Abstract
In mammals, the continuous production of hematopoietic cells (HCs) is sustained by a small number of hematopoietic stem cells (HSCs) residing in the bone marrow. Early HSC activity arises in the aorta-gonad mesonephros region, within cells localized to the ventral floor of the major blood vessels, suggesting that the first HSCs may be derived from cells capable of giving rise to the hematopoietic system and to the endothelial cells of the vasculature. TIE1 (TIE) and TIE2 (TEK) are related receptor tyrosine kinases with an embryonic expression pattern in endothelial cells, their precursors, and HCs, suggestive of a role in the divergence and function of both lineages. Indeed, gene targeting approaches have shown that TIE1, TIE2, and ligands for TIE2, the angiopoietins, are essential for vascular development and maintenance. To explore possible roles for these receptors in HCs, we have examined the ability of embryonic cells lacking both TIE1 and TIE2 to contribute to developmental and adult hematopoiesis by generating chimeric animals between normal embryonic cells and cells lacking these receptors. We show here that TIE receptors are not required for differentiation and proliferation of definitive hematopoietic lineages in the embryo and fetus; surprisingly, however, these receptors are specifically required during postnatal bone marrow hematopoiesis.
Aclose cell lineage relationship between hematopoietic cells (HCs) and endothelial cells (ECs) has long been recognized (1, 2). During embryogenesis, both cell types emerge in a spatially and temporally tightly linked manner. In the yolk sac of the mouse embryo, primitive erythrocytes differentiate juxtaposed with EC precursors from extraembryonic mesoderm by embryonic day (E) 7.5, suggestive of origin from a common cellular precursor known as the hemangioblast. A second wave of hematopoiesis is initiated in the embryo around E10–E11, when adult-type (definitive) hematopoietic stem cells (HSCs) arise in the aorta gonad mesonephros (AGM) region in close association with the ventral portion of the dorsal aorta and umbilical and vitelline arteries (3). Fate mapping studies in the chick demonstrated that the ECs and HCs that form the ventral floor of the dorsal aorta share a common cellular origin in the splanchnopleural mesoderm (4), and the para-aortic splanchnopleure (P-Sp) of the mouse contains the progenitors of cells that are capable of long-term reconstitution (5). Little is known regarding the in vivo signaling mechanisms that assign both vascular and HSC properties to these anatomically localized mesodermal progenitors.
By E11–E12, definitive HSCs leave the AGM region and colonize the fetal liver (FL) (6), where they proliferate and produce differentiated progeny that are released into the circulation, as well as lymphoid progenitors that seed the fetal thymus and spleen (7). The FL is the main hematopoietic organ until the end of gestation; after E15, HSCs migrate to and seed the bone marrow (BM) (8, 9). In contrast to the migratory nature of fetal HSCs, adult stem cells are maintained throughout life by close interaction with the extracellular matrix as well as other cell types, including ECs. In the adult, HCs can promote EC differentiation (10) and participate in postnatal neovascularization (11, 12). Thus, taken together, the available evidence supports a close developmental as well as physiological link between ECs and HCs both during embryogenesis and in the adult. The molecular signaling pathways mediating this relationship are only beginning to be elucidated.
TIE1 and TIE2 are related receptor tyrosine kinases with expression patterns suggestive of function in both EC and HC lineages. In addition to expression in virtually all ECs in development and in the adult, TIE1 and TIE2 are expressed in the HSCs of the AGM region, FL and adult BM, as well as in several differentiated HC types (13–17). Furthermore, TIE2 and the angiopoietins have been implicated in recruitment and mobilization of adult HSCs from BM (18). Targeted mutations in both receptors as well as in angiopoietin 1 (Ang-1) and Ang-2, ligands for TIE2, have demonstrated their essential roles in developmental angiogenesis and postnatal vascular maintenance (19–24). Here, we have investigated the role of TIE1 and TIE2 in developmental and BM hematopoiesis by examining the ability of cells doubly deficient for TIE1 and TIE2 to contribute to blood lineages in chimeric mice generated in both WT and Rag2-deficient backgrounds. Our results demonstrate that TIE receptors are dispensable for fetal hematopoiesis but are required specifically for hematopoiesis in the adult BM. These findings suggest a number of possible mechanisms by which signaling through the TIE receptors controls hematopoiesis and may have implications for therapeutic interventions involving engraftment or mobilization of stem and progenitor cells.
Methods
Generation of Chimeras. Single mutant alleles of Tie1 and Tie2 and the generation of recombinant doubly heterozygous (DH) strains have been described (19, 20, 22). Chimeras were generated by diploid morula–morula aggregation (22). Donor morulae from a cross between tie1lcz/+ tie2Δsp/+ and tie1lczn–/+ tie2Δsp/+ [glucose-phosphate isomerase (GPI)-AA] were aggregated with outbred ICR WT (GPI-BB) or Rag2–/– host morulae and transferred to the uteri of foster mothers. Screening of embryonic or adult chimera tail tissue for tie1lcz and tie1lczn– alleles was performed as described (21) to genotype chimeras as WT, DH, or double mutant (DM) donor cell-derived. The extent of donor cell content for individual tissues was determined by using GPI analysis as described (22) or by screening for the relative presence of the mutant and WT Tie2 alleles by Southern blot analysis (19) of genomic DNA.
Hematopoietic Progenitor Cell Assays. Single cell suspensions of FL and BM cells were prepared in Iscove's modified Dulbecco's medium (IMDM, GIBCO/BRL). Trypan blue-excluding cells were enumerated by using a hemocytometer. FL (1 × 104) and BM (2 × 104) cells were cultured in triplicate in six-well dishes in methylcellulose (M3434, StemCell Technologies, Vancouver) in the absence or presence of G418 (pH 7.5) at a final concentration of 10 μg/ml. Cells were grown at 37°C and non-red myeloid colony-forming unit in culture (CFU-C) and erythroid burst-forming unit (BFU-E) progenitors were scored on day 8. For yolk sac HPC assays, embryos were dissected on day 8.5 of gestation. Tissue from the embryo proper was used for genotyping by PCR (22). Whole yolk sacs were rinsed several times in PBS, incubated in 0.1% trypsin/EDTA for 15 min at 37°C, resuspended as single cells in IMDM, and plated in methylcellulose (M3434, StemCell Technologies). Yolk sac-derived colonies were scored by microscopy as described (25). Erythroid colonies were enumerated according to positive benzidine staining.
Flow Cytometry. Single cell suspensions of FL and BM cells were collected in IMDM, depleted of red cells by hypotonic lysis, and purified on a Ficoll gradient (Pharmacia). Cells (1 × 106) were labeled with phycoerythrin- or FITC-conjugated antibodies against B220, CD4, CD8, Ter119, Mac-1, Gr-1, CD45, and IgM (all from BD Pharmingen). Dead cells were excluded with propidium iodide.
Results
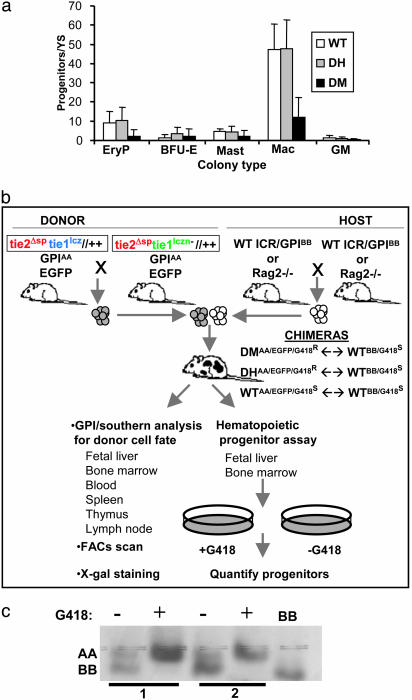
We previously reported that embryos deficient for TIE1 and TIE2 receptor tyrosine kinases display a severe impairment of the circulatory system, growth delay, and lethality in early postimplantation gestation (22). Many mutant embryos appeared pale, suggesting a possible deficiency in blood cell maturation in addition to, or as a consequence of, the vascular defects. To confirm this observation quantitatively, tissue from Tie1/Tie2 DM embryos and their control DH and WT littermates were analyzed for their content of hematopoietic progenitor cells (HPCs). Because DM embryos exhibit growth delay and lethality by E9.5, the yolk sacs of E8.5–E9.0 (15–20 somites) embryos were analyzed. As demonstrated in Fig. 1a, there was a reduction, but not absence of, yolk sac-derived HPCs for primitive erythroid and macrophage lineages in embryos deficient for both TIE1 and TIE2. This result suggests that loss of the TIE1 and TIE2 signaling pathways disrupts the normal program of primitive HC differentiation in the postgastrulation embryo, supporting and extending an earlier observation that in vitro-cultured explants of P-Sp mesoderm from Tie2–/– embryos do not produce normal numbers of differentiated HCs (16).
Fig. 1.
Analysis of Tie1/Tie2 deficiency in the hematopoietic system. (a) Tie1/Tie2 doubly deficient embryos display reduced HPC numbers in the yolk sac at E8.5. Reduced primitive erythroid (EryP) and macrophage (Mac) colony numbers in embryos doubly deficient for Tie1 and Tie2 (DM, n = 7) relative to control littermates (WT, n = 9, and DH, n = 18). No differences in colony numbers were observed for granulocyte–macrophage (GM) colonies, definitive erythrocytes (BFU-E), or mast cells (Mast), likely because of the low numbers of colonies produced. (b) Analysis of hematopoiesis in Tie1/Tie2 chimeras. Chimeras were generated by morula ↔ morula aggregation of donor embryos derived from intercross of recombinant Tie1/Tie2 DH parents and WT ICR or Rag2–/– host embryos. Donor cells carry the AA isoform of the GPI enzyme (GPIAA), whereas ICR host cells carry the BB isoform (GPIBB), allowing the fate of donor cells to be followed in chimeric tissue by colorimetric assay for GPI isoforms after electrophoresis. Donor cell fate in BM HCs was monitored by flow cytometry because donor animals express enhanced GFP (EGFP) in these cells. For quantitative analysis of donor-versus host-derived HPCs, a G418 selection strategy was implemented. Donor cells are resistant to G418 (G418R), whereas WT host cells are sensitive to G418 (G418S). (c) Validation of G418 HPC selection in chimeras. HC colonies were harvested from methylcellulose cultures of FL cells grown in the presence (+) or absence (–) of G418. Cells sensitive to G418 (derived from WT host, GPIBB) are selected against, but G418-resistant DH donor cells (lanes 1 and 2, GPIAA) carrying the PGK–neomycin-resistance gene survive selection.
Because mice doubly homozygous for null mutations in both TIE1 and TIE2 die at or before the emergence of definitive HSCs in the AGM region, an alternate strategy was required to explore the role of these receptors in subsequent HC development. Moreover, because of the coordinated and interdependent differentiation of the hematopoietic and cardiovascular lineages, it was important to ask whether the hematopoietic defects described in Fig. 1a were a primary cause of embryonic lethality or were secondary to the onset of circulatory defects. Accordingly, we used in vivo chimeric analysis combined with in vitro hematopoietic progenitor assays to examine the hematopoietic capacity of Tie1/Tie2 DM cells versus that of DH control cells in the context of a physiologically intact animal and in competition with normal cells contributed by the WT host (Fig. 1b). To distinguish host- and donor-derived progenitors, we used the presence of an actively transcribed neomycin-resistance gene in the targeted DM or DH cells. In the presence of G418, only hematopoietic colonies derived from DH or DM donor progenitors would be expected to survive, and host-derived WT cells would die, providing a quantitative readout of the ability of Tie1/Tie2-deficient cells to constitute the hematopoietic system. The validity of this strategy was demonstrated by the full selection against host-derived WT cells and the continued presence of donor-derived DH and DM cells in G418 (Fig. 1c and data not shown).
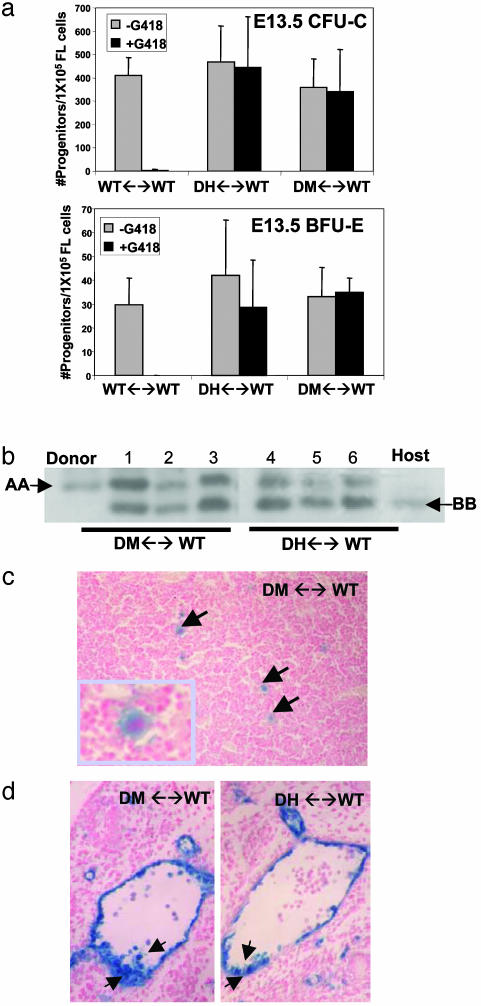
Based on the reduced hematopoietic potential of Tie1/Tie2 doubly deficient yolk sac cells, we expected that mutant cells would be compromised in their ability to contribute to hematopoiesis in chimeras. We tested this hypothesis by assaying for the presence of TIE1 and TIE2 doubly deficient HPCs in the FLs of chimeras. As shown in Fig. 2a, DM- and DH-derived erythroid (BFU-E) and non-red myeloid (CFU-C) progenitors in E13.5 FL were observed at comparable frequencies. The contribution of DH or DM cells to the hematopoietic system was comparable to that in the limb (Fig. 2b) as well as the tail, brain, and skin (data not shown). In addition to contributing to erythro-myelopoiesis in chimeras, DM-derived megakaryocytes were readily detected in the livers of E13.5 chimeric fetuses (Fig. 2c), indicating that the loss of Tie1/Tie2 function has no effect on fetal megakaryopoiesis. We further made use of the knocked-in LacZ gene in the TIE1 locus (20) to monitor in situ by X-gal staining the ability of DM cells to contribute to the early ventral islands of HCs and ECs in the developing vasculature. As shown in Fig. 2d, both DM and DH cells had equivalent potential to constitute both the ventral endothelium and the HC clusters of the dorsal aorta at E10.5–E11.0. In all DH (n = 8) and DM (n = 7) chimeric embryos in which distinct clusters could be identified, we observed donor-derived cells comprising both the endothelium and luminally associated HCs. We conclude that TIE receptors are not required for hematopoiesis at the fetal stage of development. These findings demonstrate that signaling through TIE receptors is not an intrinsic requirement for either the specification of the hematopoietic lineage or the early differentiation and maturation of HCs.
Fig. 2.
Tie1/Tie2 doubly deficient cells contribute to fetal hematopoiesis. (a) E13.5 FL cells from WT (n = 9), DH (n = 15), and DM (n = 8) chimeras were cultured in methylcellulose containing cytokines for non-red myeloid (CFU-C) and erythroid (BFU-E) differentiation in the presence and absence of G418. Mutant and control colony numbers (adjusted for the overall extent of chimerism) show equivalent contribution. (b) Representative GPI analysis demonstrating equivalent donor (AA) versus host (BB) contribution to tail (lanes 1 and 4), FL (lanes 2 and 5), and erythro-myeloid cells (lanes 3 and 6) in DH and DM chimeric fetuses. Cells in lanes 3 and 6 were collected from methylcellulose cultures in a. (c) X-gal stained FL of E15.5 DM ↔ WT chimera reveals β-galactosidase-positive, mutant cell-derived megakaryocytes identified by their large size and multilobed nucleus. (d) Tie1/Tie2-deficient cells contribute to hemogenic endothelium in E10.5 chimeras. ECs and HCs of the ventral aorta contain equivalent β-galactosidase positive donor cells in DM and DH chimeras.
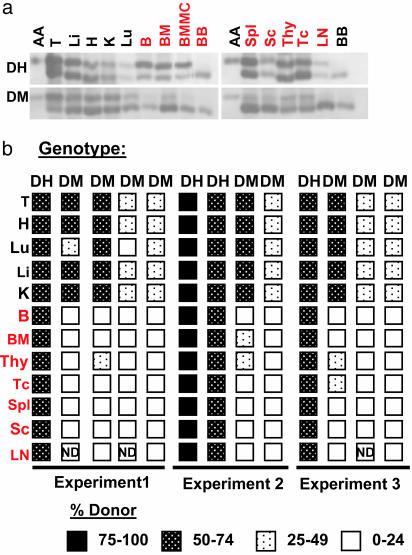
From the late prenatal to early postnatal period of development, the site of hematopoiesis shifts from the FL to the BM, which in the mature animal is the primary source of multipotential progenitors that seed other hematopoietic sites via circulation (26). We assessed the capacity of Tie1/Tie2-deficient cells to contribute to postnatal hematopoiesis by analyzing the donor cell content in several mutant and control chimeras aged 8 weeks and older. As demonstrated in Fig. 3 a and b, both DM and DH cells contributed to nonhematopoietic tissues including heart, lung, liver, and kidney. Equivalent contribution to hematopoietic tissues was also observed for DH chimeras. In contrast, a striking reduction in the donor cell content of DM-derived cells was noted in the thymus, spleen, and BM, as well as in peripheral blood (Fig. 3). These findings were consistent for several individual chimeras derived from independent embryo aggregation experiments (Fig. 3b).
Fig. 3.
Tie1/Tie2-deficient cells are at a competitive disadvantage in the adult hematopoietic system. (a) Cells lacking Tie1 and Tie2 contribute poorly to adult hematopoietic tissues in chimeras. GPI analysis was performed on lysates from tissues as indicated, demonstrating a reduced presence of the donor-derived GPI-AA isoform in cells from hematopoietic organs of DM chimeras (DM ↔ WT) but not DH chimeras (DH ↔ WT). (b) Distribution of DM relative to DH cells in chimeras from three independent aggregation experiments. DH and DM chimeras with low donor cell contribution (<15% in nonhematopoietic tissues) were eliminated from the analysis. T, tail; H, heart; Lu, lung; Li, liver; K, kidney; B, blood; BMMC, BM mononuclear cells; Spl, spleen; Sc, splenocytes; Thy, thymus; Tc, thymocytes; LN, lymph node.
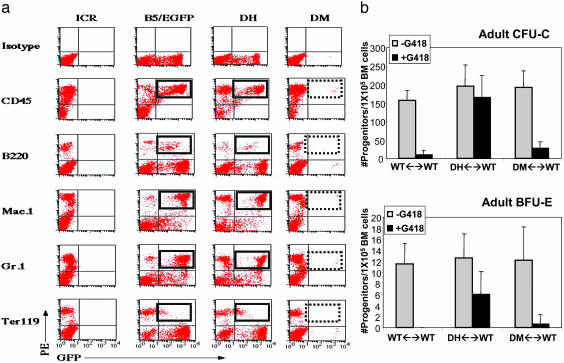
The absence of DM-derived donor cells in adult hematopoietic tissue prompted us to examine more closely individual hematopoietic lineages in the BM of chimeras in which donor cells could be identified by expression of EGFP (ref. 27; Fig. 1b). BM cells from DH and DM chimeras were labeled with markers for specific blood lineages and analyzed by flow cytometry for the relative presence of donor-derived cells. In control chimeras, a significant proportion of HCs specific to the erythroid, granulocytic, and B cell lineages were of donor origin, whereas in Tie1/Tie2-deficient chimeras, donor-derived, lineage marker-positive cells were absent (Fig. 4a). Thus, Tie1/Tie2 deficiency affects the major HC lineages in the BM, suggesting a critical role for TIE receptors in the HSC or multipotential progenitor population within the BM niche.
Fig. 4.
Tie1/Tie2-deficient HCs and progenitors are absent from adult chimeric BM. (a) Tie1/Tie2-deficient cells do not contribute to mature HC lineages in the BM. Flow cytometry was performed on BM cells isolated from DH and DM chimeras, a WT non-GFP-expressing control (ICR), and a control for the EGFP-expressing strain (B5/EGFP) by using anti-CD45 to mark all leukocytes, anti-B220 as a marker for B cells, anti-Gr-1 and anti-Mac1 for granulocytes and macrophages, respectively, and Ter119 to label erythroid cells. The hatched rectangles highlight the reduced frequency of donor-derived lymphocytes in DM versus DH BM. Results are representative of two DM ↔ WT and three DH ↔ WT chimeras. (b) BM-derived non-red myeloid (CFU-C) and erythroid (BFU-E) progenitors were enumerated from WT, DH, and DM chimeras after culture in cytokine-supplemented methylcellulose in the presence or absence of G418. DM ↔ WT, n = 8; DH, ↔ WT, n = 8; WT ↔ WT, n = 8.
To ascertain quantitatively whether HC progenitors were affected by the absence of TIE receptors, we performed colony assays in the presence of G418. BM cells from 6- to 8-week-old DH chimeras contained G418-resistant myeloid and erythroid colonies, whereas BM cells from DM chimeras did not (Fig. 4b).
Based on these findings, we infer that in chimeras with WT cells, Tie1/Tie2-deficient HPCs are at a competitive disadvantage in the BM. The absence of DM-derived cells might reflect a requirement for the TIE family of receptors for migration to the BM during late embryogenesis. To address this possibility, we examined BM cells from 7- to 10-day-old chimeric mice and determined that DM HPCs were indeed present, albeit at a reduced level relative to DH chimeras (data not shown). Taken together, these observations suggest that TIE1- and TIE2-deficient cells retain the capacity to home to the BM from the FL during ontogeny, but that they fail to be maintained in this environment for the lifetime of the animal.
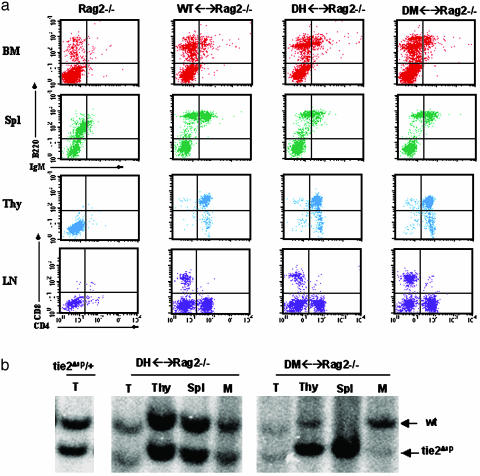
To elucidate further the function of TIE1 and TIE2 in hematopoiesis, we next generated aggregation chimeras by using Rag2-deficient morulae as the host (28). Rag2–/– mice do not produce mature lymphocytes; thus, the ability of Tie1/Tie2-deficient cells to contribute to lymphopoiesis in the absence of competing host cell differentiation could be rigorously assayed. Using fluorescence-activated cell sorter analysis, we compared the contribution of DH and DM cells to B and T cell lineages in adult Rag2–/– chimeras. As demonstrated in Fig. 5a, the frequency of B220+ cells in the BM and spleen was comparable between DM, DH, and WT chimeras. In addition, the number of immature CD4+/CD8+ T cells in the thymus was not significantly different between Tie1/Tie2-deficient and control chimeras. Furthermore, mature single CD4+ and CD8+ T cells were present in the lymph nodes of DM chimeras, suggesting that DM T cells retain the capacity to differentiate and relocate to the lymph node in the Rag2–/– strain. This finding suggests that in the absence of competing WT host cells, Tie1/Tie2-deficient lymphoid progenitors execute the correct program of lymphoid differentiation, including migration to peripheral lymphoid organs. Because DM cells are clearly reduced in lymphoid tissue in chimeras with WT cells (Fig. 3 a and b), we conclude that B- and T-lymphoid progenitors deficient for TIE1 and TIE2 are not defective in their ability to differentiate but fail to survive in the BM and thymus relative to WT competitors. It is important to note that in myelopoiesis in the BM where Rag2–/–-derived cells provide a competitive WT HPC component, DM cells were greatly reduced in their contribution relative to control DH counterparts and relative to B and T lineage reconstitution (Fig. 5b).
Fig. 5.
Tie1/Tie2-deficient cells reconstitute lymphoid lineages in Rag2–/– host. (a) Flow cytometry was performed on HCs of DM ↔ Rag2–/–, control DH ↔ Rag2–/–, and WT ↔ Rag2–/– chimeras. Anti-B220 versus anti-IgM identified mature B cells in the BM and spleen (Spl) of mutant and control chimeras. Anti-CD4 and -CD-8 antibodies were used as T cell markers for thymus (Thy) and lymph node (LN) cells. Results are representative of DM (n = 8), DH (n = 11), and WT (n = 8). (b) DM cells contribute poorly to myelopoiesis in Rag2–/– host. Genomic DNA from tail (T), thymus (Thy), spleen (Spl), and myeloid (M) cells from a CFU-C assay were screened for the relative presence of the donor-specific tie2Δsp allele (19) in Rag2–/– chimeras reconstituted with DM or DH cells.
Discussion
There is increasing evidence for a close developmental and cell lineage relationship between the early cells that give rise to the vasculature and the stem cells of the hematopoietic system (2). This evidence raises the possibility that there may be overlapping molecular pathways that regulate both HSCs and ECs. Here we have combined mosaic analysis and well defined in vitro assays for the hematopoietic system to dissect the temporal and lineage-specific dependence of HCs on TIE1- and TIE2-mediated signaling pathways, which are known to have essential functions in angiogenesis. We have demonstrated that TIE1 and TIE2 are not required for the emergence of definitive HSCs from hemogenic endothelium or for their relocation to and differentiation in the FL, despite their expression and functional correlation with this process (refs. 14, 16, and 22 and Fig. 1a). These results extend our earlier findings from chimeric analysis that TIE1 and TIE2 are also dispensable for the initial differentiation of ECs (22). We infer that defects in embryonic hematopoiesis in TIE1/TIE2-deficient embryos are a secondary consequence of compromised circulation and growth delay.
Whereas TIE1 and TIE2 are dispensable for the early differentiation of HCs, surprisingly, the chimeric analysis demonstrates that there is a cell-autonomous requirement for TIE receptors in the adult hematopoietic system. Previous reports demonstrated that TIE1-deficient cells (expressing WT levels of TIE2) contribute robustly to hematopoiesis in both WT (21) and Rag2–/– chimeras (29). These findings suggested that TIE1 alone is either not required or has a functionally redundant role with TIE2 in the formation and differentiation of HSCs, where it is expressed. In contrast, our present analysis examined the behavior of Tie1/Tie2 double null cells in the hematopoietic system. We infer that the reduced capacity of DM cells to contribute to the adult hematopoietic system reflects the loss of TIE2 in HSCs or committed progenitors.
Our findings in both WT and Rag2–/– chimeras demonstrate that TIE1/TIE2-deficient HSCs and multipotential progenitors arise and home normally to their appropriate niche in embryogenesis but in the presence of WT competitor cells fail to survive and/or expand in the adult microenvironment. Determination of the precise hematopoietic stem or progenitor population(s) affected by Tie1/Tie2 deficiency requires investigation with inbred strain chimeras. Nevertheless, our results support other studies that have identified differences in the nature of HSCs within alternate anatomic locations during ontogeny and in their responses to varying microenvironments (30–32). The BM is composed of diverse cell types, including adipocytes, fibroblasts, macrophages, ECs, and the network of the extracellular matrix, that together constitute a supportive niche that allows for the maintenance and differentiation of HSCs. Angiopoietin 1, the ligand for TIE2, facilitates HPC adherence to fibronectin, a major extracellular matrix component of the BM (16). The results presented here provide in vivo support for an adhesion function for TIE2 in the adult BM, either directly or by modulating the expression and/or function of adhesion receptor molecules such as integrins β1 and α4, which are also known to play a role in postnatal hematopoiesis (33, 34). It is interesting to speculate that the TIE family of receptor tyrosine kinases might be essential for establishing and maintaining adult-specific conditions for HSCs in the BM.
Acknowledgments
We thank Dr. Andras Nagy for the EGFP mouse line, Sandra Tondat for aggregations, Shirly Vesely and Catherine Wassenaar for technical assistance, Gisele Knowles for flow cytometry, Ken Harpal for preparation of histological sections, and Dr. William Stanford and members of the Bernstein laboratory for helpful discussions. This work was supported by the National Cancer Institute of Canada.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BM, bone marrow; DH, doubly heterozygous; DM, double mutant; En, embryonic day n; EC, endothelial cell; EGFP, enhanced GFP; FL, fetal liver; GPI, glucose-phosphate isomerase; HC, hematopoietic cell; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell.
References
- 1.Sabin, F. R. (1920) Contrib. Embryol. 9 214–262. [Google Scholar]
- 2.Kubo, H. & Alitalo, K. (2003) Genes Dev. 17 322–329. [DOI] [PubMed] [Google Scholar]
- 3.de Bruijn, M. F., Ma, X., Robin, C., Ottersbach, K., Sanchez, M. J. & Dzierzak, E. (2002) Immunity 16 673–683. [DOI] [PubMed] [Google Scholar]
- 4.Pardanaud, L., Luton, D., Prigent, M., Bourcheix, L. M., Catala, M. & Dieterlen-Lievre, F. (1996) Development (Cambridge, U.K.) 122 1363–1371. [DOI] [PubMed] [Google Scholar]
- 5.Delassus, S. & Cumano, A. (1996) Immunity 4 97–106. [DOI] [PubMed] [Google Scholar]
- 6.Muller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F. & Dzierzak, E. (1994) Immunity 1 291–301. [DOI] [PubMed] [Google Scholar]
- 7.Keller, G., Lacaud, G. & Robertson, S. (1999) Exp. Hematol. 27 777–787. [DOI] [PubMed] [Google Scholar]
- 8.Jordan, C. T., Astle, C. M., Zawadzki, J., Mackarehtschian, K., Lemischka, I. R. & Harrison, D. E. (1995) Exp. Hematol. 23 1011–1015. [PubMed] [Google Scholar]
- 9.Sanchez, M. J., Holmes, A., Miles, C. & Dzierzak, E. (1996) Immunity 5 513–525. [DOI] [PubMed] [Google Scholar]
- 10.Takakura, N., Watanabe, T., Suenobu, S., Yamada, Y., Noda, T., Ito, Y., Satake, M. & Suda, T. (2000) Cell 102 199–209. [DOI] [PubMed] [Google Scholar]
- 11.Lyden, D., Hattori, K., Dias, S., Costa, C., Blaikie, P., Butros, L., Chadburn, A., Heissig, B., Marks, W., Witte, L., et al. (2001) Nat. Med. 7 1194–1201. [DOI] [PubMed] [Google Scholar]
- 12.De Palma, M., Venneri, M. A., Roca, C. & Naldini, L. (2003) Nat. Med. 9 789–795. [DOI] [PubMed] [Google Scholar]
- 13.Batard, P., Sansilvestri, P., Scheinecker, C., Knapp, W., Debili, N., Vainchenker, W., Buhring, H. J., Monier, M. N., Kukk, E., Partanen, J., et al. (1996) Blood 87 2212–2220. [PubMed] [Google Scholar]
- 14.Hamaguchi, I., Huang, X. L., Takakura, N., Tada, J., Yamaguchi, Y., Kodama, H. & Suda, T. (1999) Blood 93 1549–1556. [PubMed] [Google Scholar]
- 15.Sato, A., Iwama, A., Takakura, N., Nishio, H., Yancopoulos, G. D. & Suda, T. (1998) Int. Immunol. 10 1217–1227. [DOI] [PubMed] [Google Scholar]
- 16.Takakura, N., Huang, X. L., Naruse, T., Hamaguchi, I., Dumont, D. J., Yancopoulos, G. D. & Suda, T. (1998) Immunity 9 677–686. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, H. C., Ema, H., Osawa, M., Nakamura, Y., Suda, T. & Nakauchi, H. (2000) Blood 96 3757–3762. [PubMed] [Google Scholar]
- 18.Hattori, K., Dias, S., Heissig, B., Hackett, N. R., Lyden, D., Tateno, M., Hicklin, D. J., Zhu, Z., Witte, L., Crystal, R. G., et al. (2001) J. Exp. Med. 193 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumont, D. J., Gradwohl, G., Fong, G. H., Puri, M. C., Gertsenstein, M., Auerbach, A. & Breitman, M. L. (1994) Genes Dev. 8 1897–1909. [DOI] [PubMed] [Google Scholar]
- 20.Puri, M. C., Rossant, J., Alitalo, K., Bernstein, A. & Partanen, J. (1995) EMBO J. 14 5884–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partanen, J., Puri, M. C., Schwartz, L., Fischer, K. D., Bernstein, A. & Rossant, J. (1996) Development (Cambridge, U.K.) 122 3013–3021. [DOI] [PubMed] [Google Scholar]
- 22.Puri, M. C., Partanen, J., Rossant, J. & Bernstein, A. (1999) Development (Cambridge, U.K.) 126 4569–4580. [DOI] [PubMed] [Google Scholar]
- 23.Suri, C., Jones, P. F., Patan, S., Bartunkova, S., Maisonpierre, P. C., Davis, S., Sato, T. N. & Yancopoulos, G. D. (1996) Cell 87 1171–1180. [DOI] [PubMed] [Google Scholar]
- 24.Gale, N. W., Thurston, G., Hackett, S. F., Renard, R., Wang, Q., McClain, J., Martin, C., Witte, C., Witte, M. H., Jackson, D., et al. (2002) Dev. Cell 3 411–423. [DOI] [PubMed] [Google Scholar]
- 25.Palis, J., Robertson, S., Kennedy, M., Wall, C. & Keller, G. (1999) Development (Cambridge, U.K.) 126 5073–5084. [DOI] [PubMed] [Google Scholar]
- 26.Morrison, S. J., Uchida, N. & Weissman, I. L. (1995) Annu. Rev. Cell Dev. Biol. 11 35–71. [DOI] [PubMed] [Google Scholar]
- 27.Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. (1998) Mech. Dev. 76 79–90. [DOI] [PubMed] [Google Scholar]
- 28.Chen, J., Lansford, V., Stewart, F., Young, F. & Alt, F. W. (1993) Proc. Natl. Acad. Sci. USA 90 4528–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodewald, H. R. & Sato, T. N. (1996) Oncogene 12 397–404. [PubMed] [Google Scholar]
- 30.Godin, I. & Cumano, A. (2002) Nat. Rev. Immunol. 2 593–604. [DOI] [PubMed] [Google Scholar]
- 31.Wang, L. C., Swat, W., Fujiwara, Y., Davidson, L., Visvader, J., Kuo, F., Alt, F. W., Gilliland, D. G., Golub, T. R. & Orkin, S. H. (1998) Genes Dev. 12 2392–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikkola, H. K., Klintman, J., Yang, H., Hock, H., Schlaeger, T. M., Fujiwara, Y. & Orkin, S. H. (2003) Nature 421 547–551. [DOI] [PubMed] [Google Scholar]
- 33.Potocnik, A. J., Brakebusch, C. & Fassler, R. (2000) Immunity 12 653–663. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo, A. G., Yang, J. T., Rayburn, H. & Hynes, R. O. (1996) Cell 85 997–1008. [DOI] [PubMed] [Google Scholar]