Abstract
Albatrosses (Diomedeidae) do not occur in the North Atlantic Ocean today except as vagrants, although five species were present in the early Pliocene. No fossil breeding sites of albatrosses were known previously. The timing of extinction of albatrosses in the North Atlantic was likewise unknown. Deposits that formed near present-day sea level along the southeastern shore of Bermuda contain remains of a former breeding colony and include intact eggshells and bones of embryos, juveniles, and adults of Short-tailed Albatross (Phoebastria albatrus), a critically endangered species now confined to a few islets in the northwestern Pacific Ocean. These deposits are correlated with the middle Pleistocene Lower Town Hill Formation, which at other sites have a radiometric age of ≈405,000 years ago. This equates with the marine isotope stage 11 interglacial, which culminated in a rise in sea-level to >+20 m. Bones of a juvenile Short-tailed Albatross were also found in beach deposits at +21.3 m from this same interglacial. We interpret the extirpation of albatrosses on Bermuda as probably resulting from lack of nesting sites protected from storm surges over the little emergent land that remained at the height of the marine isotope stage 11 sea level rise.
Albatrosses (Diomedeidae), the largest of volant seabirds, do not occur in the North Atlantic today except as accidental wanderers from the southern oceans. Scarce fossils of small and probably primitive albatrosses are known from the late Oligocene to middle Miocene of South Carolina, Maryland, and France (1, 2). By the early Pliocene, there was much greater diversity, with five species being recorded from offshore marine deposits in North Carolina exposed in phosphate mining operations (3). Three of these have been identified with modern species-lineages (Short-tailed, Laysan, and Black-footed Albatrosses: Phoebastria albatrus, Phoebastria immutabilis, and Phoebastria nigripes, respectively) that occur today only in the North Pacific. The other two are extinct species as yet known only from the North Atlantic (3).
Thus far there has been no evidence of former breeding colonies of any of these albatrosses in the North Atlantic, or for the timing of extinction of the family as a whole in that ocean. Albatrosses require oceanic islands free of mammalian predators to establish breeding colonies. They also require breeding sites that include relatively flat areas well exposed to wind to take off and land effectively. Because of these specialized requirements, evidence of former breeding colonies of albatrosses would be preserved in the geological record only under exceptional circumstance. Middle Pleistocene rocks in Bermuda reveal not only the former presence of a breeding population of albatrosses in the North Atlantic, but also provide evidence of the probable cause of its demise.
Geological Setting, Age, and Paleontology of Bermuda Albatross Colonies
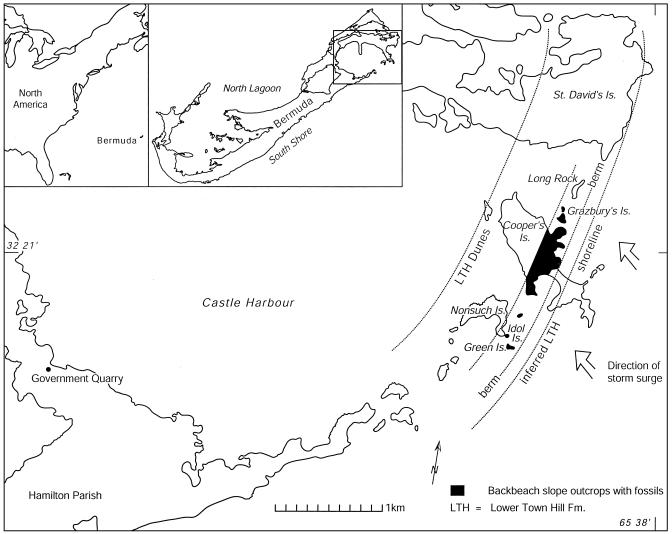
Sea-Level Deposits. From the extent and homogeneity of the shoreline deposits along southern Bermuda, a general picture emerges of a broad, largely vegetated coastal strandplain during the middle Pleistocene. Fossilized eggs and bones of albatrosses have been recovered or observed in a narrow band of intertidal and supratidal limestone along the southeastern margin of the Bermuda platform. This outcrop composes several islets south and east of Nonsuch Island, a large portion of the middle of Cooper's Island, and Grazbury's Island (Fig. 1). The fossils are contained in densely lithified coarse skeletal carbonate sediments with abundant shell fragments.
Fig. 1.
Maps showing location of Bermuda (Left Inset), present subaerial portions of Bermuda showing portion magnified (Right Inset), and detail map of southeastern Bermuda with the fossiliferous outcrops of the middle Pleistocene Lower Town Hill Formation with remains of an albatross colony.
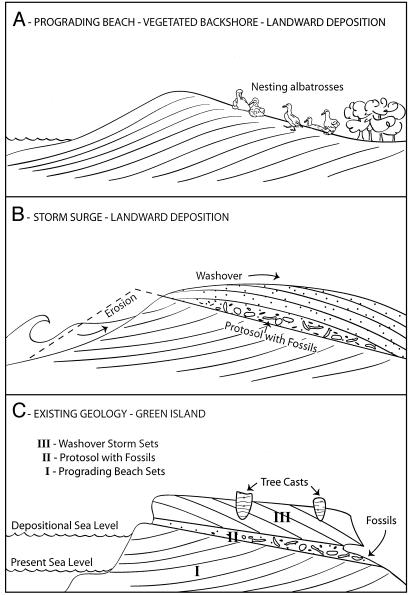
The lowermost beds (Unit I, Fig. 2C) at the base of the fossil unit probably originated as a prograding beach ridge during a sea level slightly higher than present (≈+1 m). As the coastline advanced seaward, the uppermost zone ≈0.5 m thick of the back beach was quickly vegetated and was colonized by a variety of terrestrial organisms as evidenced by the abundance of rhizomorphs and burrows. The vegetation trapped fine sand blown onshore from the beach, ultimately forming Unit II, a characteristic protosol (4) composed of fine sand and silt with organic material and faint coloration from brown to tan. Biological activity, including bioturbation by crabs and deeply rooted vegetation, destroyed most of the primary bedding at the top of Unit II.
Fig. 2.
Paleoenvironmental interpretation of the Short-tailed Albatross fossil site on Green Island. The geology shows three phases of deposition. (Unit I) An initial episode of shoreline progradation during a stillstand of sea level around +1 m. (Unit II) With seaward progradation, vegetation and terrigenous organisms became established and formed a weak soil called a protosol (4). It was among this nearshore vegetated environment that the albatross colony was established. (Unit III) An intense storm event from the SE that deposited several meters of beach sand in the supratidal environment of the albatross colony. Only a single wave event must have occurred because eggshells and articulated embryos are preserved.
It was within this landward-sloping, vegetated, but windblown and exposed supratidal environment of Unit II that the albatrosses nested. Their bones and eggs were entombed when a major storm apparently struck the island from the south, most likely generated by late summer hurricanes, as the site is well protected from northerly winter storms. Marine sand 2–4 m deep was washed landward over the beach ridge onto the nesting area, scattering and burying the eggs and either living birds or desiccated carcasses. We infer burial to be the result of a single rapid event because eggs were preserved intact, as well as articulated skeletons of embryos or recently hatched chicks and older juveniles whose soft, porous bones would have been quickly abraded and pulverized under any other conditions.
The uppermost Unit III (Fig. 2C) is composed of large, sweeping, landward-dipping, low-angle planar beds that contain abundant fenestral porosity (beach bubbles), indicative of a storm beach and washover environment. Casts of tree trunks, possibly the native palmetto Sabal bermudana, situated at the crest of the beach ridge at >+6 m elevation, suggest that the washover beds were at least briefly vegetated after their deposition.
The major concentration of fossils in Unit II on the eastern side of Green Island lies near and slightly above present sea level. Occasional bones were observed on Idol Island and on the western side of Cooper's Island. Molds of eggs, some still with eggshell preserved, have been found on the eastern and western sides of Cooper's Island but not on Green Island, further confirming the probable direction of wave action from southeast to northwest, with the more buoyant eggs, which may have been addled, being carried unbroken for the greatest distance.
The fossil bones on Green Island occurred in the low supratidal zone at the extremely indurated, sculpted limestone surfaces, where they were partially exposed by a variety of physical, chemical, and biological weathering processes. Bones are usually aligned with the horizontal axes parallel with the bedding plane and frequently occur in articulation. The limestone's high degree of induration is probably due to numerous stages of cementation from repeated inundation after deposition of the unit. Fossils were removed with a gas-powered abrasive wheel, and were later etched out of the matrix in a bath of dilute acetic acid. Only a fraction of the bones encountered were collected.
This may not have been a catastrophic assemblage, however, because the fossils may have been deposited as desiccated carcasses and addled eggs left after adults and fledgling young had departed at the end of a breeding cycle. Addled eggs may persist in an albatross colony for months after laying has ceased (5). Dried, hardened carcasses of young and adult seabirds can accumulate over more than one breeding season (6). Thus, little can be inferred about either the seasonality of breeding of albatrosses on Bermuda or of the storm that covered their remains, apart from the likelihood that intense storm waves originated from a southerly direction.
A Higher Elevation Site. Further remains of albatross on Bermuda were collected by Olson in 1981 from a beach deposit surveyed by the Ministry of Public Works of Bermuda at +21.3 m. Among the seabird remains were a few bones of probably a single prefledging juvenile albatross (Fig. 3). These deposits occupied a 0.5-m-deep horizontal notch (Calonectris Quarry, named for the abundance of bones of Mediterranean Shearwater Calonectris diomedea) on the west side of Government Quarry, situated on the opposite side of a narrow ridge within a few meters of previously described terrace deposits and sea caves (7, 8). In addition to the fossils of albatross and other marine birds, Calonectris Quarry contained beach sand and conglomerate of identical composition and texture to that in Dead End Caves, and beach-worn but colored shells of marine mollusks. Terrestrial vertebrates and pulmonate snails were also present.
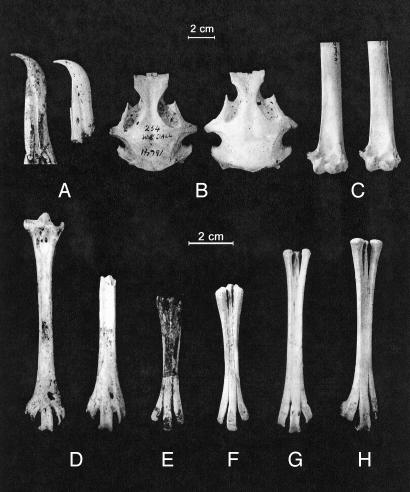
Fig. 3.
(A–D) Fossils of Short-tailed Albatross (Phoebastria albatrus) from the middle Pleistocene of Bermuda (on the right in each pair) compared with modern reference specimens (on the left in each pair). (A) Premaxillae, lateral view. (B) Crania, dorsal view. (C) Distal ends of humeri, palmar view. (D) Adult tarsometatarsi, anterior view. (E–H) Fossils of juvenile tarsometatarsi of Short-tailed Albatross from Bermuda from Green Island, near modern sea level (E, F, and H), and from beach deposit in Government Quarry, Bermuda, surveyed at 21.3 m above present sea level (G).
Age of the Fossil-Bearing Deposits. All of the outcrops on the southeast shore of Bermuda in which we found albatross remains were mapped as part of the early Pleistocene Walsingham Formation (9), possibly by extrapolation rather than on-site inspection by boat. Our first-hand observations of lithology and stratigraphy on each of several islands on the eastern margin of Castle Harbor indicate, however, that they are referable to the middle Pleistocene Lower Town Hill Formation. Conspicuous outcrops of the deeply weathered upper surface of the Walsingham Formation and Castle Harbor paleosol may be observed along the south and east headlands of St. David's Island beneath the albatross-bearing rocks. The Walsingham is typically vuggy and weakly to cryptically bedded because of complete recrystallization (7, 9), whereas the Lower Town Hill is distinctly bedded and has undergone only moderate recrystallization. Lower Town Hill beds can be traced in outcrop along the series of islands to the southwest that provided the fossils described in this study. On a small island called Long Rock, the deeply karstified upper surface of the Walsingham outcrops one or two meters above present sea level. From Long Rock, the overlying deposits of the Lower Town Hill can be seen on the Walsingham contact within 150 m on an unnamed islet to the south, and may be traced continuously from there to Cooper's, Idol, and Green islands. We therefore conclude that the deposits containing the fossils were mismapped as Walsingham, and are instead are referable to the Lower Town Hill Formation (see ref. 9).
The middle Pleistocene Lower Town Hill Formation has been correlated with marine isotope stage (MIS) 11 (420–362 thousand years ago, kyr) (10) on the basis of stratigraphy, amino acid ratios, and constraining thermal ionization mass spectrometric uranium dates (8). Many deep sea oxygen isotope records (11–13) identify MIS 11, in an oceanographic sense, as one of the longest and warmest interglaciations of the past million years (14). This duration of oceanic warmth apparently effected the melting or collapse of major ice sheets in Greenland and Antarctica, which resulted in some of the highest global sea levels of the Quaternary (15–17).
In Bermuda, other Lower Town Hill sites have been confirmed as MIS 11 by aminostratigraphy and thermal ionization mass spectrometric dating, and include a +5- to +7-m shoreline sequence on Front Street East, now mostly obscured by development, large dune deposits in Bierman and Wilkinson Quarries, and beach conglomerate in the remnants of two sea caves (Dead End Caves) at +20 ± 3 m and the Calonectris Quarry deposit mentioned above at +21 m in the western wall of Government Quarry. Land et al. (7) described a terrace with marine sands and conglomerate at this level in Government Quarry, but the site was later destroyed by quarry expansion.
From the high elevation sites, marine (Glycymeris sp.) and terrestrial (Poecilozonites sp.) mollusks yielded alloisoleucine/ isoleucine (A/I) ratios of 0.99 ± 0.02 (n = 2) and 0.96 ± 0.04 (n = 4), respectively. Whole-rock A/I ratios from the marine sands in the caves average 0.67 ± 0.05 (n = 3). The ratios of both shell species and whole-rock A/I values are concordant with Lower Town Hill averages (18), and support placement in MIS 11 with an age of 400 kyr (8, 17). Three samples of flowstone directly overlying the +20-m beach deposits in the Dead End Caves produced thermal ionization mass spectrometric ages of 420 ± 30, 409 ± 15, and 405 ± 28 kyr (ref. 8 and R. L. Edwards, personal communication).
Identity of Macrofossils from Green Island. Virtually all of the macrofossils found in the deposits on Green Island are bones of a single species of albatross. Most of the major skeletal elements are represented, in varying states of incompleteness, from numerous individuals (at least 15–25) that vary in age from what may have been embryos to adults, although the decided majority come from prefledging juveniles.
There are no macrofossils of marine invertebrates. Other vertebrates include the tip of the rostrum of a great auk (Pinguinus sp.), perhaps a vagrant individual, the sclerotic bones from the eye of a swordfish (Xiphias gladius), and the fused dentaries of a spiny puffer (Diodon sp.). The billfish fossil is essentially spherical, and the puffer jaw may have come from a spherical inflated carcass, with both objects having perhaps been rolled over the beach ridge by wind or water. A few smaller fish bones include those of flying fish (Exocoetidae), which are prey of the modern Short-tailed Albatross and doubtless were brought to the site as food for young albatrosses. Flying fish indicate a subtropical or warm temperate marine environment.
All of the albatross bones from Bermuda belong to a single species with the slender proportions of the tarsometatarsus found in the genera Diomedea and Phoebastria (generic nomenclature follows ref. 19), as opposed to the robust tarsometatarsus with expanded ends characteristic of the southern genera Thallassarche and Phoebetria (3). They were compared with the large series (>500) of albatross fossils recovered from early Pliocene deposits in North Carolina (3) and with modern skeletal material of the species of Diomedea and Phoebastria in the collections of the Smithsonian Institution.
The Bermuda fossils conform in all respects of size and qualitative characters with comparative material of Short-tailed Albatross P. albatrus (Fig. 3). This species is in a unique size-class, being smaller than the great albatrosses (Diomedea, sensu stricto) or the fossil species Phoebastria anglica, but larger than any of the other species of Phoebastria. Bill shape in the Bermuda fossils and P. albatrus is very different from the wide, inflated rostrum seen in the next-largest species, the Black-footed Albatross P. nigripes.
Length and width measurements (in mm) from three specimens of fossilized eggs in the Bermuda Aquarium and Museum and another measured in the field were 118, 118, 120, 122 (average 119.5) × 76, 78, 80, 80 (average 78.5), respectively; 11 eggs of modern Short-tailed Albatross P. albatrus from Torishima had a range (and mean) of 110.5–124.7 (118.3) × 69.8–79.4 (73.4) (H. Hasegawa, personal communication).
The Short-tailed Albatross is known historically only from the North Pacific, where it was once the common inshore member of its family. It once bred on at least nine islands of various island groups in the western North Pacific (20). Through human depredation in the 19th and 20th centuries and destruction of nesting sites by volcanic activity, the species was virtually exterminated. It is now critically endangered, with the principal remaining nesting colony being on Torishima in the Izu Islands chain, 580 km south of Tokyo (20).
Extinction of Albatrosses in the North Atlantic. The great diversity of seabirds in the North Atlantic in the early Pliocene (3) stands in marked contrast with the depauperate marine avifauna of today. In the past 5 million years, many species have retreated from the North Atlantic or have become completely extinct. The causes remain speculative and the timing of extinction is essentially unknown, as the fossil record between the early Pliocene and the present is extremely sparse.
The Bermuda discovery shows that the Short-tailed Albatross persisted for ≈4 million years after the early Pliocene and was breeding on Bermuda 400 kyr in the middle Pleistocene. Today, Black-footed Albatrosses are known to nest in proximity to Short-tailed Albatrosses (20), so the absence of any additional species in the Bermuda deposits may indicate that other albatrosses were already extinct in the North Atlantic by the middle Pleistocene. The more pelagic species, such as Black-footed and Laysan albatrosses, may have been more adversely affected by changes in the North Atlantic caused by the closing of the Panamanian seaway at the end of the Pliocene (3, 21) than was the inshore-feeding Short-tailed Albatross (20).
Determining the precise cause of extinction of any species presents many difficulties, even when the process is under direct historical observation. In the case of the Short-tailed Albatross in the North Atlantic, we have only four points of reference: the species was present at sea in the early Pliocene, it is absent now, and in Bermuda during the middle Pleistocene MIS 11 interglacial, it was present and breeding when sea level was close to the modern level and also when it was >20 m higher. There is as yet no direct stratigraphic or radiometric evidence to determine whether the remains of the thriving colony near modern sea level were deposited before or after the >+20 m maximum of MIS 11.
Nevertheless, several other lines of evidence strongly suggest that the sea-level colony preceded the +20-m rise and thus would have been preserved at the onset of MIS 11 ≈420 kyr. No remains of albatrosses have been found on Bermuda in any younger deposits, so the MIS 11 interglacial maximum presents the most plausible cause yet known to explain the extirpation of albatrosses on Bermuda. Transgression of the sea generally inundates former shorelines, but does not bury them, as is the case at Green Island, whereas slow regressions would tend to bury older shorelines with eolian deposits and faster ones might leave them high and dry. Sea levels in MIS 11 rose in a stepping-up progression with periods of sea level stability at +2 m early in the stage, +7.5 m in mid to late stage, and ending at a level >20 m above present (8, 17, 22). The preexisting middle and early Pleistocene topography of Bermuda would have been vastly reduced in area by a +20-m sea level to <20–30% of modern land area. Albatrosses were still breeding on Bermuda, perhaps in considerably reduced numbers, up until the beach deposits formed at +21.3 m, in which a few juvenile bones were found. Because there is no evidence of reef buildup to any level approaching this elevation, the small remaining land area would have had little protection from storm waves and any possible breeding sites for albatrosses would have been subject to periodic storm flooding during the 8- to 9-month-long breeding cycle of this species.
Inshore marine conditions in the North Atlantic may have continued to be suitable as feeding grounds for Short-tailed Albatrosses, but once their breeding colony had been eliminated, there was no longer any possibility of recolonization of the Atlantic because Pacific populations would have been isolated by the isthmian land connection ≈3 million years ago (23).
To account for a +20-m rise of global sea level, it is necessary to melt all of Greenland ice (5–6 m of ocean volume), all of West Antarctic ice (5–6 m), and to draw down a substantial volume of East Antarctic ice in glacial drainages shared with West Antarctica. The complete absence of ice rafted debris for 23 kyr early on in MIS 11 in cores off the coast of Greenland (16) appears to indicate that Greenland ice was the first to disappear, likely followed by West, then a portion of East Antarctica (17). Such dramatic melting events suggest that Earth was operating in a fully fledged “greenhouse” world for several tens of thousands of years during MIS 11. Thus, we raise the possibility of the 20-m sea level rise during MIS 11 in the middle Pleistocene as the probable cause of extinction of the last albatrosses in the North Atlantic.
Acknowledgments
We are greatly indebted to David Wingate (Bermuda Department of Parks), who showed us all of the fossil sites, facilitated the collection of specimens, suggested investigation of the caves in the walls of Government Quarry, provided transportation and accommodations, and shared his incomparable knowledge of the island with us. The Green Island bones were called to David Wingate's attention by B. Spurling. The Calonectris Quarry site was surveyed by Sean G. Johnson (Bermuda Public Works Department). We are also most grateful to Wolfgang Sterrer and the Bermuda Zoological Society for a grant permitting us to visit and collaborate in Bermuda in 1999. Frederick V. Grady (Department of Paleobiology, National Museum of Natural History, Smithsonian Institution) greatly assisted in collecting of fossils and particularly in their preparation. Data from Hai Cheng, R. L. Edwards, and D. Kaufman were used to develop the geochronology. We thank Hiroshi Hasegawa for providing measurements of Short-tailed Albatross eggs and photographs. Bruce Collette, David Johnson, and James Tyler assisted with identification of fish remains. For assistance with graphic material, we thank Molly Ryan, Brian Schmidt, and the Office of Photographic Services, Smithsonian Institution. Partial funding for fieldwork was from the Seward Johnson Fund, Smithsonian Institution, administered by Ross Simons. For comments on the manuscript, we thank Lloyd Burckle, Steven D. Emslie, Gary R. Graves, Kenneth I. Warheit, and David Wingate. This is contribution number 46 of the Bermuda Biodiversity Project of the Bermuda Aquarium, Natural History Museum, and Zoo.
Abbreviations: MIS, marine isotope stage; kyr, thousand years ago.
References
- 1.Cheneval, J. (1984) Palaeovertebrata 14 33–115. [Google Scholar]
- 2.Olson, S. L. (1985) in Avian Biology, eds. Farner, D. S., King, J. R. & Parkes, K. C. (Academic, New York), Vol. 8, pp. 79–252. [Google Scholar]
- 3.Olson, S. L. & Rasmussen, P. C. (2001) Smithsonian Contr. Paleobiol. 90 233–365. [Google Scholar]
- 4.Vacher, H. L. & Hearty, P. J. (1989) Q. Sci. Rev. 8 159–168. [Google Scholar]
- 5.Olson, S. L. (1996) Atoll Res. Bull. 433 1–210. [Google Scholar]
- 6.Emslie, S. D. (1995) Polar Rec. 31 409–418. [Google Scholar]
- 7.Land, L. S., Mackenzie, F. T. & Gould, S. J. (1967) Geol. Soc. Am. Bull. 78 993–1006. [Google Scholar]
- 8.Hearty, P. J., Kindler, P., Cheng, H. & Edwards, R. L. (1999) Geology 27 375–378. [Google Scholar]
- 9.Vacher, H. L., Rowe, M. & Garrett, P. (1989) Geologic Map of Bermuda (Public Works Department, Bermuda Government, Hamilton, Bermuda).
- 10.Imbrie, J., Hays, J. D., Martinson, D. G., McIntyre, A., Mix, A. C., Morley, J. J., Pisias, N. G., Prell, W. L. & Shackleton, N. J. (1984) in Milankovitch and Climate, eds. Berger, A. L., Imbrie, J., Hays, J., Kukla, G. & Saltzman, B. (Riedel, Amsterdam), pp. 269–305.
- 11.Shackleton, N. J., Berger, A. & Peltier, W. R. (1990) Trans. R. Soc. Edinburgh Earth Sci. 81 251–261. [Google Scholar]
- 12.Raymo, M. E., Ruddiman, W. F., Shackleton, N. J. & Oppo, D. (1990) Earth Planetary Sci. Lett. 97 353–368. [Google Scholar]
- 13.Oppo, D. W., Fairbanks, R. G. & Gordon, A. L. (1990) Paleoceanography 5 43–54. [Google Scholar]
- 14.Burckle, L. H. (1993) Q. Sci. Rev. 12 825–831. [Google Scholar]
- 15.Poore, R. Z., Burkle, L., Droxler, A & McNulty, W. E., eds. (1999) U.S. Geological Survey Open File Report 99-312 (U.S. Geol. Surv., Washington, DC).
- 16.Stanton-Frazee, C., Warnke, D. A., Venz, K. & Hodell, D. A. (1999) in U.S. Geological Survey Open File Report 99-312, eds. Poore R. Z., Burkle, L., Droxler, A & McNulty, W. E. (U.S. Geol. Surv., Washington, DC), p. 75.
- 17.Hearty, P. J. (2002) Pacific Sci. 56 65–82. [Google Scholar]
- 18.Hearty, P. J., Vacher, H. L. & Mitterer, R. M. (1992) Geol. Soc. Am. Bull. 104 471–480. [Google Scholar]
- 19.Nunn, G. B., Cooper, J., Jouventin, P., Robertson, C. J. R. & Robertson, G. G. (1996) Auk 113 784–801. [Google Scholar]
- 20.Hasegawa, H. & DeGange, A. R. (1982) Am. Birds 36 806–814. [Google Scholar]
- 21.Emslie, S. D. (1995) J. Vert. Paleontol. 15 313–330. [Google Scholar]
- 22.Hearty, P. J. (1998) Q. Sci. Rev. 17 333–355. [Google Scholar]
- 23.Coates, A., ed. (1987) Central America: A Natural and Cultural History (Yale Univ. Press, New Haven, CT).