Abstract
We present new hypotheses and report experimental evidence for powerful selective forces impelling the evolution of both eusociality and the soldier caste in termites. Termite ancestors likely had a nesting and developmental life history similar to that of the living family Termopsidae, in which foraging does not occur outside the host wood, and nonsoldier helpers retain lifelong options for differentiation into reproductives. A local neighborhood of families that live exclusively within a limited resource results in interactions between conspecific colonies, high mortality of founding reproductives, and opportunities for accelerated inheritance of the nest and population by offspring that differentiate into nondispersing neotenic reproductives. In addition, fertile reproductive soldiers, a type of neotenic previously considered rare and docile, frequently develop in this intraspecific competitive context. They can be highly aggressive in subsequent interactions, supporting the hypothesis that intercolonial battles influenced the evolution of modern sterile termite soldier weaponry and behaviors.
The origin and maintenance of eusociality, cooperative societies composed mainly of subfertile or sterile members, are evolutionary paradoxes, because they seem to conflict with Darwin's concept of reproductive self interest (1–5). Progress in understanding the evolution of eusociality was incisively advanced by Hamilton's (2) theory of kin selection as applied to Hymenoptera (ant, bee, and wasp) societies. Female Hymenoptera are diploid and males haploid, a circumstance that affects relatedness, control of sex ratio, and aspects of genetic structure that, in combination with various ecological features, may promote eusocial evolution (3, 6–9). Highly social thrips are similarly haplodiploid (10), but eusociality also occurs in all termites and some other fully diploid animal species, including aphids (11), beetles (12), shrimp (13, 14), and naked mole rats (15). Kin selection is less potent in these groups that lack relatedness asymmetries between sexes and generations, indicating that other factors must also be important in explaining eusociality (3, 4, 14, 16, 17). Previous nonmutually exclusive theories regarding the evolution of eusociality in termites (4, 17–22) have been constructive but indecisive; the current consensus is that termite eusocial evolution was driven by a suite of selective forces (4, 17, 21, 23).
Many hypotheses regarding how and why eusociality evolved in phylogenetically diverse animals, including termites, profitably focus on shared aspects of biology and ecology, identifying commonalities in selective regimes that apparently favored social evolution (3, 4, 14, 17, 24). The chance for nest inheritance, either by unrelated helpers or through philopatric reproduction (succession to a breeding position within the natal group), is considered a fundamental element of many theories on the evolution of both eusociality (3, 4, 14, 17, 25, 26) and cooperative breeding in insects, fish, birds, and mammals (27–29). Incentives for helping without inheritance occur through kin selection if offspring can sufficiently enhance reproduction by their parents or relatives; however, opportunities for inheritance can further promote helping behaviors and life history modifications. Moreover, the survival and fitness payoffs of inheriting an established nest and resource area may well exceed those realized by dispersing offspring in solitary species, thus favoring helpers that remain as “hopeful reproductives” (30) in social units, even if that means delaying or forgoing reproduction. In philopatry, a system of serial reproductive inheritance by kin, all individuals in the group also gain inclusive fitness benefits. The inheritance hypothesis, productively applied to the evolution of altruistic behavior in other social groups, was logically extended to termites, supported by the fact that philopatric reproduction is common through helper differentiation into secondary (replacement or supplementary) reproductives on death or senescence of established reproductives (4, 17, 26). An apparent problem with this application to termites, however, is that founding kings and queens are long-lived in captivity and in some field colonies (31), even in relatively primitive taxa (32), suggesting that early orphaning of helpers (or originally, nonhelpers) and thus opportunities for inheritance might be rare in the young families that must have characterized the evolutionary transition to termite eusociality (23). Founder life spans may have been shorter in prototermites than in modern groups (4), but timing of inheritance opportunities would be important if parents survived past the time their first offspring could reach sexual maturity and if remaining in the natal nest resulted in progeny delaying or forgoing direct reproduction. Demise of founding reproductives might also occur through predation or parasitism, but it is difficult to imagine preferential attack of the king and/or queen while leaving healthy helpers capable of assuming reproductive roles in a robust colony. Thus the question remained, why would early brood offspring remain as helpers in prototermite families if their probability of inheriting the nest resource and assuming reproductive status depended on the apparently unlikely circumstance of death of a parent but not the whole colony?
All of the >2,600 living species of termites are eusocial, and solitary ancestors are sufficiently distant to obscure prototermite selective regimes. No “stepping-stone” intermediate taxa exist for comparative study of transitional states from solitary to social to eusocial species; we must instead draw evolutionary inferences based on theoretical constructs and/or the biology and socioecology of the most primitive living lineages. Modern species belonging to even the most basal groups represent blended assemblages of primitive and derived traits. Recent phylogenetic analyses differ in topology but include the same three families as the most basal living termite clades: Mastotermitidae, Hodotermitidae, and Termopsidae (33). Although living Mastotermitidae and Hodotermitidae retain pleisiomorphic morphological characters, their social organization appears to be derived. Among extant taxa, Termopsidae are widely viewed as the closest available approximation to ancestral termites in socioecological features such as colony size, social organization, nesting biology, and caste polyphenism (4, 17, 22, 34–37). Termopsid colonies live and feed exclusively within a single log, facing eventual resource limitation (38, 39). Fertile alates, produced seasonally, are the only colony members to leave their natal wood. They fly to find mates and nest under the bark of a recently dead tree to found new colonies. Tens or hundreds of typically monogamous “royal pairs” (king and queen) may settle synchronously in the same piece of wood, frequently using beetle holes to colonize a tree trunk at an appropriate stage of decay. As they consume the wood, initially preferring the soft, narrow, nutrient-rich decaying phloem layer under bark, they create nest chambers, often in close proximity to neighboring colonies (Fig. 1) (32, 40).
Fig. 1.
Natural chambers of young Zootermopsis colonies revealed under the bark of a Ponderosa pine tree; note close proximity of neighboring families and therefore high probability of interactions in the field.
Development is exceptionally flexible in Termopsidae, with all individuals except soldiers and reproductives capable of molting into any other caste (4). Two types of nondispersing secondary reproductives can develop to replace or supplement the founding king and queen. Neotenic reproductives typically differentiate in response to the death or senescence of reproductives; multiple males and females may persist in the same colony (41). Fertile reproductive soldiers (soldier neotenics), individuals of either sex with soldier-like heads and neotenic gonad development, have been recorded in six species of termopsids (4, 42, 43). Soldiers of all other families of modern termites, as well as normal soldiers within the Termopsidae, are sterile,† and the significance of reproductive soldiers has heretofore been obscure.
Using the termopsid species Zootermopsis nevadensis (Hagen), we test the Accelerated Inheritance hypothesis that intraspecific interactions, occurring as families grow and meet within a limited resource, can result in high mortality of established reproductives and opportunities for helpers to differentiate into reproductives. We further demonstrate that these interactions frequently prompt production of reproductive soldiers, a caste unique to primitive termites, and previously considered rare, incidental, and docile. We show that reproductive soldiers can be highly aggressive in intercolony interactions, supporting the postulate that they represent an evolutionary precursor to the sterile soldiers characteristic of modern termite societies.
Methods
Intercolony Interactions. To simulate the circumstance of intraspecific competition in which growing colonies meet one another within their natural shared food and nesting resource, we arranged interactions between 14- to 16-month-old complete Z. nevadensis colonies of similar sizes. The protocol for interactions between colonies was to provide a Tygon tube connection between Petri plates containing each colony and their nest material (detailed methods described as even-age interactions in ref. 32). We report on data from 57 such interactions, each involving two similarly sized colonies containing their founding king and queen and an average of 46.2 (±2.69 SE; median 43) “workers”‡ and soldiers (range of one to five soldiers). Seventeen unmanipulated colonies were monitored as controls. Time intervals are reported from the time of the interaction. Statistical comparisons were made by using χ2 analyses. Each of the six replicates of interactions between already interacted, merged “colonies” and simple families originated from colonies 16 months old at the time of the first interaction. Results from 14 interactions between colonies ≈1 yr apart in age are also discussed (the protocol is described as uneven interactions in ref. 32). Colonies used in all experiments were bred from alates that emerged from colonies collected near Placerville, CA (El Dorado County) and were thus complete families and social units (rearing methods described in ref. 32).
Intracolony Reproductive Soldier Behavior. We observed laboratory colonies (complete families) ranging in age from 1 to 2 years and containing 100–300 individuals in 15-cm-diameter Petri dishes covered with red Plexiglas. One of the colonies had a queen, and the other two had three and four female neotenics; all had a male reproductive soldier (mRS) and two to five normal soldiers. Position data were recorded at 30-min intervals for 10 h on 4 days.
Interactions Between Colonies Containing Reproductive Soldiers. Data are reported on 11 paired interactions involving 22 complete families that had not been involved in a previous interaction. The experimental set-up was identical to that described above for intraspecific interactions. One treatment involved six replicates of interactions between similarly sized colonies headed by a king and queen and colonies containing a mRS and a queen. The second treatment, five replicates, involved meetings between two similarly sized colonies that each contained a mRS and reproductive females (female neotenics were in 9 of 10 colonies; 3 also contained a queen). Behavioral observations were made for 2 h after the beginning of an interaction. We defined aggression as a continuum of behaviors ranging from mandible flaring (mild agonism) to biting.
Results and Discussion
Intraspecific Interactions: Opportunities for Accelerated Inheritance. In the interactions between complete 14- to 16-month-old colonies, staged as an experimental test of this Accelerated Inheritance hypothesis, termites typically explore the connection between the two families immediately. Agonistic behavior ensues, directed primarily at reproductives, although some other individuals on one or both sides may be injured or killed. Workers, soldiers, kings, and queens all act as aggressors toward reproductives during intercolony interactions. In 72 interindividual attacks witnessed during the first 2 h of 18 interactions, reproductives were aggressors in 22 (30.5%) of the strikes, and in all but one of those cases (95.4%), the target was another king or queen. Workers attacked reproductives in 35/72 = 48.6% of observed cases, and soldiers were the aggressors in the remaining 15/72 = 20.8% of attacks.
Twenty-four hours after the interaction, at least one of the four original kings or queens was killed in 94.7% of the 57 replicates, and at least one king and queen survived in 87.7% of the conjoined colonies. Of the 26 interactions in which we know the colony of origin of the reproductives due to identifying paint marks, 92% of the king and queen pairs that survived were from the same original colony. At 24 h after an interaction, all castes from both original colonies appear to intermingle freely in the newly merged “colony.” At 6 months after the interactions, at least one king and queen persisted in 61.1% of interactions compared with 94.1% of 17 unmanipulated controls; a king and queen survived in 46.3% of interaction colonies at 12 months (88.2% of controls).
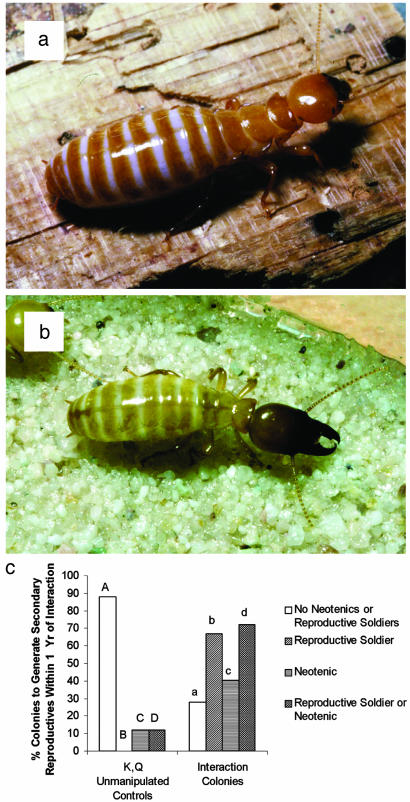
A remarkable result of intercolony interactions is that reproductive soldiers appeared in 58% of merged colonies within the first 3 months and in 66.7% of them within the first year (never within control colonies within 12 months). Normal neotenic reproductives differentiated in 40.4% of interaction colonies within the first year but in only two (11.8%) control colonies, both of which had lost one or both founding reproductives. One or both types of secondary reproductives, normal neotenics or reproductive soldiers, occurred in 41/57 = 71.9% of interaction colonies within the first 12 months, and in 27/41 = 65.8% of those cases, one or more of these secondaries appeared when at least one king and queen remained alive in the combined colony. Secondary reproductives occurred only rarely in isolated control colonies within the same time period (2/17 = 11.8%) and never when both original reproductives were healthy (Fig. 2). The higher mortality of founding reproductives in groups involved in intraspecific interactions vs. controls (P < 0.0001) and development of secondary reproductives in a statistically greater proportion of such colonies in comparison to controls (P < 0.0001; Fig. 2c, D and d) are critical contrasts in support of the Accelerated Inheritance hypothesis. Molecular genetic analyses are in progress to determine the colony of origin of reproductives that differentiate after interactions and the long-term genetic structure of the growing populations. Experiments regarding colony recognition cues and how they are adjusted to facilitate “merging” within interaction colonies are also underway.
Fig. 2.
Occurrence of secondary reproductives in control vs. interaction colonies. Normal neotenic (a), reproductive soldier (soldier neotenic) (b), and secondary reproductives (neotenics and/or reproductive soldiers) (c) differentiate significantly more frequently after intercolony interactions than in control colonies (and only in controls in which founding reproductives died). Compare columns with the same letters; if case differs, they are significantly different. χ2 levels of significance: Aa, P < 0.0001; Bb, P < 0.0001; Cc, P < 0.05; Dd, P < 0.0001.
Survivorship of founding reproductives is reduced after intercolony interactions, and even if they live, the inhibition that normally restrains development of secondary reproductives in the presence of a functional king and queen (41) is muted, allowing helpers a chance to molt into fertile replacement or supplementary reproductives. This is not simply a phenomenon of weakened inhibitory control by kings and queens due to larger colony size. In long-term rearing of control colonies, families that retained a viable king and queen never developed a secondary reproductive (n = 12, up to 7 years of age, colony population sizes exceeding 1,000 individuals). In control colonies that lost the king or queen, a neotenic or reproductive soldier of the same sex as the surviving primary developed only once in 7 years; a male neotenic was recorded in a census immediately before the death of the king.
The merged associations that result from interactions appear to function as stable groups and are much larger units than same-aged nuclear family neighbors. Six months after the original interaction, we arranged meetings (n = 6) between those merged colonies and simple family colonies of the same age. The previously interacted colonies were now at least twice as large as unmanipulated families. In all replicates, the second series of interactions resulted in death of all reproductives in the smaller simple family colonies within 24 h, whereas only one of 15 reproductives (a female neotenic) died in the merged colonies. This demonstrates a competitive advantage due to size conferred on groups resulting from interactions [note parallels to brood raiding and associated behaviors in ants, e.g., Adams and Tschinkel (45), although those examples involve no philopatry or colony inheritance].
Disparity in colony size has a similar effect on the outcome of interactions between nuclear families of different ages, with consequences for colonists of the same resource in different years or same-aged colonies with slower growth rates. Of 14 interactions involving meetings between 14- and 19-month-old colonies and 3- to 4-month-old incipient colonies (each 40–62% the size of the older colony), all primary reproductives in the younger colonies were killed, but only a single king died among founders of the larger colonies. All of the younger colonies were decimated in entirety by the larger families, i.e., no “merging” occurred, as was typical in the more evenly sized interactions described above. Pairs and extremely young colonies often use protective tactics to avoid contact with other colonies. In 8 of the 14 replicates, the smaller 3-month-old colonies invested in building “fecal fortresses” as reinforcement barriers to intrusion by the larger colony, forcing a delay but not altering the outcome of interaction between neighbors. Thus successful early colonists in a resource gain an advantage over founders that colonize in subsequent years, nest usurpation by younger pairs or their incipient colonies is unlikely, and chance of survival is precarious if the resource is already relatively densely settled (although predators or pathogens may eliminate entire colonies, opening uncontested resource space for later arrivals).
The ecological circumstance of intraspecific termite families growing and expanding within a limited resource ensures meetings among young colonies. Among similarly sized colonies, such as a cohort of neighboring families initiated the same season, intercolony interactions often precipitate death of founding reproductives, opportunities for nest inheritance by offspring helpers, and resulting large merged colonies that have a competitive advantage in future interactions. This dynamic therefore accounts for a missing link in the logic of theories of the evolution of eusociality based on philopatric reproduction: how even the earliest brood in a family might have had opportunities for reproductive inheritance and therefore further incentive to remain as helpers, even in an insect in which parents have the potential to be long lived. Once alleles for offspring to help in the parental nest and delay or forgo dispersal spread, the selective landscape was in place for potential evolution of caste polyphenism and ultimately the evolution of sterile castes (3, 4, 30).
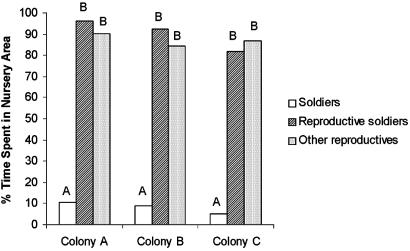
Evolution of the Soldier Caste. Intraspecific interactions between dampwood termite colonies have also facilitated insights regarding evolution of the soldier caste in termites. It is unknown whether modern sterile termite soldiers evolved as a defensive caste or from neotenic reproductives similar to modern reproductive soldiers. Myles (26, 42) postulated that reproductive soldiers are ancestral to modern sterile soldiers, having “weapons” selected in response to intracolonial competition among neotenics with the soldiers' role in colony defense as a secondary adaptation. Roisin (43) questioned this hypothesis, noting that there was no evidence of reproductive soldier aggression against any individual. Our data demonstrate a context in which reproductive soldiers frequently develop (i.e., after intraspecific interactions) and support that within their own colonies, reproductive soldiers are docile. We have never seen a reproductive soldier behave aggressively toward a nestmate and, like other reproductives, they remain with eggs and brood while normal soldiers patrol away from the nursery (Fig. 3).
Fig. 3.
Time spent in nursery area of the nest by normal soldiers and reproductives. Normal soldiers rarely associate with eggs and dependent brood in the nursery; in this regard, reproductive soldiers behave like other reproductives. Compare columns with the same letters; if case differs, they are significantly different (χ2 analysis; P < 0.0001).
Although reproductive soldiers are passive within their own colonies, we documented multiple cases of reproductive soldier aggression during intraspecific interactions. We set up two interaction treatments, the first (n = 6) between families headed by a king and queen and colonies containing a mRS and a queen. The second treatment (n = 5) involved meetings between two colonies that each contained a mRS and one or more reproductive females (female neotenics were in nine of 10 colonies; three also contained a queen). None of the colonies used in this series of experiments had been involved in previous interactions.
As in the experimental interactions described above, we observed marked mortality of established reproductives as a result of interactions involving mRSs: 45.8% of the 12 reproductives in treatment I and 34.8% of the 46 reproductives in treatment II were killed within the first 24 h after interaction. In 10 of 11 cases (91%, combined treatments), some or all reproductives from one colony were killed during the interaction, whereas all reproductives from the opposing colony survived in the merged colony. In all 11 cases (both treatments), only one of the two male reproductives originally present in the interaction remained in the merged colony after 24 h.
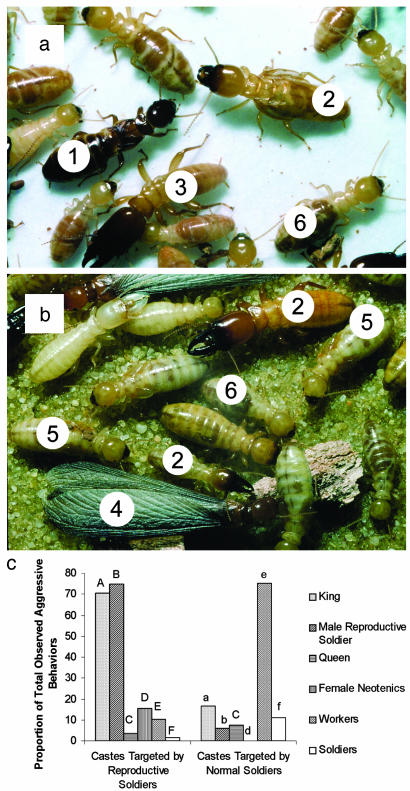
We observed aggression toward noncolony members by 5 of 6 (83.3%) mRSs in the queen–mRS/queen–king treatment and 7 of 10 (70%) mRSs in the female–mRS/female–mRS treatment. Notably, the four reproductive soldiers not observed as aggressive were never seen in the same colony chamber as the other male reproductive, hence their reaction in that context could not be assessed. No aggressive acts by mRSs were aimed toward colony mates in any trial. All five aggressive mRSs from the first treatment were observed directing aggressive behaviors toward the opposing king and occasionally members of other castes. Strong aggressive behaviors (biting and/or killing another termite) were correlated with mRS survivorship in the queen–mRS/queen–king treatment. All four reproductive soldiers observed displaying strong aggression did so toward the opposing king and survived to 24 h after the interaction, whereas the remaining two relatively passive mRSs died within 24 h of the interaction. mRSs directed most of their biting and lunging behaviors toward male reproductives in the other colony, a significant difference from targets of aggression by normal soldiers (Fig. 4).
Fig. 4.
Differential targeting of castes by reproductive soldiers vs. normal soldiers. (a and b) Castes and polyphenism within colonies. Number indicates caste: 1, queen; 2, reproductive soldier; 3, normal soldier; 4, alate; 5, nymph; and 6, worker. (c) mRSs selectively attack other male reproductives during intercolony interactions (treatments I and II combined, thus categories are context-dependent and proportions do not add to 100). χ2 levels of significance: Aa, P < 0.001; Bb, P < 0.0001; Cc, not significant; Dd, no attacks by normal soldiers on female neotenics; Ee, P < 0.0001; Ff, P < 0.05. Compare columns with the same letters; if case differs, they are significantly different (P ≤ 0.05).
Therefore, instead of soldier morphology and agonistic behavior evolving as an adaptation to intracolonial aggression among neotenics (26, 42), our study supports the new hypothesis that termite soldier weaponry and aggressive behaviors evolved in the context of intercolonial fighting among reproductives with modern sterile soldiers and their roles in colony defense having evolved secondarily. Fertile “soldiers” in the basal family Termopsidae, found commonly on our Zootermopsis collecting trips, are retained in extant species due to nesting biology (coincidence of food and nest/habitat) and intraspecific competition. A similar sequence of reproductive forms with soldier-like morphologies and behaviors evolving prior to sterile soldiers apparently occurred in social aphids (11) and thrips (46); ant soldiers also appear derived from reproductives (47).
Thus the same ecological context, intraspecific interactions between colonies nesting within a limited resource, may have influenced the evolution of both eusociality and the soldier caste in termites. The hypothesis of Accelerated Inheritance fortifies the theory that the evolution of termite eusociality was promoted by a suite of ecological conditions providing advantages to family living and long-term helping behavior by offspring that retain remarkable developmental plasticity. Those offspring gain inclusive fitness advantages by helping to produce fertile siblings. They also benefit from opportunities to become replacement reproductives as their young families grow, meet, and interact with neighboring colonies, often resulting in early death of established reproductives. Helping behavior by offspring that forgo or delay risky dispersal options, even if in a young family, results in their being profitably situated to become a reproductive upon the death of founding reproductives. After interactions, they would belong to a larger association, conferring a competitive advantage in future intraspecific meetings.
Acknowledgments
We thank J. Anchan, J. Carlson, A. Costa-Leonardo, M. Fox, M. Haverty, C. Long, P. Miller, M. Suarez, and M. TerAvest for assistance in this research; and C. Mitter, A. Greene, C. Long, J. Traniello, and three anonymous reviewers for constructive comments on the manuscript. This work was supported by the National Geographic Society, the U.S. Forest Service, and a grant from the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: mRS, male reproductive soldier.
Footnotes
In the most primitive living Termopsid, Archotermopsis wroughtoni Desneux, gonads of all soldiers are as well developed as in alates (44), but their fertility is not established.
In formal termite terminology, Termopsidae do not have true workers because those individuals retain substantial developmental plasticity (35); however, in the functional sense that they help and work within the colony, we refer to them as helpers or workers in this paper.
References
- 1.Darwin, C. (1859) The Origin of Species (John Murray, London).
- 2.Hamilton, W. D. (1964) J. Theor. Biol. 7 1–52. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, R. D., Noonan, K. M. & Crespi B. J. (1991) in The Biology of the Naked Mole Rat, eds. Sherman, P. W., Jarvis, J. U. M. & Alexander, R. D. (Princeton Univ. Press, Princeton), pp. 1–44.
- 4.Thorne, B. L. (1997) Annu. Rev. Ecol. Syst. 28 27–54. [Google Scholar]
- 5.Duffy, J. E., Morrison, C. L. & Rijos, R. (2000) Evolution (Lawrence, Kans.) 54 503–516. [DOI] [PubMed] [Google Scholar]
- 6.Gadagkar, R. (1991) J. Genet. 70 1–31. [Google Scholar]
- 7.Reeve, H. K. (1993) Philos. Trans. R. Soc. London B 342 335–352. [Google Scholar]
- 8.Bourke, A. F. G. & Franks, N. R. (1995) Social Evolution in Ants (Princeton Univ. Press, Princeton).
- 9.Hunt, J. (1999) Evolution (Lawrence, Kans.) 53 225–237. [Google Scholar]
- 10.Crespi, B. J. (1992) Nature 359 724–726. [Google Scholar]
- 11.Stern, D. L., Foster, W. A. (1997) in The Evolution of Social Behavior in Insects and Arachnids, eds. Choe, J. C. & Crespi, B. J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 150–165.
- 12.Kent, D. S. & Simpson, J. A. (1992) Naturwissenschaften 79 86–87. [Google Scholar]
- 13.Duffy, J. E. (1996) Nature 381 512–514. [Google Scholar]
- 14.Duffy, J. E. (2002) in Genes, Behavior, and Evolution in Social Insects, ed. Kikuchi, T. (Univ. of Hokkaido Press, Sapporo, Japan), pp. 1–38.
- 15.Sherman, P. W., Jarvis, J. U. M. & Alexander, R. D. (1991) The Biology of the Naked Mole Rat (Princeton Univ. Press, Princeton).
- 16.Choe, J. C. & Crespi, B. J. (1997) The Evolution of Social Behaviour in Insects and Arachnids (Cambridge Univ. Press, Cambridge, U.K.).
- 17.Shellman-Reeve, J. S. (1997) in The Evolution of Social Behaviour in Insects and Arachnids, eds. Choe, J. C. & Crespi, B. J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 52–93.
- 18.Bartz, S. H. (1979) Proc. Natl. Acad. Sci. USA 76 5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalepa, C. A. (1994) in Nourishment and Evolution in Insect Societies, eds. Hunt, J. H. & Nalepa C. A. (Westview, Boulder, CO), pp. 57–104.
- 20.Roisin, Y. (1994) Am. Nat. 143 751–765. [Google Scholar]
- 21.Rosengaus, R. B., Maxmen, A. B., Coates L. E. & Traniello, J. F. A. (1998) Behav. Ecol. Sociobiol. 44 125–134. [Google Scholar]
- 22.Higashi, M., Kamamura, N. & Aloe, T. (2000) in Termites: Evolution, Sociality, Symbioses, Ecology, eds. Abe, T., Bignell, D. E. & Higashi, M. (Kluwer, Dordrecht, The Netherlands), pp. 169–187.
- 23.Roisin, Y. (1999) Insect Soc. 46 297–305. [Google Scholar]
- 24.Crespi, B. J. (1994) Insect Soc. 41 395–400. [Google Scholar]
- 25.Andersson, M. (1984) Annu. Rev. Ecol. Syst. 15 165–189. [Google Scholar]
- 26.Myles, T. G. (1988) in The Ecology of Social Behavior, ed. Slobodchikoff, C. N. (Academic, New York), pp. 379–423.
- 27.Kokko, H. & Johnstone, R. A. (1999) Proc. R. Soc. London B 266 571–578. [Google Scholar]
- 28.Pen, I. & Weissing, F. J. (2000) Proc. R. Soc. London B 267 2411–2418. [Google Scholar]
- 29.Queller, D. C., Zacchi, F., Cervo, R., Turillazzi, S., Henshaw, M. T., Santorelli, L. A. & Strassmann, J. E. (2000) Nature 405 784–787. [DOI] [PubMed] [Google Scholar]
- 30.West-Eberhard, M. J. (1978) Proc. Am. Philos. Soc. 123 222–234. [Google Scholar]
- 31.Keller, L. (1998) Insect Soc. 45 235–246. [Google Scholar]
- 32.Thorne, B. L., Breisch, N. L. & Haverty, M. I. (2002) J. Anim. Ecol. 71 1030–1041. [Google Scholar]
- 33.Eggleton, P. (2001) Insect Soc. 48 187–193. [Google Scholar]
- 34.Emerson, A. E. & Krishna K. (1975) Am. Mus. Nov. 2570 1–31. [Google Scholar]
- 35.Noirot, C. & Pasteels, J. M. (1987) Experientia 43 851–860. [Google Scholar]
- 36.Noirot, C. (1989) Ethol. Ecol. Evol. 1 1–17. [Google Scholar]
- 37.Thorne, B. L. & Traniello, J. F. A. (2003) Annu. Rev. Entomol. 48 283–306. [DOI] [PubMed] [Google Scholar]
- 38.Abe, T. (1987) in Evolution and Coadaptation in Biotic Communities, eds. Dawano, S., Connell, J. H. & Hidaka, T. (Univ. of Tokyo Press, Tokyo), pp. 125–148.
- 39.Lenz, M. (1994) in Nourishment and Evolution in Insect Societies, eds. Hunt, J. H. & Nalepa, C. A. (Westview Press, Westview, CO), pp. 159–209.
- 40.Shellman-Reeve, J. S. (1994) J. Anim. Ecol. 63 921–932. [Google Scholar]
- 41.Light, S. F. (1943) Q. Rev. Biol. 18 46–63. [Google Scholar]
- 42.Myles, T. G. (1986) Pan-Pac. Entomol. 62 293–299. [Google Scholar]
- 43.Roisin, Y. (2000) in Termites: Evolution, Sociality, Symbioses, Ecology, eds. Abe, T., Bignell, D. E. & Higashi, M. (Kluwer, Dordrecht, The Netherlands), pp. 95–119.
- 44.Imms, A. D. (1919) Philos. Trans. R. Soc. London B 209 75–180. [Google Scholar]
- 45.Adams, E. S. & Tschinkel, W. R. (1995) J. Anim. Ecol. 64 315–324. [Google Scholar]
- 46.Chapman, T. W., Kranz, B. D., Bejah, K.-L., Morris, D. C., Schwarz, M. P. & Crespi, B. J. (2002) Behav. Ecol. 13 519–525. [Google Scholar]
- 47.Baroni Urbani, C. & Passera, L. (1996) Nature 383 223. [Google Scholar]