Abstract
The potential consequences of biodiversity loss for ecosystem functioning and services at local scales have received considerable attention during the last decade, but little is known about how biodiversity affects ecosystem processes and stability at larger spatial scales. We propose that biodiversity provides spatial insurance for ecosystem functioning by virtue of spatial exchanges among local systems in heterogeneous landscapes. We explore this hypothesis by using a simple theoretical metacommunity model with explicit local consumer–resource dynamics and dispersal among systems. Our model shows that variation in dispersal rate affects the temporal mean and variability of ecosystem productivity strongly and nonmonotonically through two mechanisms: spatial averaging by the intermediate-type species that tends to dominate the landscape at high dispersal rates, and functional compensations between species that are made possible by the maintenance of species diversity. The spatial insurance effects of species diversity are highest at the intermediate dispersal rates that maximize local diversity. These results have profound implications for conservation and management. Knowledge of spatial processes across ecosystems is critical to predict the effects of landscape changes on both biodiversity and ecosystem functioning and services.
The potential consequences of biodiversity loss for ecosystem functioning and services have received considerable attention during the last decade (1–3). Several controlled experiments have now established that species diversity influences ecosystem-level processes such as primary production in grasslands (4–7) and heterotrophic consumption of suspended resources in streams (8). These experiments, however, have typically been performed over small spatial and temporal scales relative to the size and generation time of the organisms involved. At these small scales, uncontroversial effects of biodiversity due to functional complementarity among species with different traits have been shown only at low to moderate levels of diversity (1).
It is possible that the functional significance of biodiversity will fully appear only at larger spatial and temporal scales. The insurance hypothesis (9) proposes that species or phenotypes that are functionally redundant for an ecosystem process at a given time may show temporal complementarity because of asynchronous responses to environmental fluctuations. In this case, biodiversity acts as insurance for ecosystem functioning against temporal environmental change because functional compensations among types provide enhanced and more predictable aggregate ecosystem properties. A number of theoretical studies have provided support for this hypothesis (9–15) although experimental evidence remains scanty and controversial (reviewed in ref. 16). Similarly, species or phenotypes may complement each other in space, such that a more diverse species pool allows better occupation of spatial gradients (17).
This theoretical work has usually not considered explicitly the ecological conditions that allow the maintenance of biodiversity within ecosystems. Whereas environmental fluctuations may promote species diversity (17), the frequency of these fluctuations is critical because frequencies that are either too low or too high may prevent coexistence (18). If this is the case, local diversity may be maintained by immigration from surrounding sites (19–22), which provides the source of variation on which “selection” can act to favor “adapted” types, and thereby both enhance and buffer ecosystem processes locally (14, 23). Because immigration into a system corresponds to emigration from some other systems, the maintenance of diversity and its functional consequences have then to be considered at the scale of a metacommunity, i.e., a set of local communities connected by dispersal at the landscape or regional scale (24–28). In such a metacommunity, we propose that the insurance hypothesis has a specific spatial component. If different systems experience different environmental conditions and fluctuate asynchronously, different species are expected to thrive in each system at each point in time, and dispersal ensures that the species adapted to the new environmental conditions locally are available to replace less adapted ones as the environment changes. As a result, biodiversity may enhance and buffer ecosystem processes by virtue of spatial exchanges among local systems in a heterogeneous landscape even when such effects do not occur in a closed homogeneous system. This hypothesis, however, is complicated by the fact that dispersal has nonintuitive, nonmonotonic effects: both low and high dispersal rates lead to competitive exclusion whereas local species diversity is maximized at intermediate dispersal rates (27, 28).
In this paper, we explore the spatial insurance hypothesis by using a simple theoretical model of a metacommunity in which different systems experience asynchronous environmental fluctuations, different species have different but constant traits, and the degree of matching between environmental values and species trait values determines the species' abilities to exploit local resources at each point in time. We show that intermediate dispersal rates are critical for the operation of spatial insurance effects of biodiversity, which stresses the importance of appropriate levels of connectivity in fragmented landscapes.
The Model
Our metacommunity model differs from previous ones (27, 28) in that it describes consumer–resource dynamics explicitly, which allows us to measure ecosystem processes such as productivity in a more straightforward way. Let Nij(t) be the biomass of species i (e.g., a plant) and Rj(t) be the amount of limiting resource (e.g., a nutrient such as nitrogen) in community j at time t. The metacommunity consists of M communities and S species in total. Species i consumes the resource at a rate cij(t), converts it into new biomass with efficiency eij, and dies at rate mij in community j. We assume that the resource is renewed locally through a constant input flux Ij and is lost at a rate lj. We ignore nutrient cycling for simplicity; incorporating it does not change the behavior of our system qualitatively. Last, species disperse at a rate a, such that dispersal is global and dispersers are redistributed uniformly across the landscape. We assume identical dispersal rates for all species to simplify the interpretation of the results because dispersal rate is a critical parameter that governs the behavior of the whole system. Our model thus reads:
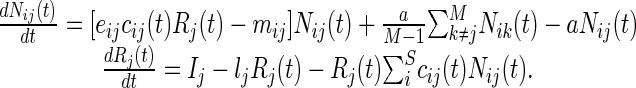 |
[1] |
We further assume that the consumption rates cij(t) vary as local environmental conditions change through time and reflect the matching between species traits and environmental conditions (29). Let the constant trait value of species i be Hi, which may be interpreted as its niche optimum along an environmental gradient, and the fluctuating environmental value of community j be Ej(t). We assume that both species trait and environmental values vary between 0 and 1, and that a species' consumption rate is highest when the environmental value matches its niche optimum as measured by its trait value. Specifically, consumption rates are given by:
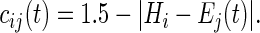 |
[2] |
These variations in consumption rates describe the species responses to environmental fluctuations (9).
Ecosystem productivity at time t is defined as the production of new biomass per unit time, which, averaged across the metacommunity, is:
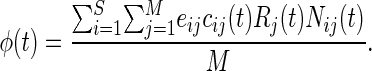 |
[3] |
Simulations for various scenarios and parameter values led to qualitatively similar results. In the simulations reported below, we considered a metacommunity consisting of seven species and seven communities with the following parameters: eij = 0.2; mij = 0.2; Ij = 150; lj = 10; H1 = 1; and Hi = Hi–1 – 1/6 for i = 2 to 7. We set an extinction threshold to Nij(t) = 0.1; local populations lower than this threshold biomass were regarded as extinct (22). We assumed autocorrelated, sinusoidal fluctuations of local environmental values with period T:
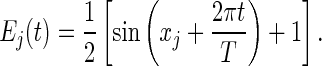 |
[4] |
Environmental conditions were assumed to be out of phase in the various communities, by choosing xj such that E1(0) = 1 and Ej(0) = Ej–1(0) – 1/6 for j = 2 to 7. This assumption ensured that each species was the best competitor in a different community at the beginning of the simulation (species 1 in community 1, etc.). With this assumption, there is always a community in which each species is superior, but, because of temporal fluctuations, that community shifts in space over time, thus requiring some dispersal for long-term coexistence. The period T was chosen to be large enough (T = 40,000) so that there was rapid competitive exclusion in the absence of dispersal. This choice avoided confounding effects due to coexistence mechanisms, such as the intermediate disturbance hypothesis, other than the “source–sink” or “mass” effect that is specific to our metacommunity approach (27, 28).
Results
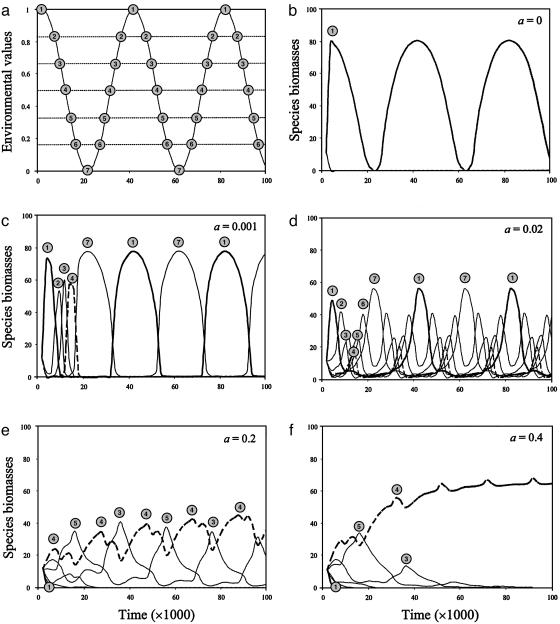
Fig. 1 shows the dynamics of the various species for different dispersal rates in one particular community (community 1, in which species 1 is the best competitor initially). Environmental fluctuations in this community and species trait values are illustrated in Fig. 1a. The moment when each species is potentially the best competitor is indicated by its number in a gray circle. When there is no dispersal (Fig. 1b), local coexistence is impossible. The species that has the initial competitive advantage excludes all of the other species, and its biomass fluctuates with local environmental conditions. In contrast, regional diversity is maximal because different species persist in different communities. As dispersal increases to intermediate levels, local diversity increases because of source–sink effects among communities (Fig. 1 c and d). Regional diversity, however, drops before increasing again (see Fig. 3a). This nonlinear response occurs because specialist species 1 and 7 are adapted to the stationary parts of the sinusoidal environmental curve (Fig. 1a) and thus have the longest contiguous time periods in which they are locally dominant. These species tend to outcompete the other species at low dispersal. As dispersal further increases, however, species that are closer to the intermediate type (represented by species 4, Fig. 1a) and hence are better adapted to the average environmental conditions across the metacommunity become increasingly more competitive. Source–sink effects then gain in magnitude and allow local and regional coexistence of an increasing number of species. When all species coexist (Fig. 1d), shifts in dominance reflect the matching between species trait values and environmental conditions. As dispersal increases to high levels (Fig. 1 e and f), the metacommunity tends to behave as a single large community. The species that has an average trait value (species 4) becomes the best competitor at the scale of the metacommunity and excludes all of the other species. Both regional and local diversity then decrease (see Fig. 3a).
Fig. 1.
Environmental fluctuations (a) and dynamics of species biomasses for various dispersal rates (b–f) in community 1. In a, the time when each species is potentially the best competitor is indicated by its number in a gray circle, and the dashed horizontal lines show constant species trait values. In b–f, the numbers in gray circles indicate species identity, the bold solid line corresponds to the species that has the initial competitive advantage (species 1), and the dashed line corresponds to the species that has an average trait value (species 4).
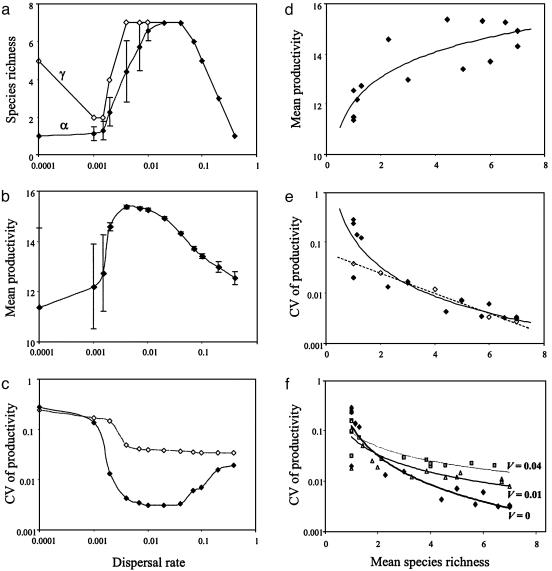
Fig. 3.
Regional (γ) and mean local (α) species richness (a), temporal mean of ecosystem productivity (b), and coefficient of variation (CV) of ecosystem productivity through time (c) as functions of dispersal rate (mean ± SD across communities), and the resulting relationships between the temporal mean of ecosystem productivity (d) or the CV of ecosystem productivity (e and f) and mean local species richness. In d, the curve fitted to the data is a logarithmic function (r2 = 0.71). In c and e, filled diamonds correspond to the original model in which both dispersal rate and species diversity are allowed to vary. In c, open diamonds show the results for the case in which the dispersal rate varies while local species richness is held constant at a single species (species 1). In e, open diamonds show the results for the case in which local species richness varies while the dispersal rate is held constant at an intermediate value (a = 0.02). In this scenario, species richness was varied by removing species sequentially from species 7 to species 2. The curves fitted to the data are a power function for filled diamonds (solid line, r2 = 0.84) and an exponential function for open diamonds (dotted line, r2 = 0.98). In f, a normally distributed random deviate with zero mean and variance (V) has been superimposed onto the periodic environmental fluctuations Ej(t) at each time step. Filled diamonds, V = 0 (no noise); open triangles, V = 0.01; gray squares, V = 0.04.
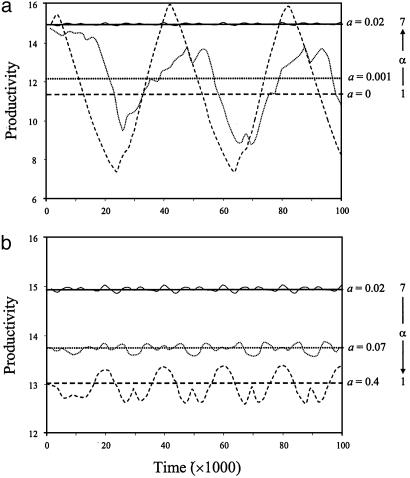
These fluctuations in species biomasses generate corresponding fluctuations in ecosystem productivity (Fig. 2). As the dispersal rate increases from zero to intermediate values and local species diversity accordingly increases from its minimum to its maximum value, the temporal mean of productivity increases whereas its temporal variability decreases (Fig. 2a). As the dispersal rate further increases from intermediate to high values and local species diversity accordingly declines back to a minimum, the temporal mean of productivity decreases whereas its temporal variability increases (Fig. 2b). As a consequence, variations in dispersal rate generate strongly nonlinear, broadly correlated variations in local species diversity, average productivity, and the coefficient of variation of productivity, a common standardized measure of variability (11–13, 15) (Fig. 3 a–c).
Fig. 2.
Temporal fluctuations and temporal mean of ecosystem productivity for various dispersal rates and corresponding levels of local species diversity, α. In a, the dispersal rate a increases from zero to intermediate values (a = 0, dashed line; a = 0.001, dotted line; a = 0.02, solid line). In b, it further increases from intermediate to high values (a = 0.02, solid line; a = 0.07, dotted line; a = 0.4, dashed line). Time averages (calculated when species biomasses settled into a regular pattern in time) are indicated by horizontal lines. Note that a and b have different scales on the y axis, for clarity.
Thus, changes in species diversity driven by changes in dispersal rates generate two kinds of effects on ecosystem productivity at the metacommunity scale: an increase in its temporal mean and a decrease in its temporal variability. The first effect is apparent in the positive correlation between average productivity and local species diversity (Fig. 3d). The second is apparent in the negative correlation between the coefficient of variation of productivity and local species diversity (Fig. 3e). Variability, however, declines most rapidly at low values of diversity (note the log scale in Fig. 3e).
Also, species diversity is not the only factor that changes as the dispersal rate varies. The intermediate-type species that dominates across the landscape when dispersal is high has a much lower variability than do the species that dominate local communities when dispersal is low (compare Fig. 1 b and f), which leads to a higher mean and a lower variability of productivity in a highly connected than in a poorly connected metacommunity despite the fact that local species diversity is minimum in both cases (compare Fig. 2 a and b). This result occurs because the intermediate-type species averages out spatial and temporal environmental variations in highly connected metacommunities.
To separate out the effects of species diversity and spatial averaging, which are both generated by changes in dispersal, on the temporal variability of productivity, we varied local species richness by removing some species while keeping the dispersal rate fixed at an intermediate value (Fig. 3e, open diamonds), and contrasted the coefficients of variation of productivity in this new scenario and in the previous scenario in which dispersal was allowed to vary (Fig. 3e, filled diamonds). The two scenarios differ only when local diversity is close to minimum (1 species), which shows that spatial averaging plays a significant role only at such low values of local diversity. We also contrasted dispersal-induced changes in the coefficient of variation of productivity in the two scenarios where local species diversity is either allowed to vary (Fig. 3c, filled diamonds) or kept at a constant minimum value of 1 species (Fig. 3c, open diamonds). This comparison shows that both spatial averaging and species diversity contribute to decrease the temporal variability of productivity, but the spatial averaging effect is roughly constant beyond a threshold dispersal rate. In contrast, the spatial insurance effect of species diversity is strong enough to produce a wide divergence between the two scenarios at the intermediate dispersal rate that maximizes diversity.
Lastly, we checked the robustness of our results to environmental stochasticity by superimposing a high-frequency white noise onto our periodic environmental fluctuations, i.e., by adding a normally distributed random deviate with zero mean to Ej(t) at each time step. The pattern of decreasing variability with increasing species diversity was still apparent, but increasing the variance of the noise reduced the buffering effect of diversity (Fig. 3f). For a given variance, however, increasing the temporal autocorrelation of the noise (from white to red) had the opposite effect of restoring the buffering effect of diversity (results not shown).
Discussion
Biodiversity can act as biological insurance for local ecosystem functioning by allowing functional compensations between species or phenotypes in time (9, 12–14). A prerequisite for this effect, however, is that local diversity be maintained through time. Maintenance of species diversity can occur through the same local mechanism of temporal niche partitioning that allows these functional compensations (17), but it can also hinge on immigration from outside the local system considered (14, 19–22). If such is the case, there is a strong spatial component to the insurance effects of biodiversity. Our model shows that species diversity maintained by dispersal among ecosystems in heterogeneous landscapes can have the two kinds of insurance effects previously identified (9): i.e., an increase in the temporal mean of ecosystem productivity across the metacommunity and a decrease in its temporal variability. These effects occur despite the fact that local coexistence is impossible, and thus no temporal insurance effect can occur, within a closed system. Therefore, the insurance effects showed by our model are entirely generated by the spatial dynamics of the metacommunity.
Accordingly, these effects are also strongly dependent on the dispersal rate, which determines the system's connectivity. Local species diversity and the insurance effects that are related to it collapse at both low and high dispersal rates. When dispersal is very low, each local community behaves as a closed system in which competitive exclusion proceeds; and, when dispersal is high, the whole metacommunity behaves as a single large closed system in which competitive exclusion also proceeds (27, 28). At both ends of the dispersal gradient, functional compensations and adaptive shifts between species are prevented, which leads to a relatively low average productivity as well as large fluctuations in productivity as the single surviving species tracks environmental fluctuations. Local species diversity is highest at an intermediate dispersal rate. Dispersal is then high enough to prevent local competitive exclusion by the dominant species, and low enough to prevent homogenization of the entire metacommunity, and hence global competitive exclusion by the intermediate-type species. It is also this intermediate dispersal rate that maximizes the insurance effects of species diversity.
Dispersal, however, also has significant effects on the mean and variability of ecosystem productivity on its own, independently of species diversity, as single-species metapopulation and source–sink models also show (25, 30). Although average productivity decreases and the variability of productivity increases as dispersal increases beyond its diversity-maximizing value, average productivity is higher and the variability of productivity is lower in a highly connected system than in a poorly connected system. This result occurs because the intermediate-type species that dominates a highly connected system is always able to find suitable conditions somewhere in the landscape and averages out environmental variations across the various local sites. Thus, dispersal has a spatial averaging effect for this species, which buffers associated ecosystem processes against local environmental fluctuations. Our work shows that both spatial averaging and species diversity contribute to buffer ecosystem productivity. Spatial averaging has a roughly constant effect beyond an intermediate threshold dispersal rate and plays a significant role mainly when local species diversity declines at high dispersal rates. In contrast, the insurance effects of species diversity are highest at the intermediate dispersal rate that maximizes local diversity.
Our model is obviously simple, and deliberately so. Our goal in this work was to explore theoretically the potential for specific spatial insurance effects of biodiversity in heterogeneous landscapes. Accordingly, we removed the effects of other factors that may influence species coexistence and ecosystem functioning, such as temporal partitioning of environmental fluctuations due to local nonequilibrium coexistence in the absence of dispersal. In real systems, however, it is clear that many factors will operate in combination so that the spatial insurance effects investigated here will be mixed with local insurance as well as other effects. Our simple scenario in which there are periodic environmental fluctuations in each community and a regular phase lag of these fluctuations among neighboring communities is of course rather extreme and unlikely to occur in nature. Superimposing high-frequency noise on these fluctuations limits the buffering effect of diversity on ecosystem productivity because it creates a background level of variability for which no functional compensations among species can occur. Increasing the autocorrelation or decreasing the frequency of these random fluctuations tends to restore the buffering effect of diversity because it provides more time for species abundances to respond to these environmental fluctuations, thus allowing functional compensations to occur. Also, spatial insurance effects are expected to become weaker for a given diversity as environmental fluctuations become more synchronized across communities, such as may occur with seasonal forcing. However, because the spatial autocorrelation of any given environmental fluctuation typically decays with distance, population fluctuations across communities will not be perfectly synchronous (31), thereby ensuring the role of spatial insurance effects. The rate at which the spatial autocorrelation of the environmental fluctuation decays will determine the scale at which the spatial insurance effects are most pronounced.
Although increasing synchrony among local systems will decrease spatial insurance effects for a given species diversity, it will conversely increase the species diversity necessary to generate the same level of spatial insurance as in less synchronized systems. Two species that fluctuate with opposite phases are sufficient to strongly buffer productivity, whereas a much higher diversity is required to generate the same effect as species responses become more synchronous within a local ecosystem (9). Similarly, in a spatially heterogeneous landscape, more species will be necessary to achieve the same spatial insurance effects as environmental fluctuations become more synchronized among communities. This finding explains why insurance effects occur at relatively low levels of diversity in our model, in which asynchrony among communities is maximized. Also, it should be recalled that our model deliberately ignores local functional complementarity among species because all species are assumed to perform the same function and hence are unable to coexist within a closed local system. Thus, the mechanisms that operate in our model add to other mechanisms that generate functional effects of biodiversity. For all these reasons, our model provides a conservative view of the functional significance of biodiversity in heterogeneous landscapes.
Our work emphasizes the spatial dimension of the relationship between biodiversity and ecosystem functioning and stability, which has been largely ignored so far (1, 32–34). The spatial dynamics of populations and ecosystems within landscapes extend functional effects of biodiversity beyond the boundaries of local ecosystems. These results have profound implications for conservation and management. Biodiversity can affect ecosystem functioning and stability in heterogeneous landscapes in ways that could not be detected by local-scale experiments. Furthermore, changes in landscape connectivity after fragmentation or other anthropogenic and natural perturbations may substantially alter both species diversity and ecosystem processes at local and regional spatial scales (28, 34). Both increasing and decreasing landscape connectivity can either increase or decrease species diversity and the average magnitude and temporal variability of ecosystem processes, depending on the initial level of landscape connectivity and the dispersal abilities of the organism considered. Knowledge of spatial processes across ecosystems is therefore critical to predict the effects of landscape changes on both biodiversity and ecosystem functioning and services.
Acknowledgments
We thank Bob Holt and an anonymous reviewer for helpful comments on the manuscript. This paper is a contribution of the “Metacommunities” working group at the National Center for Ecological Analysis and Synthesis (U.S.A.). M.L. and A.G. acknowledge financial support by the Quantitative Ecology Incentive Coordinated Action of the Ministry of Research (France). N.M. was supported by the School of Computational Science and Information Technology at Florida State University.
References
- 1.Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., Hector, A., Hooper, D. U., Huston, M. A., Raffaelli, D., Schmid, B., et al. (2001) Science 294 804–808. [DOI] [PubMed] [Google Scholar]
- 2.Kinzig, A. P., Pacala, S. W. & Tilman, D., eds. (2002) The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions (Princeton Univ. Press, Princeton).
- 3.Loreau, M., Naeem, S. & Inchausti, P., eds. (2002) Biodiversity and Ecosystem Functioning: Synthesis and Perspectives (Oxford Univ. Press, Oxford).
- 4.Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M. & Siemann, E. (1997) Science 277 1300–1302. [Google Scholar]
- 5.Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., et al. (1999) Science 286 1123–1127. [DOI] [PubMed] [Google Scholar]
- 6.Loreau, M. & Hector, A. (2001) Nature 412 72–76, and erratum (2001) 413, 548. [DOI] [PubMed] [Google Scholar]
- 7.Tilman, D., Reich, P. B., Knops, J., Wedin, D., Mielke, T. & Lehman, C. (2001) Science 294 843–845. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale, B. J., Palmer, M. A. & Collins, S. C. (2002) Nature 415 426–429. [DOI] [PubMed] [Google Scholar]
- 9.Yachi, S. & Loreau, M. (1999) Proc. Natl. Acad. Sci. USA 96 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naeem, S. (1998) Conserv. Biol. 12 39–45. [Google Scholar]
- 11.Doak, D. F., Bigger, D., Harding, E. K., Marvier, M. A., O'Malley, R. E. & Thomson, D. (1998) Am. Nat. 151 264–276. [DOI] [PubMed] [Google Scholar]
- 12.Ives, A. R., Gross, K. & Klug, J. L. (1999) Science 286 542–544. [DOI] [PubMed] [Google Scholar]
- 13.Lehman, C. L. & Tilman, D. (2000) Am. Nat. 156 534–552. [DOI] [PubMed] [Google Scholar]
- 14.Norberg, J., Swaney, D. P., Dushoff, J., Lin, J., Casagrandi, R. & Levin, S. A. (2001) Proc. Natl. Acad. Sci. USA 98 11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ives, A. R. & Hughes, J. B. (2002) Am. Nat. 159 388–395. [DOI] [PubMed] [Google Scholar]
- 16.Loreau, M., Downing, A., Emmerson, M., Gonzalez, A., Hughes, J., Inchausti, P., Joshi, J., Norberg, J. & Sala, O. (2002) in Biodiversity and Ecosystem Functioning: Synthesis and Perspectives, eds. Loreau, M., Naeem, S. & Inchausti, P. (Oxford Univ. Press, Oxford), pp. 79–91.
- 17.Chesson, P., Pacala, S. & Neuhauser, C. (2002) in The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions, eds. Kinzig, A. P., Pacala, S. W. & Tilman, D. (Princeton Univ. Press, Princeton), pp. 213–245.
- 18.Hutchinson, G. E. (1961) Am. Nat. 95 137–145. [Google Scholar]
- 19.Caswell, H. (1978) Am. Nat. 112 127–154. [Google Scholar]
- 20.Shmida, A. & Ellner, S. (1984) Vegetatio 58 29–55. [Google Scholar]
- 21.Comins, H. N. & Noble, I. R. (1985) Am. Nat. 126 706–723. [Google Scholar]
- 22.Loreau, M. & Mouquet, N. (1999) Am. Nat. 154 427–440. [DOI] [PubMed] [Google Scholar]
- 23.Loreau, M. (2000) Oikos 91 3–17. [Google Scholar]
- 24.Wilson, D. S. (1992) Ecology 73 1984–2000. [Google Scholar]
- 25.Holt, R. D. (1993) in Species Diversity in Ecological Communities: Historical and Geographical Perspectives, eds. Ricklefs, R. E. & Schluter, D. (Univ. of Chicago Press, Chicago), pp. 77–88.
- 26.Hubbell, S. P. (2001) The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton).
- 27.Mouquet, N. & Loreau, M. (2002) Am. Nat. 159 420–426. [DOI] [PubMed] [Google Scholar]
- 28.Mouquet, N. & Loreau, M. (2003) Am. Nat., in press.
- 29.Mouquet, N., Moore, J. L. & Loreau, M. (2002) Ecol. Lett. 5 56–65. [Google Scholar]
- 30.Gonzalez, A. & Holt, R. D. (2002) Proc. Natl. Acad. Sci. USA 99 14872–14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanski, I. & Woiwood, I. (1993) J. Anim. Ecol. 62 656–668. [Google Scholar]
- 32.Bengtsson, J., Engelhardt, K., Giller, P., Hobbie, S., Lawrence, D., Levine, J., Vilà, M. & Wolters, V. (2002) in Biodiversity and Ecosystem Functioning: Synthesis and Perspectives, eds. Loreau, M., Naeem, S. & Inchausti, P. (Oxford Univ. Press, Oxford), pp. 209–220.
- 33.Bond, E. M. & Chase, J. M. (2002) Ecol. Lett. 5 467–470. [Google Scholar]
- 34.Gonzalez, A. & Chaneton, E. (2002) J. Anim. Ecol. 71 594–602. [Google Scholar]