Abstract
Previous studies showed that i.p. administration of C75, a potent inhibitor of fatty acid synthase (FAS), blocked fasting-induced up-regulation of orexigenic neuropeptides and down-regulation of anorexigenic neuropeptides in the hypothalami of mice. As a result, food intake and body weight were drastically reduced. Here we provide evidence supporting the hypothesis that hypothalamic malonyl-CoA, a substrate of FAS, is an indicator of global energy status and mediates the feeding behavior of mice. We use a sensitive recycling assay to quantify malonyl-CoA to show that the hypothalamic malonyl-CoA level is low in fasted mice and rapidly (≤2 h) increases (≈5-fold) on refeeding. Intracerebroventricular (i.c.v.) administration of C75 to fasted mice rapidly (≤2 h) increased (by 4-fold) hypothalamic malonyl-CoA and blocked feeding when the mice were presented with food. Moreover, prior i.c.v. administration of an acetyl-CoA carboxylase inhibitor, 5-(tetradecyloxy)-2-furoic acid, rapidly (although only partially) prevented the C75-induced rise of hypothalamic malonyl-CoA and prevented the C75-induced decrease of food intake. These effects correlated closely with the rapid (≤2 h) and reciprocal effects of i.c.v. C75 on the expression of hypothalamic orexigenic (NPY and AgRP) and anorexigenic (proopiomelanocortin) neuropeptide mRNAs. Previous results showing that C75 administered i.c.v. rapidly activates hypothalamic neurons of the arcuate and paraventricular nuclei are consistent with the results reported in this paper. Together these findings suggest that level of hypothalamic malonyl-CoA, which depends on the relative activities of acetyl-CoA carboxylase and FAS, is an indicator of energy status and mediates feeding behavior.
Keywords: C75, acetyl-CoA carboxylase, fatty acid synthase, neuropeptides, obesity
The hypothalamus monitors global energy status in higher animals (1–4). Specific regions within the hypothalamus, notably the arcuate nucleus, respond to changes in energy status by altering the expression/secretion of neuropeptides that affect energy intake and expenditure. Thus, when energy intake exceeds expenditure expression of the orexigenic neuropeptides, i.e., NPY and AgRP, decreases whereas the expression of anorexigenic neuropeptides, i.e., proopiomelanocortin (POMC) and CART, increases (1). Signals triggered by these changes are transmitted to higher brain centers through second-order neurons that affect behavior leading to decreased food intake. Conversely, when energy expenditure exceeds intake, the inverse response occurs. Despite considerable progress in identifying many of the neuropeptides and circuits involved (1–4), the signaling mechanisms by which energy status is initially monitored by neurons of the hypothalamus are incompletely understood.
Recent evidence (5, 6) has implicated malonyl-CoA, an intermediate in fatty acid biosynthesis, as a possible mediator in the hypothalamic signaling pathway that monitors energy status. We and others have detected both acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) (6, 7), enzymes that catalyze the formation and utilization of malonyl-CoA, respectively, in a subset of hypothalamic neurons. A potent inhibitor of FAS, i.e., C75 (8), that would be expected to increase cellular malonyl-CoA, suppresses food intake and appropriately alters expression of the hypothalamic neuropeptide mRNAs described above (9). Also consistent with the “malonyl-CoA hypothesis” is a recent report of Gilbert et al. (10), who found that carotid infusion of obese (Zucker) rats with glucose and insulin suppressed food intake and this effect was prevented by the ACC inhibitor, 5-(tetradecyloxy)-2-furoic acid (TOFA), administered intracerebroventricularly (i.c.v.). Although these indirect lines of evidence support the hypothesis that malonyl-CoA participates in monitoring energy status in the hypothalamus, direct proof is still lacking.
Most previous studies (5–7, 9, 11) compared the effects of C75 administered by i.p. injection to mice that had been fasted to increase appetite when presented with food. These studies were conducted for relatively long time periods, i.e., ≈24 h, sufficient to achieve the fasted state. It was found that C75 prevented fasting-induced up-regulation of key orexigenic neuropeptides and down-regulation of anorexigenic neuropeptides in the hypothalamus, which correlated well with the suppression of food intake (9). Because C75 was administered i.p., some of the effects observed could have been exerted centrally and/or peripherally or could have resulted from conditioned taste aversion (CTA).¶ To limit the effects of C75 to the brain, the studies presented here were conducted primarily by i.c.v. (a route that does not produce CTA; ref. 12) injection of low levels of C75, after which hypothalamic responses (malonyl-CoA level and neuropeptide mRNA expression) and feeding behavior were rapidly, i.e., ≤2 h, assessed.
Crucial to the present investigation was an extremely sensitive recycling assay (13) with which to quantify malonyl-CoA in the hypothalamus after fasting, refeeding, and i.c.v. administration of FAS and ACC inhibitors. Our findings provide compelling evidence that malonyl-CoA is a mediator in the hypothalamic system that monitors global energy status.
Materials and Methods
Handling of Mice and Administration of C75 and TOFA. Animal experiments were conducted in accordance with guidelines of the Johns Hopkins University School of Medicine Institutional Animal Care and Use Committee. Male BALB/c mice (20–25 g) from Charles River Breeding Laboratories were acclimated for 1 week to a 12-h light (6:00 a.m. to 6:00 p.m.)/12-h dark (6:00 p.m. to 6:00 a.m.) cycle at 22°C. Mice were either fed standard laboratory chow ad libitum or fasted for the time indicated. In some experiments controls, were pair-fed; i.e., food intake was limited to that of C75-treated mice.
C75 was administered either by i.p. (30 mg/kg of body weight) or i.c.v. (10 μg in 2 μl of RPMI medium 1640) injection. TOFA was administered by i.c.v. (2 μg in 2 μl of DMSO) injection. All i.p. injections were given 2 h before the start of the dark cycle. Cumulative food intake was measured 24 h after i.p. injection, after which hypothalami were removed for analysis. For i.c.v. administration, mice were fasted for 23 h, then anesthetized with metofane and injected with 2 μl of RPMI medium 1640 (control) or C75 in RPMI medium 1640 into the lateral ventricle with a calibrated 10-μl Hamilton syringe. All i.c.v. injections of C75 were given 1–1.5 h before the start of the dark cycle and, where indicated, mice were refed 30 min later. Two and a half hours after i.c.v. injection, cumulative food intake was measured and hypothalami were removed. On average, ≈85% of mice receiving C75 i.c.v. exhibited drastically (>90%) reduced food intake.
Malonyl-CoA Recycling Assay. Mouse hypothalami were quickly (<1 min) removed and frozen in liquid nitrogen. Frozen tissue was weighed, pulverized, transferred to 1 ml of ice-cold 0.3 M sulfuric acid, sonicated for 10 min and centrifuged at 10,000 × g for 5 min at 4°C. Supernates were carefully adjusted to pH 6.0 on ice. The reaction mixture, which is based on the method of Takamura et al. (13), contained the appropriate amount of neutralized tissue extract, 50 mM Tris·HCl (pH 7.2), 10 mM magnesium sulfate, 1 mM 2-mercaptoethanol, 2 mM oxaloacetate, and 1 unit of citrate synthase (Sigma) in a total volume of 200 μl. After 20 min at 25°C, the reaction was terminated by placing the tube in an ice slush. Citrate synthase does not interfere the malonyl-CoA determination (see Fig. 1C). The “cycling” reaction is then initiated by addition of 50 mM malonate, 10 mM ATP, and 1.0 unit of malonate decarboxylase (from Pseudomonas putida; ref. 13) and incubated at 30°C for 20 min followed by the addition of 1.0 unit of acetate kinase (EC 2.7.2.1). After 20 min of incubation, 100 μl of 2.5 M neutralized hydroxylamine were added and the incubation was continued for an additional 20 min at 25°C. The reaction was terminated by addition of 0.3 ml of 10 mM ferric chloride dissolved in 25 mM trichloroacetic acid and 1 M HCl. The quantity of acetylhydroxamate formed was measured at A540.
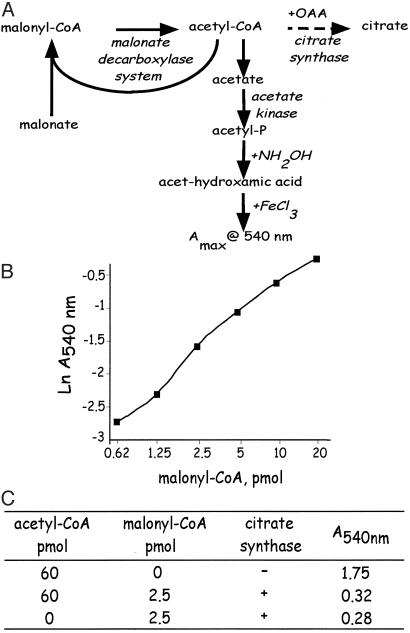
Fig. 1.
Enzymatic recycling assay for malonyl-CoA. (A) Protocol for determination of hypothalamic malonyl-CoA by using bacterial malonyl-CoA decarboxylase. Acetyl-CoA in the tissue extract was first eliminated with citrate synthase in the presence of oxaloacetate. Then the cycling reaction was initiated by addition of excess malonate, ATP, and malonate decarboxylase, followed by the addition of acetate kinase. The malonate decarboxylase multicomponent enzyme first decarboxylates malonyl-CoA to produce acetyl-CoA and then transfers CoA from acetyl-CoA to malonate to regenerate malonyl-CoA. Repetition of these events amplifies accumulation of the ultimate product, acetate, which is then converted to acetyl-phosphate. After addition of hydroxylamine, the acetyl-hydroxamate formed is quantified at A540. (B) Standard curve for the malonyl-CoA assay. (C) Verification that contaminating acetyl-CoA does not interfere with quantification of malonyl-CoA in the recycling assay. An excess (60 pmol) of acetyl-CoA (to mimic acetyl-CoA contaminant in tissue extracts) was added (or not) to the reaction mixture with or without 2.5 pmol of malonyl-CoA in the presence or absence of citrate synthase. The amount of acetyl-hydroxamate formed (as indicated by A540) was not affected by added acetyl-CoA, verifying lack of interference in the recycling assay.
Cloning of Partial cDNA Fragments and Preparation of Probes. Partial cDNAs encoding fragments of NPY and POMC were obtained by RT-PCR (with Qiagen OneStep RT-PCR; Qiagen, Valencia, CA) from the first-strand cDNA with mouse hypothalamic total RNA as a template. Primers used for reverse transcription-PCR were 5′-61TGT TTG GGC ATT CTG GCT GAG G82-3′ (5′ primer) and 5′-265TTC TGG GGG CGT TTT CTG TGC T244-3′ (3′ primer) for NPY (GenBank accession no. AF273768), 5′-261CGG CCC CAG GAA CAG CAG CAG T282-3′ (5′ primer) and 5′-564GGG CCC GTC GTC CTT CTC C546-3′ (3′ primer) for POMC (GenBank accession no. NM008895). The amplified sequence was from the conserved sequence region based on an alignment of the sequence information of human (GenBank accession no. M57703). Each PCR product was cloned into pCR II-TOPO vector by using a TOPO TA Cloning kit Dual Promoter (Invitrogen) and sequenced. A plasmid DNA with the correct sequence was prepared by using the Qiagen Plasmid Maxi kit (Qiagen), purified, linearized with HindIII or XbaI, extracted with phenol/chloroform, and stored at –80°C. pT7 Blue plasmid (Novagen) containing a partial sequence of mouse AgRP cDNA (396 bp, 1–396 of GenBank accession no. U89494, gift from Tina M. Hahn, University of California, Davis) was linearized with EcoRI and amplified with T7 RNA polymerase to generate antisense RNA, which was used for RNase protection assays.
RNA Isolation. Frozen hypothalami (paired hypothalami from each group) was pulverized with a Bio-Pulverizer II (Research Products International) chilled in liquid nitrogen. The fine powder was transferred into 1.5-ml microcentrifuge tube with tethered cap and o-ring (Denville Scientific, Metuchen, NJ) chilled on dry ice. One milliliter of TRIzol (Invitrogen) was added, vortexed, and suspended with a 1-ml syringe with 25G5/8 needle (Becton Dickinson), and total RNA was extracted according to the manufacturer's procedure (Invitrogen). RNA concentration was determined at A260, adjusted to a constant concentration (≈100 ng/μl) with RNase-free 1× TE buffer (10 mM Tris/1 mM EDTA, pH 8.0), and stored at –80°C before analysis.
RNase Protection Assay. Antisense RNA was transcribed with [α-32P]UTP by using a MAXIscript SP6/T7 kit (Ambion, Austin, TX). In brief, 20-μl in vitro transcription reaction contains 1 μg of linearized DNA template, 0.5 mM each ATP, CTP, and GTP, 6 μl of [α-32P]UTP [800 Ci/mmol/20 mCi/ml (1 Ci = 37 GBq); 1 μl UTP for cyclophilin probe synthesis], and 1 μl of 10 μM unlabeled (“cold”) UTP for NPY (4 μl of 200 μM cold UTP for Cyclophilin; no addition for AgRP and POMC), and 1 μl of SP6 or T7 RNA polymerase (15 units/μl) in transcription buffer. After incubation for 1 h, 1 μl of DNase I (RNase-free, 2 units/μl) was added, incubated for 30 min, and extracted with phenol/chloroform. Each of the ethanol-precipitated probes was gel-purified according to the manufacturer's manual (Ambion), and radioactivity was measured. RNase protection assays were performed with a HybSpeed RPA kit (Ambion). Aliquots of hypothalamic total RNA (5 μg) were mixed with 32P-labeled probe mixtures (8 × 104 cpm for each probe) for mouse AgRP, NPY, POMC, and Cyclophilin. Yeast RNA (50 μg) and GlycoBlue (50 μg/ml final) were added and RNA was precipitated with ethanol at –80°C for 20 min. After centrifugation, pellets were dissolved in a 10-μl HybSpeed buffer and denatured at 95°C for 5 min. Samples were then hybridized at 68°C for 10 min. After digestion with RNase A/T1, protected fragments corresponding to mouse AgRP (396 bp), NPY (205 bp), POMC (304 bp), and Cyclophilin (103 bp) were separated on an 8 M urea/5% polyacrylamide gel. Gels were dried at 65°C for 2 h and exposed to a PhosphoImaging plate (BAS-IP MP 2040) (Fuji) for 24 h. Relative mRNA levels of neuropeptides were quantified with a Fuji Bio-Imaging analyzer 1500, and the data were normalized with an intensity of signal of cyclophilin mRNA.
Determination of Blood Glucose. Blood (–30 μl) was collected in a heparinized microcentrifuge tube and centrifuged at 12,000 × g for 3 min, and plasma was stored at –80°C before analysis. Glucose concentration was determined by using a Glucose Trinder-100 kit (Sigma).
Statistical Analysis. Results were statistically analyzed by the Student's t test and are presented as means ± SEM of multiple determinations
Results
Previous studies (5, 9) led to the hypothesis that administration of C75, a potent FAS inhibitor, causes an increase of malonyl-CoA in hypothalamic nuclei that monitor energy status and initiate signals that alter food intake. To test this hypothesis, it was necessary to determine the effect of C75 on hypothalamic malonyl-CoA concentration. Because the amount of hypothalamic tissue in the mouse is small (15–20 mg per hypothalamus) and its malonyl-CoA concentration is low, the usual enzymatic methods to quantify this metabolic intermediate were not sufficiently sensitive (Z.H. and M.D.L., unpublished results). Therefore, a more sensitive method with which to assay malonyl-CoA was adapted to hypothalamic tissue.
Recycling Assay for Malonyl-CoA. The substrate-recycling malonate decarboxylase method of Takamura et al. (13), which amplifies signal output by several thousand-fold, was used to assay malonyl-CoA in hypothalamic tissue extracts. Bacterial (Pseudomonas putida) malonate decarboxylase is a multienzyme complex that contains both malonyl-CoA decarboxylase (EC 4.1.1.9) and acetyl-CoA: malonate CoA-transferase (EC 2.8.3.3). The principle of the method, illustrated in Fig. 1 A, is the transfer/“recycling” of CoA from acetyl-CoA, generated by the decarboxylation of malonyl-CoA, to exogenous malonate. Initially, it is necessary to remove any free acetyl-CoA or propionyl-CoA in tissue extracts with citrate synthase and oxaloacetate. Typical standard curves (Fig. 1B) are linear between 0.62 and 20 pmol malonyl-CoA. The presence of citrate synthase and oxaloacetate does not interfere with subsequent steps in the assay (as shown in Fig. 1C), as acetyl-CoA produced during decarboxylation of malonyl-CoA is directly channeled to the CoA transferase component of the enzyme complex.
Long-Term Effect of C75 Administered i.p. on Food Intake, Hypothalamic Malonyl-CoA, and Blood Glucose. Male BALB/c mice fed ad libitum were given i.p. injections of C75 or vehicle (control). An additional control group, pair-fed to the intake of the C75-treated mice, was injected i.p. with vehicle. As shown in Fig. 2A, C75 suppressed food intake by ≈90% compared with that of ad libitum-fed controls during the 24-h period after injection.
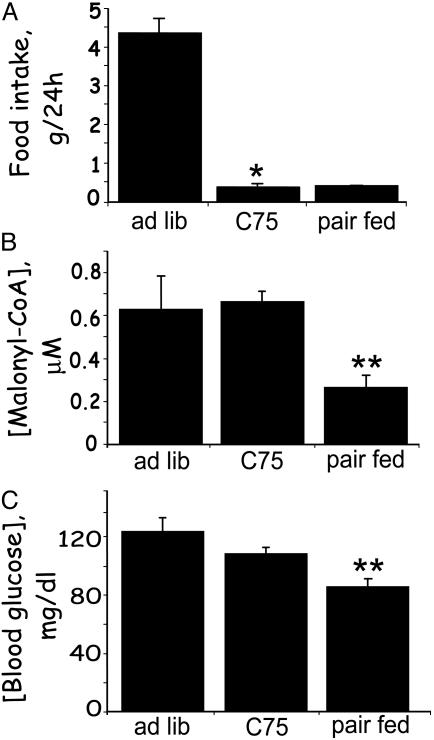
Fig. 2.
Long-term effects of C75 administered i.p. on blood glucose, hypothalamic malonyl-CoA, and food intake. Male BALB/c mice were acclimated for 1 week to a 12-h light/12-h dark cycle at 22°C and fed standard laboratory chow ad libitum. C75 or vehicle was administered by i.p. injection 2 h before the start of the dark cycle. Cumulative food intake over the next 24 h was measured. At that point, blood samples were drawn and hypothalami were quickly removed for malonyl-CoA analysis. Another group of mice was given mock injection and pair-fed the same quantity of food consumed by C75-treated mice. (A) Effect of C75 administered i.p. on 24-h food intake. (B) Effect of C75 administered i.p. on hypothalamic malonyl-CoA level. (C) Effect of C75 administered i.p. on blood glucose level. There were five mice per treatment. *, P < 0.001 vs. ad libitum; **, P < 0.001 vs. both ad libitum and C75.
The level of malonyl-CoA in hypothalami from pair-fed controls was markedly reduced (by ≈60%) compared with that of ad libitum-fed controls (Fig. 2B) because of restriction of food intake. Despite the same reduction of food intake, the malonyl-CoA level of C75-treated mice was the same as that of ad libitum-fed mice (Fig. 2B). Thus, i.p. administration of C75 prevented the decrease in malonyl-CoA caused by “fasting.” This result is consistent with previous experiments (9) in which C75 blocked the fasting-induced increase in orexigenic (NPY and AgRP) and decrease in anorexigenic (POMC and CART) neuropeptide mRNA expression. These findings are consistent with, but for reasons discussed below, do not validate the hypothesis that C75 suppresses food intake by up-regulating malonyl-CoA, and thereby alters expression of the orexigenic and anorexigenic neuropeptides.
As expected, blood glucose was reduced significantly by food intake restriction in the pair-fed mice when compared with that in mice fed ad libitum (Fig. 2C). Nevertheless, blood glucose levels in C75-treated mice were still significantly higher than those of pair-fed partially fasted mice. Because blood glucose levels were affected by i.p. C75 treatment and fasting, it is possible that the observed differences in hypothalamic malonyl-CoA were due, at least in part, to peripheral/hormonal effects. Blood fatty acid levels were markedly increased in the pair-fed partially fasted mice and were elevated far less in C75-treated mice compared with mice fed ad libitum (results not shown). The question was addressed, were the any of effects of i.p. C75 indirect, due possibly to peripheral changes, e.g., a change in circulating hormone levels? Therefore, short-term experiments were conducted with C75 administered by i.c.v. injection to limit its effects to the CNS.
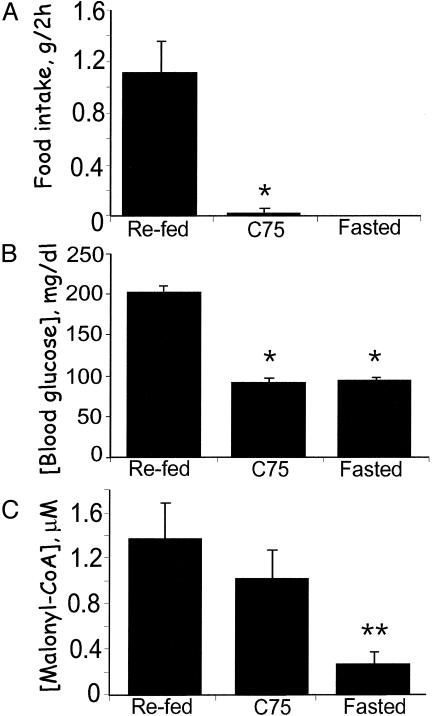
Short-Term Effects of C75 Administered i.c.v. on Food Intake, Hypothalamic Malonyl-CoA, and Blood Glucose. Central action of C75 was ensured with a low dose (≈1/100th of that administered i.p.) injected directly into the ventricular system of fasted mice. The mice were then either given free access to food (refed and C75-treated) or fasting was continued. Food intake by refed control mice over the next 2 h was substantial, whereas food intake by mice given C75 i.c.v. was almost totally (≥98%) suppressed (Fig. 3A). As expected, blood glucose levels of the fasted mice were reduced and within 2 h of being given access to food (refed controls) the levels increased >2-fold (Fig. 3B). Food intake was almost totally blocked by C75 treatment, and the mice maintained the same low blood glucose level as fasted mice. This observation is in contrast to the long-term (24 h) effect of C75, where blood glucose levels were higher (Fig. 2C) and blood fatty acid levels (not shown) were lower than those of “fasted” pair-fed mice. Thus, it appears that, in the 2.5-h period after i.c.v. injection, C75 does not provoke significant peripheral metabolic effects.
Fig. 3.
Short-term effects of C75 administered i.c.v. on blood glucose, hypothalamic malonyl-CoA, and food intake. Male BALB/c mice were acclimated for 1 week to a 12-h light/12-h dark cycle at 22°C and fed standard laboratory chow ad libitum. Mice were fasted for 23 h and then given 10 μg of C75 or vehicle by i.c.v. injection at 1.5 h before the start of the dark cycle. Thirty minutes after i.c.v. injection, food was presented to the mice and cumulative food intake was measured for 2 h. Hypothalami were then quickly removed for malonyl-CoA analysis. Another group of mice previously fasted for 23 h was given an i.c.v. injection of vehicle, and 2.5 h later hypothalami were removed for analysis. (A) Effect of C75 administered i.c.v. on 2-h food intake. (B) Effect of C75 administered i.c.v. on blood glucose level. (C) Effect of C75 administered i.c.v. on hypothalamic malonyl-CoA level. There were four mice per treatment. *, P < 0.001 vs. refed; **, P < 0.001 vs. both refed and C75.
The hypothalamic malonyl-CoA level of fasted mice was much lower than that of refed mice (Fig. 3C). Moreover, this difference occurred rapidly, thus, in ≤2 h after refeeding fasted mice, hypothalamic malonyl-CoA levels increased >5-fold. The response to i.c.v. administration of C75 to fasted mice was equally rapid increasing by ≈4-fold in ≤2.5 h. Importantly, this increase was independent of food intake, as it occurred despite the C75-induced blockade of food intake. Therefore, the effect of C75 on malonyl-CoA level appears to be caused by a central action of the inhibitor, because the effect is rapid, occurs by i.c.v. administration at a low dose, and is not accompanied by changes indicative of altered insulin and/or glucagon status.
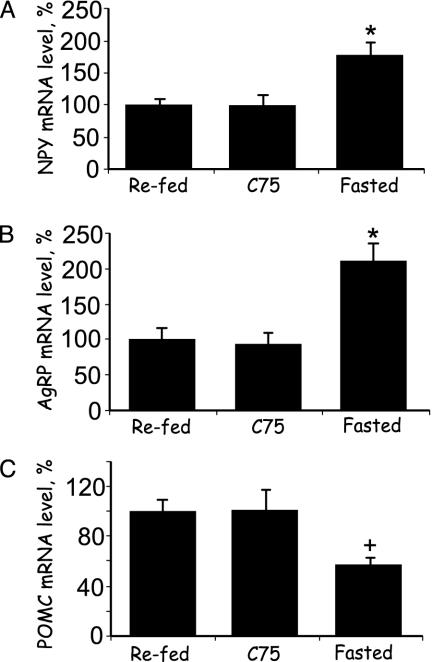
Short-Term Effects of C75 Administered i.c.v. on Expression of Hypothalamic Orexigenic and Anorexigenic Neuropeptide mRNA Levels. Experiments were conducted to determine whether C75 or vehicle administered i.c.v. rapidly affect the expression of NPY, AgRP, and POMC mRNAs in the hypothalamus. The levels of the orexigenic neuropeptide (NPY and AgRP) messages in hypothalami of fasted mice were ≈2-fold higher than those of refed mice (Fig. 4 A and B), whereas the level of the anorexigenic neuropeptide (POMC) message was ≈2-fold lower than in refed mice (Fig. 4C). The changes in expression of these mRNAs were rapid, occurring in ≤2.5 h. Likewise, the changes in neuropeptide message levels caused by i.c.v. injection of C75 to fasted mice were rapid and occurred without a change in food intake (Fig. 4), i.e., the mice remained in a fasted state (as in Fig. 3A). Thus, i.c.v. C75 blocked the fasting-induced changes in expression of the orexigenic and anorexigenic neuropeptides. The fact that C75 provoked a rapid (≤2.5 h) increase in hypothalamic malonyl-CoA level under the same conditions (Fig. 3C) is consistent with the view that an increased malonyl-CoA level is responsible for the observed changes in expression of the hypothalamic orexigenic and anorexigenic neuropeptide mRNAs (Fig. 4).
Fig. 4.
Short-term effects of C75 administered i.c.v. on the expression of orexigenic and anorexigenic neuropeptides in the hypothalamus. Mice were treated as described in Fig. 3. Two and a half hours after i.c.v. injection of C75, hypothalami were removed, and RNA was isolated and subjected to RNase protection analysis by using antisense probes specific for each neuropeptide mRNA. (A) NPY. (B) AgRP. (C) POMC. There were four mice per treatment. *, P < 0.001 vs. both refed and C75. +, P < 0.01 vs. C75 and P < 0.001 vs. refed. Results are presented as percentage relative to the level in refed mice.
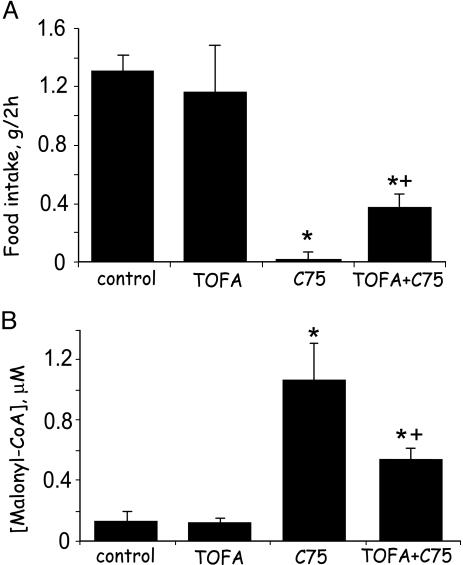
Prevention of the Effects of C75 Administered i.c.v. on Food Intake and Hypothalamic Malonyl-CoA Level by an ACC Inhibitor, TOFA, Administered i.c.v. In previous long-term experiments (5) we showed that i.c.v. administration of the ACC inhibitor, TOFA, partially prevented the suppression of food intake caused by C75 administered i.p. Experiments were conducted to determine whether TOFA administered i.c.v. could rapidly prevent the effect of i.c.v. C75 on food intake and to assess the correlation to hypothalamic malonyl-CoA. As expected, C75 rapidly (≤2.5 h) suppressed food intake (Fig. 5A). The suppressive effect of i.c.v. C75 on food intake was rapidly prevented, albeit partially, by i.c.v. TOFA, the increase over that of mice injected i.c.v. with C75 alone being >10-fold (Fig. 5A). It is likely that the reason for the incomplete restoration of food intake to the level of fasted-refed mice is incomplete inhibition of ACC by TOFA.
Fig. 5.
i.c.v. administration of an ACC inhibitor rapidly blocks i.c.v. C75-induced up-regulation of hypothalamic malonyl-CoA and suppression of food intake. Male BALB/c mice were first fasted for 24 h. Where indicated, TOFA (2 μg), an ACC inhibitor, or vehicle was injected i.c.v. 2.5 h before the dark cycle. Where indicated, C75 (10 μg) or vehicle was injected i.c.v. 1 h before the dark cycle. In some cases, both TOFA and C75 were injected sequentially. (A) To determine the effects of TOFA or C75 or both (TOFA, then C75) on food intake, mice were given food 0.5 h before the dark cycle, after which food consumption was measured during the next 2 h. (B) Hypothalami were removed for malonyl-CoA analysis 2.5 h after C75 or 4 h after TOFA injection. Mice for analysis of hypothalamic malonyl-CoA did not receive food. There were four mice per treatment. *, P < 0.001 vs. control or TOFA alone; +, P < 0.001 vs. C75.
TOFA also rapidly prevented (albeit partially) the effect of i.c.v. C75 administration on hypothalamic malonyl-CoA (Fig. 5B) to approximately the same extent as TOFA prevented the effect of C75 on food intake. As illustrated in Fig. 5B, both fasted and TOFA-treated mice exhibited low hypothalamic malonyl-CoA levels. i.c.v. administration of C75 to fasted mice caused a dramatic (≈8-fold) and rapid increase in hypothalamic malonyl-CoA. i.c.v. administration of TOFA 1.5 h before C75 decreased hypothalamic malonyl-CoA by 40–50% (Fig. 5B), a decrease inversely proportional to the TOFA-provoked change of food intake (Fig. 5A). The fact that the extents of these TOFA-induced changes are inversely proportional is consistent with malonyl-CoA acting as a negative mediator of food intake.
Discussion
Strong precedents exist for a connection between fatty acid synthesis and the inhibition of food intake. Fatty acid synthesis occurs only during periods of energy surplus when excess physiological fuel is channeled into energy storage pathways, circumstances that cause down-regulation of orexigenic (NPY and AgRP) neuropeptides and up-regulation of anorexigenic (POMC/αMSH and CART) neuropeptides in the hypothalamus (1). These neuropeptides are an integral part of the system that monitors and responds to changes in energy balance (2). The enzymes, ACC and FAS, that carry out fatty acid synthesis are expressed by certain hypothalamic neurons (6, 7), including a subset in the arcuate nucleus (6), a major site of orexigenic and anorexigenic neuropeptides expression. Relevant to the present investigation, the key regulatory enzyme of fatty acid synthesis (14, 15), ACC, catalyzes the formation of malonyl-CoA, a substrate for FAS.
Recent studies (5, 16) show that potent inhibitors of FAS, e.g., C75 and cerulenin, that would be expected to increase hypothalamic malonyl-CoA, suppress food intake. The fact that the level of malonyl-CoA increases in lipogenic tissues, such as the liver, at times of active fatty acid synthesis (5) and appears to do so in the hypothalamus, argues against an effect of the end-product of the pathway (fatty acid) as mediator. Rather, it suggests that the intermediate preceding FAS in the pathway, malonyl-CoA, may mediate the signal that causes suppression of food intake. Indeed, a role for malonyl-CoA as metabolic mediator in other tissues is already firmly established (17–19). In other tissues, e.g., the liver, malonyl-CoA functions as a negative mediator of fatty acid oxidation under conditions of energy surplus by inhibiting carnitine/palmitoyl-CoA transferase-1 (CPT-1), thereby blocking entry of fatty acids into the mitochondrion (17–19). The present investigation provides compelling evidence that, under conditions of energy surplus, hypothalamic malonyl-CoA acts as a negative mediator of food intake.
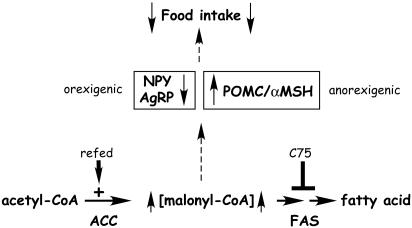
Thus, when energy expenditure exceeds intake, as in the fasted state, the level of hypothalamic malonyl-CoA decreases (Fig. 3B). Conversely, when fasted mice are refed, hypothalamic malonyl-CoA rapidly increases by ≈4-fold (Fig. 3B). The fact that administration of C75 by i.c.v. injection both provokes a rapid (≤2.5 h) increase in hypothalamic malonyl-CoA and blocks food intake suggests a causal relationship. Consistent with this view, an inhibitor of ACC, TOFA, which should lower the rate of formation of malonyl-CoA, prevented the C75-induced increase in malonyl-CoA level and prevented the C75-induced suppression of food intake (Fig. 5 A and B). Although food intake and malonyl-CoA were not completely restored to the levels of fasted-refed mice, the fact that these changes were inversely proportional lends credence to the “malonyl-CoA hypothesis.” A model supported by the findings presented in this paper is illustrated in Fig. 6.
Fig. 6.
Model for hypothalamic malonyl-CoA as mediator of expression of orexigenic and anorexigenic neuropeptides and food intake.
Although the results presented in this paper implicate malonyl-CoA as a mediator linking hypothalamic fatty acid synthesis to the control of food intake, they do not define the signaling mechanism beyond malonyl-CoA in the pathway. Two possible mechanisms are envisioned: (i) that malonyl-CoA interacts directly with a signaling protein that regulates expression of the orexigenic and anorexigenic neuropeptides, or (ii) that malonyl-CoA acts indirectly by inhibiting carnitine/palmitoyl-CoA transferase-1, thereby preventing entry of long-chain fatty acyl-CoAs into the mitochondrion. This would be expected to increase cytoplasmic fatty acyl-CoA, which could interact with a signaling protein that regulates expression of the orexigenic and anorexigenic neuropeptides. Evidence supporting the latter mechanism has been presented by Rossetti and colleagues (20) and was interpreted to indicate that fatty acyl-CoA is the mediator in the signaling pathway controlling feeding behavior. To distinguish between these mechanisms, it will be necessary to know the effects of fasting and refeeding on the cytoplasmic level of fatty acyl-CoA in the hypothalmus. Further studies will be necessary to address these issues.
Acknowledgments
This research was supported by the Yamanouchi Pharmaceutical Co., Ltd. (Tokyo).
Abbreviations: ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; TOFA, 5-(tetradecyloxy)-2-furoic acid; i.c.v., intracerebroventricular(ly).
Footnotes
It should be noted that, although some studies with C75 have been conducted by i.c.v. injection indicating its central action, most studies have used the i.p. route of administration. There is lack of agreement as to whether some of the effects of i.p. administration of C75 are caused by conditioned taste aversion (6, 12).
References
- 1.Schwartz, M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404 661–671. [DOI] [PubMed] [Google Scholar]
- 2.Williams, G., Bing, C., Cai, X. J., Harrold, J. A., King, P. J. & Liu, X. H. (2001) Physiol. Behav. 74 683–701. [DOI] [PubMed] [Google Scholar]
- 3.Morton, G. J. & Schwartz, M. W. (2001) Int. J. Obes. Relat. Metab. Disord. 25 Suppl. 5, S56–S62. [DOI] [PubMed] [Google Scholar]
- 4.Mercer, J. G. & Speakman, J. R. (2001) Neurosci. Biobehav. Rev. 25 101–116. [DOI] [PubMed] [Google Scholar]
- 5.Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D. & Kuhajda, F. P. (2000) Science 288 2379–2381. [DOI] [PubMed] [Google Scholar]
- 6.Kim, E. K., Miller, I., Landree, L. E., Borisy-Rudin, F. F., Brown, P., Tihan, T., Townsend, C. A., Witters, L. A., Moran, T. H., Kuhajda, F. P. & Ronnett, G. V. (2002) Am. J. Physiol. 283 E867–E879. [DOI] [PubMed] [Google Scholar]
- 7.Gao, S. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhajda, F. P., Pizer, E. S., Li, J. N., Mani, N. S., Frehywot, G. L. & Townsend, C. A. (2000) Proc. Natl. Acad. Sci. USA 97 3450–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimokawa, T., Kumar, M. V. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert, M., Magnan, C., Turban, S., Andre, J. & Guerre-Millo, M. (2003) Diabetes 52 277–282. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, M. V., Shimokawa, T., Nagy, T. R. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99 1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clegg, D. J., Wortman, M. D., Benoit, S. C., McOsker, C. C. & Seeley, R. J. (2002) Diabetes 51 3196–3201. [DOI] [PubMed] [Google Scholar]
- 13.Takamura, Y., Kitayama, Y., Arakawa, A., Yamanaka, S., Tosaki, M. & Ogawa, Y. (1985) Biochim. Biophys. Acta 834 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Moss, J. & Lane, M. D. (1971) Adv. Enzymol. Relat. Areas Mol. Biol. 35 321–442. [DOI] [PubMed] [Google Scholar]
- 15.Lane, M. D., Moss, J. & Polakis, S. E. (1974) Curr. Top. Cell. Regul. 8 139–195. [PubMed] [Google Scholar]
- 16.Makimura, H., Mizuno, T. M., Yang, X. J., Silverstein, J., Beasley, J. & Mobbs, C. V. (2001) Diabetes 50 733–739. [DOI] [PubMed] [Google Scholar]
- 17.McGarry, J. D. & Foster, D. W. (1979) J. Biol. Chem. 254 8163–8168. [PubMed] [Google Scholar]
- 18.McGarry, J. D., Woeltje, K. F., Kuwajima, M. & Foster, D. W. (1989) Diabetes Metab. Rev. 5 271–284. [DOI] [PubMed] [Google Scholar]
- 19.McGarry, J. D. & Brown, N. F. (1997) Eur. J. Biochem. 244 1–14. [DOI] [PubMed] [Google Scholar]
- 20.Obici, S., Feng, Z., Arduini, A., Conti, R. & Rossetti, L. (2003) Nat. Med. 9 756–761. [DOI] [PubMed] [Google Scholar]