyelinated axons make up the majority of nerve fibers in the brain, and impulse traffic in them accounts for a large part of the energy consumption that underlies cerebral cortical activity (17–19). The mechanisms that link unmyelinated axon activity, glial activity, and vascular supply are ripe for further investigation and need to be incorporated into models of the cellular basis of brain energy metabolism. Further studies are likely to give fundamental insights into understanding not only the neural basis of human brain activity patterns, but also the brain's response to losses of microvascular supply that occur in stroke, trauma, and degenerative neurological diseases. New technology matched to new animal models will continue to be crucial to this endeavor.
Acknowledgments
I thank Drs. M. F. Raichle, S. B. Laughlin, and C. A. Green for valuable discussions. Our research is supported by National Institutes of Health Grant DC-00086, the Human Brain Project (National Institute of Deafness and Other Communication Disorders, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and National Institute on Aging), and the Office of Navel Research (Multiuniversity Research Initiative).
See companion article on page 13081.
References
- 1.Kennedy, C., Des Rosiers, M. H., Jehle, J. W., Reivich, M., Sharpe, F. & Sokoloff, L. (1975) Science 187 850–853. [DOI] [PubMed] [Google Scholar]
- 2.Petersen, S. E., Fox, P. T., Posner, M. I., Mintun, M. & Raichle, M. E. (1988) Nature 331 585–589. [DOI] [PubMed] [Google Scholar]
- 3.Kwong, K. K., Belliveau, J. W., Chesler, D. A., Goldberg, I. E., Weisskoff, R. M., Poncelet, B. P., Kennedy, D. N., Hoppel, B. E., Cohen M. S., Turner, R., et al. (1992) Proc. Natl. Acad. Sci. USA 89 5675–6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa, S., Tank, D. W., Menon, R., Ellermann, J. M., Kim, S. G., Merkle, H. & Ugurbil, K. (1992) Proc. Natl. Acad. Sci. USA 89 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle, M. E. (2003) J. Neurosci. 23 3959–39562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. (1998) Proc. Natl. Acad. Sci. USA 95 15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaigneau, E., Oheim, M., Audinat, E. & Charpak, S. (2003) Proc. Natl. Acad. Sci. USA 100 13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd, G. M. & Greer, C. A. (1998) in The Synaptic Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, New York), pp. 159–204.
- 9.Woolsey, T. A., Rovainen, C. M., Cox, S. B., Henegar, M. H., Liang, G. E., Liu, D., Moskalenko, Y. E., Sui, J. & Wei, L. (1996) Cereb. Cortex 6 647–660. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie, J. M. (1995) in The Axon: Structure, Function, and Pathophysiology, ed. Waxman, S. G., Kocsis, J. D. & Stys, P. K. (Oxford Univ. Press, New York), pp. 68–96.
- 11.Sharp, F. R., Kauer, J. S. & Shepherd, G. M. (l977) J. Neurophysiol. 40 800–8l3. [DOI] [PubMed] [Google Scholar]
- 12.Greer, C. A., Stewart, W. B., Kauer, J. S. & Shepherd, G. M. (1981) Brain Res. 217 279–293. [DOI] [PubMed] [Google Scholar]
- 13.Yang, X., Renken, R., Hyder, F., Siddeek, M., Greer, C. A., Shepherd, G. M. & Shulman, R. G. (1998) Proc. Natl. Acad. Sci. USA 95 7715–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magistretti, P. J. (2000) Brain Res. 886 108–112. [DOI] [PubMed] [Google Scholar]
- 15.Shulman, R. G., Hyder, F. & Rothman, D. L. (2001) NMR Biomed. 14 389–396. [DOI] [PubMed] [Google Scholar]
- 16.Benson, T. E., Burd, G. D., Greer, C. A., Landis, D. M. D. & Shepherd, G. M. (1985) Brain Res. 339 67–78. [DOI] [PubMed] [Google Scholar]
- 17.Attwell, D. & Laughlin, S. B. (2001) J. Cereb. Blood Flow Metab. 21 1133–1145. [DOI] [PubMed] [Google Scholar]
- 18.Lennie, P. (2003) Curr. Biol. 13 493–497. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis, N. K. (2003) J. Neurosci. 23 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]