Abstract
Dysregulation of the Wnt pathway and altered β-catenin expression are central early events in colorectal carcinogenesis. We studied the ortho, meta, and para (o-, m-, and p-) positional isomers of NO-donating aspirin (NO-ASA), a chemopreventive agent against colon cancer, for their effect on β-catenin/T cell factor (TCF) signaling. In human SW480 colon carcinoma cells, cell-growth inhibition by NO-ASA [IC50 values for p-, o-, and mwere 48.1 ± 4.3 (mean ± SEM), 60.4 ± 2.1, and 900 ± 50 μM, respectively] was accompanied by significant inhibition of β-catenin signaling. We determined β-catenin-dependent TCF-4 transcriptional activity by measuring the activity of the luciferase gene placed under the control of TCF-4 regulatory sequences. The IC50 values for β-catenin/TCF-4-signaling inhibition by NO-ASA were: o-, 2.6 ± 0.4; m-, 15 ± 5; p-, 1.1 ± 0.1 μM; and for ASA, >5,000 μM. Total or nuclear levels of β-catenin and its distribution in the cell were not altered by NO-ASA, as judged by protein expression levels and semiquantitative immunofluorescence analysis. NO-ASA disrupted the association of β-catenin and TCF-4 in the nucleus, whereas ASA did not affect it. NO-ASA reduced the expression of cyclin D1, a downstream target gene that plays an important role in colon carcinogenesis. In contrast, a structural analog of NO-ASA lacking the —NO2 moiety did not affect TCF-4 transcriptional activity. Thus, NO-ASA inhibits β-catenin-mediated TCF activity by preventing the formation of the β-catenin/TCF-4 complex. This effect, occurring at NO-ASA concentrations far below those required to inhibit cell growth, may be a critical early event in the chemopreventive activity of NO-ASA against colon cancer.
Colorectal cancer (CRC), the third leading cause of cancer mortality in the United States, is a highly preventable cancer. Of several strategies that can reduce colon cancer risk, chemoprevention is emerging as a promising approach (1). The aim of chemoprevention is to decrease the incidence of the cancer being targeted in an at-risk population. Nonsteroidal antiinflammatory drugs (NSAIDs) are the prototypical chemopreventive agents against CRC, as evidenced by a plethora of data, including >30 epidemiological studies, and by recent human interventional trials using aspirin (ASA) (2). The major impediments to the general application of NSAIDs as chemopreventive agents are their limited efficacy (at best 50% reduction of tumor incidence) and considerable side effects, mainly from the gastrointestinal system and the kidneys. NO-donating NSAIDs (NO-NSAIDs) have been introduced as a class of safer compounds with minimal damage to the gastric mucosa as compared with traditional NSAIDs (3). A recent study in healthy humans demonstrated that, at least in this group, NO-donating aspirin (NO-ASA) avoids gastrointestinal damage, while it maintains cyclooxygenase-1 and platelet inhibitory activity (4).
A phenomenon that is central to the early stages of colorectal carcinogenesis is dysregulation of the Wnt pathway, including altered β-catenin expression. In particular, mutations of the APC gene, which occur in 85% of human sporadic CRCs or activating mutations in the CTNNB1 (β-catenin) can lead to the accumulation of free catenin in the cytosol (reviewed in ref. 5). Current understanding indicates that free β-catenin in the cytoplasm and nucleus lead to its association with T cell factor (TCF) family of transcription factors and to subsequent transcriptional activation of target genes such as cyclin D1, resulting in increased cell proliferation. For CRC chemoprevention, agents that can modulate this pathway and control the expression of β-catenin and its downstream targets could be highly effective. In several CRC cell lines, ASA attenuates β-catenin/TCF signaling; however, the molecular mechanism behind this effect remains elusive (6). We have demonstrated that NO-ASA inhibits the growth of CRC and other cancer cell lines far more potently than ASA (7, 8). Such enhanced potency has also been demonstrated in animal models of colon cancer prevention (9).§ In the present study, we investigated the effect of NO-ASA on the β-catenin/TCF pathway in human CRC cells, examined which part of the NO-ASA molecule likely causes the enhanced effect that we observed, and explored the molecular mechanism of its chemopreventive effect against CRC.
Methods
Reagents and Cell Culture. NO-ASA; the para isomer (NCX4040), 2-(acetyloxy)benzoic acid 4-(nitrooxy methyl)phenyl ester; the ortho isomer (NCX 4060), 2-(acetyloxy)benzoic acid 2-(nitrooxy methyl)phenyl ester; and the meta isomer (NCX 4016), 2-(acetyloxy)benzoic acid 3-(nitrooxy methyl)phenyl ester were obtained from Nicox, Sophia Antipolis, France. ASA was from Sigma. Stock solutions (100 mM) were made in DMSO; final DMSO concentration was adjusted in all media to 1%. SW480 cells (CCL-228, American Type Culture Collection) were grown per American Type Culture Collection instructions.
Plasmids, Transient Transfection, and Reporter Gene Assay. Transient transfection was performed by using FuGENE 6 (Roche Molecular Biochemicals) according to the manufacturer's instructions. In brief, 105 cells were transfected with 400 ng of luciferase reporter constructs TOPflash or FOPflash (Upstate Biotechnology, Lake Placid, NY) and 200 ng of pSV-βgal vector as internal control. TOPflash contains three copies of the TCF/LEF-binding site (AAGATCAAAGGGGGT) upstream of a TK minimal promoter. FOPflash contains a mutated TCF/LEF-binding site (AAGGCCAAAGGGGGT). The plasmid S33Y-CAT (containing the cDNA for a constitutive active mutant of β-catenin) was provided by B. Vogelstein (Johns Hopkins Oncology Center, Baltimore). Two hours after transfection, cells were treated for 18 h with NO-ASA or ASA. Luciferase activity was measured according to the manufacturer's instructions, and β-galactosidase (βgal) activity was measured by using standard protocols. The activity was normalized to βgal values and expressed as relative fold compared with control.
Cell-Growth Inhibition Assay. The growth-inhibitory effect of NO-ASA on SW480 cells was measured by using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay kit (Roche Molecular Biochemicals). In brief, cells were plated in 96-well plates at a density of 13.7 × 103 cells per well and, after overnight incubation, NO-ASA was added to the culture medium. At indicated times of treatment, viable cells were quantified with MTT substrate according to the manufacturer's instructions. Growth inhibition was expressed as percentage of the corresponding control.
Western Blot Analysis and Immunoprecipitation. After treatment with the test drug, cells were harvested and lysed in 50 mM Tris·HCl buffer (pH 7.5) containing 150 mM NaCl, 1 mM EDTA, 0.5% octylphenyl-polyethylene glycol, and 10% glycerol in the presence of proteinase inhibitors. Nuclear extracts were prepared as described (10). Proteins were fractionated by SDS/PAGE and transferred to Immobilon P membrane (Millipore). Primary mouse monoclonal antibodies were against the following at the dilutions indicated: β-catenin, 1:1,000 (BD Transduction Laboratories); TCF-4, 1:500 (Upstate Biotechnology); β-tubulin, 1:1,000, and α-actin, 1:1,000 (Santa Cruz Biotechnology); and rabbit polyclonal anti-Cyclin D1, 1:1,000 (Upstate Biotechnology). Secondary antibodies conjugated to horseradish peroxidase (1:4,000) were from Sigma. Immunoreactive protein was detected by using ECL chemiluminescence (Amersham Pharmacia). For coimmunoprecipitation, TCF-4 antibody (1 μg) and 100 μl of protein A-Sepharose (3 mg/ml) were added to 100 μg of nuclear extract in the above-mentioned buffer and incubated at 4°C for 2 h. The beads were washed three times with this buffer, and the proteins were dissolved in sample buffer for SDS/PAGE.
Detection of β-Catenin by Immunofluorescence. SW480 cells (0.2 × 104), plated in Nalge Nunc slide chambers and treated as described above, were fixed with 4% paraformaldehyde and blocked with PBS containing 5% goat serum. Primary mouse monoclonal β-catenin antibody was used at a concentration of 2.5 μg/ml (BD Transduction Laboratories) in PBS containing 1% BSA and 0.2% Tween 20 (buffer B); goat anti-mouse IgG, rhodamine-conjugated, 1:200 (Pierce), diluted in buffer B, was used as secondary antibody. Ten random fields in mounted slides were observed with an Olympus Provis microscope at ×400 magnification to assess the subcellular localization of β-catenin. Each study, performed in duplicate, was repeated at least once.
Results
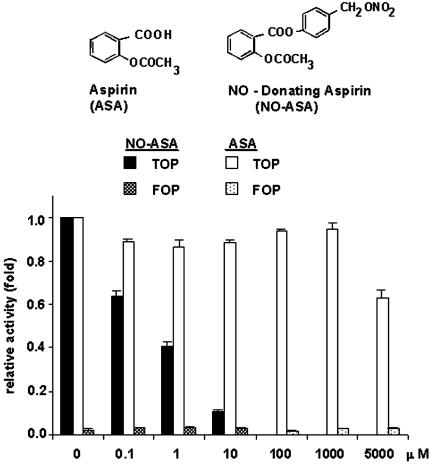
NO-ASA Inhibits β-Catenin/TCF Transcriptional Activity More Potently than ASA. To investigate whether NO-ASA modulates β-catenin-dependent TCF transcriptional activity, we used the SW480 human CRC cell line. In most CRC cell lines the β-catenin/TCF pathway is constitutively active because of defective APC or β-catenin functions. The SW480 cell line (mutant APC, wild-type CTNNB1, COX-2 negative) has elevated levels of β-catenin and is a good representative for the majority of dysplastic epithelial cells in human sporadic colorectal adenomas (11). SW480 cells were transiently transfected with the reporter plasmids TOP-flash or FOPflash and pCMV-β galactosidase to normalize for transfection efficiency and as an indicator of potential toxicity of the treatments. A range of concentrations of p-NO-ASA and ASA were added to transfected cells followed by assay for luciferase activity. Fig. 1 shows that p-NO-ASA strongly inhibits TCF activity in these cells in a concentration-dependent manner. At 18 h, p-NO-ASA (10 μM) inhibited β-catenin/TCF transcriptional activity by 90% compared with control. In contrast, a rather high concentration of ASA (5 mM) inhibited transcriptional activity by ≈35%, which is consistent with the observations made by other investigators (6). The IC50 of NO-ASA for this effect is 1.1 ± 0.1 μM (mean ± SEM), whereas for ASA it is >5,000 μM. Thus, p-NO-ASA is a much more potent down-regulator of β-catenin/TCF signaling than traditional ASA.
Fig. 1.
Inhibition of the TCF-4-responsive luciferase reporter gene by NO-ASA. SW480 cells were cotransfected with luciferase reporter plasmids that have TCF-4-binding sites (TOP) or a mutant TCF-binding site (FOP) and the lacZ expression plasmid pSV-βgal as internal control and treated with NO-ASA or ASA. Luciferase activity, normalized to the corresponding β-gal activity, was expressed as fold activation compared with control (DMSO control) cells. Values represent mean ± SEM of two representative experiments performed in triplicate.
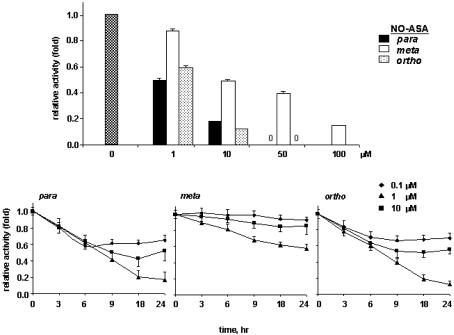
We compared the kinetics of inhibition of β-catenin/TCF activity by the three positional isomers of NO-ASA (p-, m-, and o-). SW480 cells were transiently transfected as described and treated with 1, 10, 50, and 100 μM each isomer for 18 h; luciferase activity was measured. The p- and o-isomers showed stronger inhibitory activity as compared with the m-isomer (Fig. 2A). The p- and o-isomers at 10 μM concentration reduced the transcriptional activity by 72% and 90%, respectively, compared with control; however, the m-isomer was less potent, requiring 100 μM concentration to get the same effect. Further comparison was done at lower concentrations and various times up to 18 h and beyond. A time– and concentration–response curve shows that 50% reduction in TCF activity occurred after ≈8 h of treatment with 10 μM p- and o-isomers (Fig. 2B). At this time interval, p-NO-ASA was also effective at the very low concentration of 0.1 μM, reducing the transcriptional activity by ≈40% compared with control. The IC50 for p-isomer is 1.1 ± 0.1 μM, for o-isomer, 2.6 ± 0.4 μM, and for m-isomer, 15 ± 5 μM (Table 1).
Fig. 2.
Concentration- and time-dependent inhibition TCF-4 responsive reporter gene by NO-ASA isomers. SW480 cells, cotransfected with luciferase reporter plasmids (TOP/FOP) and the pSV-βgal as in Methods, were treated with p-, m-, or o-NO-ASA, as in Methods. Relative TCF activity (fold) of treated cells is shown (DMSO control was set as 1); values are mean ± SEM of two representative experiments performed in triplicate. (Top) p- and o-NO-ASA inhibit TCF signaling more potently than the m-isomer. Cells were treated for 18 h. (Bottom) Compared with m-isomer, the p- and o-NO-ASA inhibit TCF, earlier and with lower IC50 values.
Table 1. The IC50 values for NO-ASA-induced inhibition of cell growth and β-catenin/TCF-4 transcription in SW480 colon cancer cells at 18 h.
| β-Catenin/TCF transcription |
|||
|---|---|---|---|
| Compound | Cell growth IC50, μM | IC50, μM | % IC50 for growth |
| NO-ASA | |||
| o- | 60.4 ± 2.1 | 2.6 ± 0.4 | 3.8 |
| m- | 900 ± 50 | 15 ± 5.0 | 1.1 |
| p- | 48.1 ± 4.3 | 1.1 ± 0.1 | 1.9 |
| ASA | >5,000 | >5,000 | |
Values are mean ± SEM (n ≥ 3).
Because activated β-catenin/TCF signaling modulates cell proliferation, we examined in parallel the effect of NO-ASA on SW480 cell growth. Compared with ASA, all three isomers of NO-ASA inhibited cell growth strongly (Table 1); e.g., the IC50 of p-NO-ASA for cell-growth inhibition is 48.1 ± 4.3 μM in contrast to ASA, which has a value of >5,000 μM. Of note, when the effect of NO-ASA on cell growth was compared with its effect on β-catenin/TCF signaling for all three positional isomers, the IC50 for β-catenin/TCF-signaling inhibition ranged from 1.1% to 3.8% of the IC50 for cell-growth inhibition.
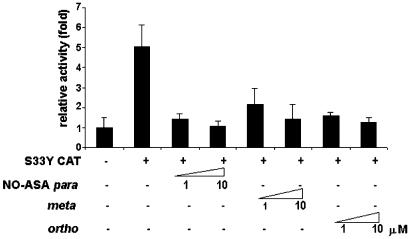
NO-ASA Acts on β-Catenin or Its Downstream Elements. An important step in the activation of TCF is its interaction with β-catenin. We investigated if NO-ASA acts by inhibiting β-catenin or other Wnt-pathway components. To distinguish these possibilities, SW480 cells were cotransfected with a constitutively active mutant of β-catenin (S33Y cat) along with the reporter constructs. This constitutively active mutant cannot be phosphorylated by GSK3, a property that leads to its accumulation in the cytosol. On cotransfection, TCF activity was substantially increased (5-fold) in these cells, confirming that the S33Y-β-catenin was expressed amply (Fig. 3). The positional isomers p- and o-NO-ASA (1 or 10 μM) essentially abrogated this increased activity, returning it to the basal level, whereas the m-isomer exhibited a somewhat weaker effect. These results indicate that NO-ASA exerts its inhibitory effect on β-catenin/TCF signaling either by acting on β-catenin itself or on components downstream of β-catenin in this pathway.
Fig. 3.
NO-ASA inhibits mutant β-catenin-mediated increase in TCF activity. SW480 cells were transiently transfected with TOP/FOP reporters and pSV-βgal as in Methods. S33Y CAT, an expression vector for constitutively active mutant of β-catenin, was cotransfected as indicated. Two hours after transfection, cells were treated with 1 or 10 μM p-, m-, or o-NO-ASA, and the relative transcriptional activity was determined.
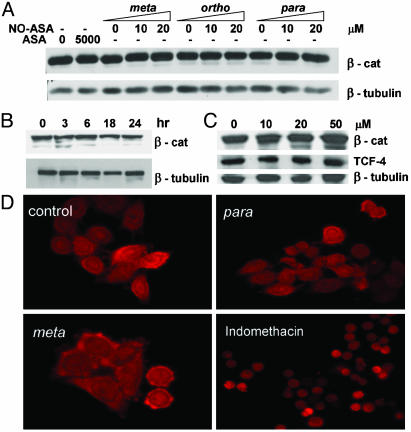
NO-ASA Does Not Affect Total Cellular Levels of β-Catenin or Its Distribution in the Cell. We considered the possibility that NO-ASA acts on β-catenin itself. If so, then it would down-regulate either its cellular levels or its translocation from the cytoplasm into the nucleus. The possibility of down-regulation of β-catenin expression by NO-ASA was examined by assessing its cellular levels after NO-ASA treatment. Lysates of SW480 cells were made after treatment with NO-ASA at concentrations causing near-maximal β-catenin/TCF inhibition. As shown in Fig. 4A, the total levels of β-catenin were not altered by 10 or 20 μM NO-ASA. All three isomers of NO-ASA and ASA (5 mM) were similar in this respect. To examine the possibility of a reduction followed by recovery of β-catenin (i.e., an oscillatory behavior), we performed a time–response study. SW480 cells were treated with NO-ASA for up to 24 h, covering times both well before and after 18 h. Fig. 4B shows that the levels of β-catenin remained essentially unaltered during the 24-h period of observation.
Fig. 4.
NO-ASA does not alter the expression and distribution of β-catenin (β-cat). β-Catenin expression was detected by immunoblotting in lysates of SW480 cells treated with p-, m-, or o-NO-ASA or ASA for 18 h (A), or with p-NO-ASA for up to 24 h (B). (C) β-Catenin and TCF-4 levels were determined by immunoblotting in nuclear lysates of SW480 cells treated with p-NO-ASA (0–50 μM) for 18 h; blots were reprobed with an anti-β-tubulin antibody as loading control. (D) The cellular distribution of β-catenin in SW480 cells treated with p-or m-NO-ASA (each 20 μM) or indomethacin (200 μM) for 18 h was determined by immunofluorescence analysis. After fixation, cells were stained with anti-β-catenin antibody as in Methods and observed under ×400 magnification.
Translocation of β-catenin into the nucleus is required for its transcriptional activity as a coactivator. Because β-catenin exists both in the cytosol and in the nucleus, we explored the possibility that NO-ASA may alter its distribution between the nucleus and the cytosol, thereby inhibiting TCF activity. SW480 cells were treated with NO-ASA for 18 h as described earlier, and β-catenin was detected by immunofluorescence. Fig. 4D shows strong nuclear and cytoplasmic expression of β-catenin in control SW480 cells; neither the distribution nor the intensity of β-catenin was significantly affected by 20 μM NO-ASA. The nuclear staining of β-catenin remains similar overall in cells treated with any one of the three positional isomers of NO-ASA. ASA (5 mM) also showed no effect on β-catenin. Similar results were obtained with lower concentrations of NO-ASA (data not shown). As a control for cell response, treatment with 200 μM indomethacin caused rounding of the cells, which remained adhered. In such cells the overall β-catenin expression was lower than control, although its cellular distribution was not significantly altered.
To strengthen our conclusion that NO-ASA does not affect the expression of nuclear β-catenin, we determined by immunoblotting the nuclear levels of both β-catenin and TCF-4. Treatment of SW480 cells with 10, 20, or 50 μM NO-ASA did not alter the nuclear levels of either β-catenin or TCF (Fig. 4C). These findings suggest that NO-ASA decreases β-catenin/TCF-dependent transcription by mechanisms other than reduced nuclear levels of either component of this complex.
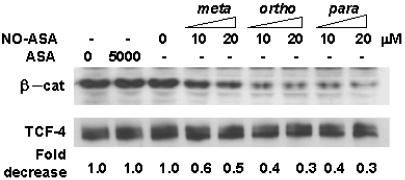
NO-ASA Disrupts the β-Catenin/TCF Complex in the Nucleus. The integrity of the β-catenin/TCF complex is required for its proper transcriptional activity. Because the nuclear levels of these two proteins remained the same after NO-ASA treatment and no evidence appeared of their degradation (no breakdown products on immunoblots), we examined the possibility that NO-ASA disrupts the association of β-catenin with TCF-4. Thus, we treated SW480 cells with either NO-ASA or ASA as described earlier. TCF-4 and β-catenin were coimmunoprecipitated from nuclear lysates by using an anti-TCF antibody. As shown in Fig. 5, in the nucleus of untreated cells, a substantial level of β-catenin is associated with TCF-4. In contrast, NO-ASA (p- and o-) used at concentrations that inhibit β-catenin/TCF-dependent transcription, disrupted significantly the association between β-catenin and TCF. Concentrations of 10 or 20 μM p-NO-ASA reduced this association to 43% and 30%, respectively, with respect to untreated control. The m-isomer inhibited this association, but less potently, in agreement with our observations with the transcription reporter assay. ASA, on the other hand, had no appreciable effect on the TCF-4–β-catenin interaction. Comparable amounts of TCF-4 were immunoprecipitated from each of the extracts. Because the total amount of nuclear β-catenin remained unchanged, NO-ASA disrupts the β-catenin–TCF interaction without interfering with the translocation of β-catenin into the nucleus.
Fig. 5.
NO-ASA disrupts the interaction of β-catenin and TCF-4 in the β-catenin/TCF complex. SW480 cells were treated with NO-ASA or ASA as indicated for 18 h. TCF-4 and β-catenin were coimmunoprecipitated from nuclear lysates by using an anti-TCF mouse monoclonal antibody, and β-catenin levels were analyzed by immunoblotting. The blot was reprobed for TCF-4 levels.
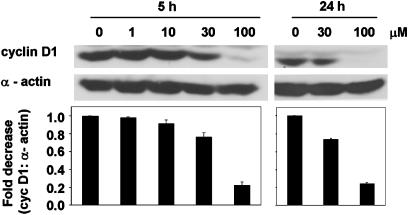
NO-ASA Modulates the Expression of β-Catenin/TCF-Dependent Genes. Of the several of the genes whose transcription is regulated by β-catenin/TCF-4, cyclin D1 plays an important role in colon carcinogenesis (12, 13). Therefore, we investigated whether NO-ASA affects the expression of cyclin D1 in SW480 cells. SW480 cells were synchronized by serum deprivation for 24 h followed by NO-ASA treatment in the presence of 15% serum for 5 or 24 h. Cyclin D1 protein expression is down-regulated in a concentration-dependent manner in response to NO-ASA (Fig. 6). For example, at 24 h, 100 μM p-NO-ASA reduced cyclin D1 expression by nearly 80% compared with control.
Fig. 6.
Cyclin D1 is down-regulated by NO-ASA. SW480 cells were serum-deprived for 24 h followed by NO-ASA treatment at the indicated concentrations, in the presence of 15% serum for 5 or 24 h. Blots were probed with anti-cyclin D1 rabbit polyclonal antibody and reprobed for α-actin levels.
The —NO2 Group of NO-ASA Is Critical for the Modulation of β-Catenin/TCF Activity. To investigate the contribution of key structural components of the NO-ASA molecule, we used two structural analogs of p-NO-ASA, one with its —NO2 group replaced by —OH and another in which the —CH3OOH is replaced by —OH. Pharmacologically, —NO2 is considered pivotal for the properties of NO-ASA beyond those of ASA. The —CH3OOH group, on the other hand, is the critical part of ASA for inactivation of cyclooxygenase (COX).
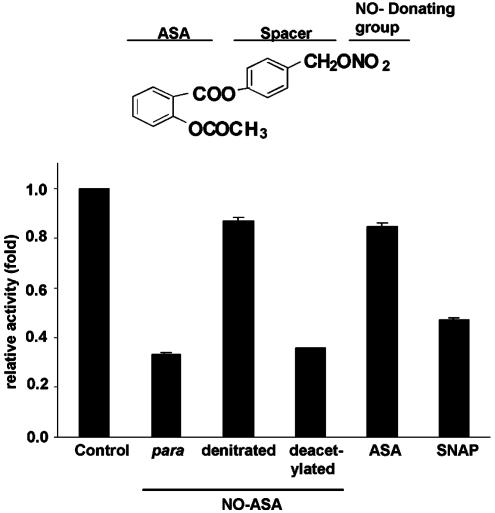
SW480 cells were treated for 18 h with 1 μM p-NO-ASA or its denitrated analog, i.e., the one that lacks —NO2 (Fig. 7). The denitrated derivative down-regulated β-catenin/TCF-dependent transcriptional activity by only 15%, compared with control. In contrast, p-NO-ASA decreased it, as expected, by 70%. Equimolar concentrations of ASA were equipotent with the denitrated analog. Of note, the exogenous NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP), administered at equimolar concentrations, inhibited β-catenin/TCF-dependent transcription by 55%, thus strengthening the conclusion that the —NO2 group is critical for the modulation of β-catenin/TCF activity. The deacetylated analog of NO-ASA was virtually equipotent with NO-ASA, indicating that the —CH3OOH group plays no role in inhibiting transcriptional activity by NO-ASA.
Fig. 7.
The effect of NO-ASA structural analogs on TCF-4-dependent transcriptional activity. SW480 cells were cotransfected with reporters TOP/FOP and pSV-βgal, and treated for 18 h with a 1 μM concentration of each of the following: p-NO-ASA, denitrated analog of p-NO-ASA, deacetylated analog of p-NO-ASA, ASA, or the NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP). Relative luciferase activity was determined as in Methods.
Discussion
The strong growth-inhibitory effect of NO-ASA on various colon cancer cell lines and its chemopreventive efficacy against CRC in animal model systems have raised the important question of the underlying molecular mechanisms. Our findings document that NO-ASA inhibits profoundly the β-catenin/TCF-signaling pathway in a human colon cancer cell line. Our work has two particularly interesting findings. First, inhibition of β-catenin/TCF signaling by NO-ASA occurs at concentrations far lower than those that inhibit cell growth, suggesting a potentially clinically important effect. Second, our results indicate that the mechanism by which this effect is brought about appears to be distinct.
The inhibition of β-catenin/TCF signaling by NO-ASA, as measured by changes in the luciferase reporter gene under the control of the TCF-4 regulatory sequences, was profound. The three main features of this inhibition are its concentration, time, and positional isomer dependence. The concentration dependence of this inhibition is best appreciated if viewed in conjunction with the biological effect of NO-ASA that is relevant to colon cancer, namely, inhibition of cell growth. For all three positional isomers, the IC50 for β-catenin/TCF-signaling inhibition ranged between 1.1% and 3.8% of the IC50 for cell-growth inhibition. The effect of NO-ASA became evident as early as 3 h and started to plateau at 9 h, becoming maximal at 24 h when the highest concentration of NO-ASA studied (10 μM) is considered. Finally, a remarkable feature was the influence of positional isomerism. The p- and o-positional isomers of NO-ASA inhibited cell growth and β-catenin/TCF signaling with similar potency, which was at least one order of magnitude lower than that for the m-isomer.
The effect of NO-ASA on β-catenin/TCF-dependent gene transcription can be important for the function of the cell. The importance of this effect is clearly indicated by the reduced expression of cyclin D1, a protein that plays an important role in cell-cycle transitions. NO-ASA led to a sustained suppression of cyclin D1 levels, which is consistent with the inhibition of G1-to-S transitions that it brings about in colon cancer cells (7).
Experiments designed to elucidate the underlying mechanism have revealed a distinct way of modulating β-catenin/TCF signaling. Expression of a constitutively active β-catenin mutant increased the expression of the reporter gene 5-fold; this β-catenin is stable because it lacks Ser-33 and therefore GSK-3β cannot phosphorylate it and degrade it by means of ubiquitination (14). That NO-ASA suppressed this enhanced expression by ≈80% suggests that NO-ASA acts either at the β-catenin level or downstream of it in the Wnt-signaling cascade. Consequently, we examined whether NO-ASA affects either the expression or the distribution of β-catenin in the colon cancer cell. Both possibilities were ruled out based on (i) the lack of change of total or nuclear β-catenin levels and (ii) the lack of changes in the distribution of this molecule in the cell, as judged (semi-quantitatively) by the immunofluorescence study. Moreover, the nuclear levels of TCF-4, a molecule with which β-catenin must interact in the nucleus to effect gene transcription, were not altered by NO-ASA.
We investigated the possibility that, under the influence of NO-ASA, these two molecules, present in their normal amounts in the nucleus, do not associate with each other, as they normally do to perform their physiological function. That this was the case was clearly shown by our finding that TCF-4 immunoprecipitated from the nuclei of NO-ASA-treated cells had significantly reduced amounts of β-catenin associated with it. Thus, 20 μM m-isomer reduced the β-catenin–TCF-4 association by 50% compared with control, the p- and o-isomers at the same concentration reduced it by 70% and 75%, respectively. At least two possible explanations exist for this effect of NO-ASA. First, NO-ASA could modify either β-catenin or TCF-4 or both in a way that such modification sterically or otherwise inhibits their physical association, thus diminishing their transcriptional activity. Two types of covalent changes of these proteins that could account for this effect are their S-nitrosylation or tyrosine nitration. This notion is consistent with the reported effects of NO-ASA on other target proteins, such as caspases, and with our observation that denitrated NO-ASA had only a marginal effect on our β-catenin/TCF gene transcription assay. A second possible explanation is that NO-ASA may induce a protein(s) that somehow inhibits the formation of a transcriptionally active complex between TCF-4 and β-catenin.
The regulation of the β-catenin/TCF signaling has been the subject of recent studies. For example, chemopreventive agents, such as traditional NSAIDs and curcumin, have been reported to down-regulate β-catenin/TCF signaling by caspase-mediated degradation of β-catenin (15). On the other hand, complex endogenous regulatory mechanisms of this signaling pathway are known, such as the ubiquitin–proteasome pathway, which controls the fate of β-catenin (16). The mechanism we have identified, i.e., down-regulation of β-catenin/TCF signaling by disruption of the physical association between β-catenin and TCF-4, perhaps represents an important pharmacological activity of NO-ASA.
The significance of the —NO2 moiety of NO-ASA in its effect on β-catenin/TCF signaling is underscored by our structure–activity study. The analog of NO-ASA that lacked —NO2 had no effect on the transcriptional activity that we monitored. Consistent with this idea is the observation that equimolar amounts of synaptosome-associated protein, an exogenous NO donor, decreased this activity by half. On the other hand, our data virtually exclude a role for COX in bringing about these results. First, the deacetylated analog of NO-ASA had the same effect on transcriptional activity as the complete NO-ASA molecule; the acetyl group is the one that inactivates COX. Second, SW480 cells have low levels of COX-1 and no detectable expression of COX-2 (17).
An interesting finding was the striking difference in potency between NO-ASA and traditional ASA with respect to their effect on β-catenin/TCF signaling, with NO-ASA being two orders of magnitude more potent than ASA. ASA, even at millimolar concentrations, had only a modest effect on β-catenin/TCF signaling. This difference cannot be ascribed to a simple variation in potency but reflects a major mechanistic difference between these two forms of aspirin. Unlike ASA, NO-ASA inhibits the association between β-catenin and TCF-4 in the nucleus. This finding is in agreement with the report of Dihlmann et al. (6), who noted that ASA treatment of SW480 cells had no effect on the binding of the β-catenin–TCF-4 complex to DNA, implying its uninhibited formation.
Our findings suggest a potential explanation for the significant chemopreventive activity of NO-ASA against CRC, which, as observed in preclinical work, is superior to that of traditional ASA. Three considerations are relevant to this view. First, activated β-catenin/TCF signaling is an early and quantitatively significant event in colon carcinogenesis. This signaling pathway affects the expression of many genes relevant to colon carcinogenesis, such COX-2, c-myc, cyclin D1, MMP-7, and others (reviewed in ref. 5). Thus, our observations suggest that the inhibition of this pathway by low concentrations of NO-ASA could represent a critical disruption in the carcinogenic process. Indeed, inhibition of several pathways may be required to prevent a change in the cell as complex as its neoplastic transformation. Second, the effect of NO-ASA on β-catenin/TCF signaling takes place at very low concentrations compared with its growth-inhibitory effect. Both these properties of NO-ASA are entirely consistent with the concept of chemoprevention, according to which successful agents act during early stages of the process they control. Third, NO-ASA is far more potent than traditional ASA. Because traditional ASA is a proven, albeit less-than-optimal, chemopreventive agent against CRC, if this pathway is, as expected, relevant to colon carcinogenesis, and if NO-ASA has a similarly superior potency in humans as in our system, then one might expect NO-ASA to be a strong chemopreventive agent against CRC. It probably requires no reiteration here that cell culture data cannot be safely extrapolated to humans and that our findings simply form the basis to explore a mechanistic possibility in humans, to whom chemoprevention is to be ultimately applied.
In conclusion, our data demonstrate that NO-ASA, at concentrations well below those needed to inhibit the growth of a human colon cancer cell line, inhibits profoundly the β-catenin/TCF-signaling pathway, thus modulating the expression of dependent genes. In this regard, NO-ASA is far more potent than traditional ASA, accounting, at least in part, for its grater biological efficacy. This effect, brought about by a commensurate inhibition of the physical association of β-catenin and TCF-4 that is required for their transcription-regulatory effect, may be a critical early event in the chemopreventive activity of NO-ASA against colon cancer.
Acknowledgments
This work was supported by National Institutes of Health Grant CA92423 and the Emmanuel Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ASA, aspirin; NO-ASA, NO-donating aspirin; CRC, colorectal cancer; NSAID, nonsteroidal antiinflammatory drug; NO-NSAID, NO-donating NSAID; βgal, β-galactosidase; COX, cyclooxygenase; TCF, T cell factor.
Footnotes
Rao, C. V., Cooma, I., Rigas, B., Kopelovich, L. & Reddy, B. S. (2002) American Association of Cancer Research Annual Meeting 2002: Frontiers in Cancer Prevention Research, Oct. 14–19, 2002, Boston, MA (abstr.).
References
- 1.Gustin, D. M. & Brenner, D. E. (2002) Cancer Metastasis Rev. 21 323–348. [DOI] [PubMed] [Google Scholar]
- 2.Levy, R. (2002) N. Engl. J. Med. 347 615. [DOI] [PubMed] [Google Scholar]
- 3.Rigas, B. & Williams, J. L. (2002) Int. J. Oncol. 20 885–890. [PubMed] [Google Scholar]
- 4.Fiorucci, S., Santucci, L., Gresele, P., Faccino, R. M., Del Soldato, P. & Morelli, A. (2003) Gastroenterology 124 600–607. [DOI] [PubMed] [Google Scholar]
- 5.Moon, R. T., Bowerman, B., Boutros, M. & Perrimon, N. (2002) Science 296 1644–1646. [DOI] [PubMed] [Google Scholar]
- 6.Dihlmann, S., Siermann, A. & von Knebel Doeberitz, M. (2001) Oncogene 20 645–653. [DOI] [PubMed] [Google Scholar]
- 7.Williams, J. L., Borgo, S., Hasan, I., Castillo, E., Traganos, F. & Rigas, B. (2001) Cancer Res. 61 3285–3289. [PubMed] [Google Scholar]
- 8.Kashfi, K., Ryann, Y., Qiao, L. L., Williams, J. L., Chen, J., Del Soldato, P., Traganos, F. & Rigas, B. (2002) J. Pharmacol. Exp. Ther. 303 1273–1282. [DOI] [PubMed] [Google Scholar]
- 9.Bak, A. W., McKnight, W., Li, P., Del Soldato, P., Calignano, A., Cirino, G. & Wallace, J. L. (1998) Life Sci. 62 L367–L373. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan, K., Singh, S., Burke, T. R., Jr., Grunberger, D. & Aggarwal, B. B. (1996) Proc. Natl. Acad. Sci. USA 93 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapple, K. S., Cartwright, E. J., Hawcroft, G., Tisbury, A., Bonifer, C., Scott, N., Windsor, A. C., Guillou, P. J., Markham, A. F., Coletta, P. L. & Hull, M. A. (2000) Am. J. Pathol. 156 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utsunomiya, T., Doki, Y., Takemoto, H., Shiozaki, H., Yano, M., Sekimoto, M., Tamura, S., Yasuda, T., Fujiwara, Y. & Monden, M. (2001) Oncology 61 226–233. [DOI] [PubMed] [Google Scholar]
- 13.Heinen, C. D., Goss, K. H., Cornelius, J. R., Babcock, G. F., Knudsen, E. S., Kowalik, T. & Groden, J. (2002) Gastroenterology 123 751–763. [DOI] [PubMed] [Google Scholar]
- 14.Morin, P. J., Sparks, A. B., Korinek, V., Barker, N., Clevers, H., Vogelstein, B. & Kinzler, K. W. (1997) Science 275 1787–1790. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal, A. S., Marlow, B. P., Gupta, N. & Narayan, S. (2002) Oncogene 21 8414–8427. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs, S. Y. (2002) Cancer Biol. Ther. 1 337–341. [PubMed] [Google Scholar]
- 17.Richter, M., Weiss, M., Weinberger, I., Furstenberger, G. & Marian, B. (2001) Carcinogenesis 22 17–25. [DOI] [PubMed] [Google Scholar]