Abstract
In group-living animals, mutual policing to suppress reproduction is an important mechanism in the resolution of conflict between selfish group members and the group as a whole. In societies of bees, ants, and wasps, policing against the production of males by other workers is expected when egg laying by workers decreases the average inclusive fitness of individual group members. This may result (i) from the relatedness of workers being lower to workerthan to queen-derived males or (ii) from a lowered overall colony efficiency. Whereas good evidence exists for policing behavior caused by genetic conflicts, policing caused by efficiency factors has not been demonstrated. We investigated the regulation of reproduction in the ant Platythyrea punctata, a species in which colonies are clones because workers are capable of producing female offspring by thelytokous parthenogenesis. Reproductive conflicts resulting from differences in genetic relatedness are therefore not expected, but uncontrolled reproduction by all workers could lead to the destruction of sociality. Here we show that worker policing by aggressive attacks against additionally reproducing workers keeps the number of reproducing workers low. Furthermore, through experimental manipulation of the number of brood items per colony, we show that worker policing can enhance group efficiency.
Animal groups are structured by a process of selection that acts on different levels. On the level of the group, traits are selected that maximize the competitive ability of the group as a whole against other such groups. At the same time, however, individuals are selected to maximize their own reproductive success, and these individuals may try to obtain benefit from the group without contributing to shared traits that enhance the group outcome. Such individual selfishness will decrease the average fitness of all other group members, resulting in a conflict between individual and group interests.
Currently, there is intense study into how such conflicts of interest are resolved in social insects, in particular, the societies of Hymenoptera (ants, wasps, and bees) (1, 2). In these societies, there are conflicts, among others, about the production of males. In most species, workers are capable of rearing their own sons from unfertilized eggs (arrhenotokous parthenogenesis). In a society with a single, once-mated queen (monogyny and monandry), workers are more closely related (life-for-life relatedness) to their own sons (r = 0.5) or the sons of other workers (r = 0.375) than to males produced by the queen (r = 0.25). It is expected that workers favor male production by workers over male production by the queen. In contrast, in a society with a multiply mated queen (polyandry), workers are still more closely related to their own sons, but at an effective queen mating frequency above two, their average relatedness to other workers' sons is lower than to the queen's sons. In this situation, workers can increase their average inclusive fitness by laying their own eggs but preventing each other from reproducing through aggression or egg eating. Behavioral mechanisms, which in this way suppress selfishness of individuals against the group interest, are termed “worker policing” (3, 4).
Good experimental evidence for worker policing through egg eating has been presented for polyandrous honey bees (5–9) and wasps (10–12). In several ant species, workers attack and immobilize nestmates whose ovaries are developing in the presence of a fertile reproductive (13–16). In contrast to honey bees, some of these latter species are monogynous and monandrous, and policing, therefore, cannot simply be explained by the degree of genetic relatedness. Instead, policing might have evolved because worker reproduction would reduce overall colony efficiency and, hence, the average inclusive fitness of workers (4). However, as of yet, it has not been documented whether the presence of additional reproductives in such societies indeed poses a cost to group productivity.
Platythyrea punctata is an unusual ant species because workers can reproduce by thelytokous parthenogenesis; i.e., they produce diploid females from unfertilized eggs (17). Although all workers in a P. punctata colony are equally capable of laying eggs, reproduction is monopolized by one (occasionally two) worker while the others forego their own reproduction and instead perform tasks necessary for nest maintenance and brood rearing (18). Because sexual reproduction and recombination appear to be extremely rare or absent, colonies are essentially clones (19). Policing behavior to suppress genetic conflict is not expected to occur in clonal species (20). In the thelytokous Cape honey bee, Apis mellifera capensis, worker policing indeed appears to be absent or at least not very efficient; in contrast to other honey bee subspecies, a large number of worker-laid eggs remain undestroyed in queenright colonies (21, 22). In a recent study, however, worker policing has been observed to occur in the Cape honey bee (23).
Uncontrolled thelytokous reproduction can be detrimental for the colony as a whole. Workers in orphaned colonies of the facultatively thelytokous ant Cataglyphis cursor produce more eggs than can be reared and thereby waste colony resources (24). Furthermore, a particular Cape honey bee clone has been observed to invade and parasitize hives of another honey bee subspecies, A. mellifera scutellata, where the clone spreads because of the lack of policing. Reproductive clone bees replace host queen and workers, and because they appear to be unable to maintain a functional colony on their own, the parasitized colony perishes within months (25–27). Thelytokous reproduction without policing has therefore been referred to as “social cancer” (28) and should be nonadaptive in the long run.
The aim of this study was to determine how reproduction is controlled among totipotent, clonal individuals of P. punctata and whether new reproductive (NR) workers are policed against. Furthermore, we investigated whether any potential policing behavior could be explained by efficiency costs from having too many reproductives per colony.
Materials and Methods
Colonies. Colonies were collected in June 2001 at the El Verde Field Station in the Luquillo Experimental Forest (18°19′ N, 65°45′ W) and near Guaynabo (18°18′ N, 66°7′ W), Puerto Rico. They were transferred to the laboratory and kept in 20 × 10 × 6-cm plastic boxes with plaster floors. Three connected chambers measuring ≈6 × 4 × 0.5 cm and covered by a glass plate served as a nesting site in the plaster. The temperature was kept constant at 27°C with a 12-h light–dark cycle. Colonies were fed pieces of cockroaches and honey water five times per week.
Regulation of Reproduction. For behavioral observations, all workers of six colonies (A, D, E, and F from Guaynabo; B and C from El Verde) were individually marked with an edding paint marker. Reproductive workers are characterized by a very high frequency of resting on the brood pile (29) and are therefore easily identified by behavioral observations. Observations were confirmed by individually isolating presumed reproductives for 12–36 h until they had laid an egg and then returning them into the nest.
To induce the development of NR workers, we used an experimental set-up used in previous studies of worker policing in other ponerine ants (14, 15). Colonies were separated into two halves, part 1 containing the old reproductive (OR) worker and part 2 consisting exclusively of nonreproductive workers. Cocoons and larvae were equally distributed between both groups, but eggs were given only to part 1. After a few weeks, first eggs were laid in part 2 and the NR individual could be identified by its behavior, by an observation of egg laying in the colony, or after isolation. The frequency of aggressive behavior in both colony parts was determined by observing the colonies six times per day for 10 min each over a period of 7 days.
After this observation period, i.e., 2–3 weeks after the first eggs had appeared in part 2, the colony parts were reunified. Four colonies (A–D) were brought together by placing individuals and brood near the nest entrance of part 1. To exclude the possibility that the enhanced aggression by workers of part 1 compared with workers of part 2 might be merely a defense reaction against intruders, a second set of experiments with two colonies (E and F) was conducted in which all workers of parts 1 and 2 were transferred into a new nest chamber, i.e., a neutral environment, for both parts. To detect any policing behavior, observations were started after reunion for the same duration as before (six 10-min scans per day over 7 days). In colonies A–C, quantitative data were recorded only for aggression involving ORs or NRs, whereas in colonies D–F, the frequency of all types of aggression was noted. We used a Wilcoxon matched-pair test (two-tailed) to compare the number of aggressions before and after reunion and the number of attacks against different individuals per 10-min scan.
After the experiment, all individuals of colonies D–F were dissected to determine their ovarian development. We dissected only the ORs and NRs in colony B and only the NRs in colonies A and C. Ovaries were sorted in the following categories: I, undeveloped ovaries without any oocytes or yellow bodies, i.e., remnants of previously laid or resorbed eggs (mean length ± SD = 1.20 ± 0.26 mm, n = 26); II, slightly developed ovaries sometimes containing one or more small immature yolky oocytes (1.51 ± 0.14 mm, n = 9); III, developed, active ovaries with one or few maturing yolky oocytes (1.36 and 1.46 mm, n = 2); and IV, fully developed ovaries with several maturing, yolky oocytes and distinct yellow bodies (3.11 ± 0.81 mm, n = 4). The length of ovarioles was significantly different among categories I, II, and IV [ANOVA, F = 60.0, df = 2, P < 0.001 (category III was excluded because of low sample size; ovariole length here was more similar to those in categories I and II)]. In a post hoc comparison, no difference could be detected between types I and II (Scheffé's test, P = 0.06), but type IV ovarioles were significantly longer than those of categories I and II (Scheffé's test, P < 0.001 for both comparisons).
Brood Manipulation. To test whether colonies can rear more brood than is produced by one single reproductive, the number of brood items was artificially increased in five colonies and colony growth was measured in comparison to four control colonies. Colonies of equal size (40 workers each) were created by establishing eight colony fractions from two large colonies collected near Guaynabo and one from a third colony collected in El Verde. This procedure was necessary because of the lack of a sufficient number of large colonies. However, because populations are in part clonal (19) and new societies presumably arise through colony fission or budding, we feel it is appropriate to treat all experimental colonies as independent data points.
Colonies were kept in the laboratory until one individual had started to lay eggs in each colony fraction. All brood was removed from these nine experimental and three additional natural colonies, mixed, and redistributed to the colonies. Mixing brood should minimize the potential risk of nepotistic brood rearing. Four control colonies were supplied with an amount of brood typical for colonies of that size (5 cocoons, 12 larvae, and 10 eggs). Five test colonies received more than double the amount of larvae and eggs (5 cocoons, 31 larvae, and 25 eggs). The number of brood items and adult workers was counted after 15, 24, 30, 39, 46, 51, 72, 80, and 92 days. To allow an easy comparison among the different colonies, the amount of brood was given in relation to the number of workers, which constantly changed because of the death of some individuals and the eclosion of callows.
Results
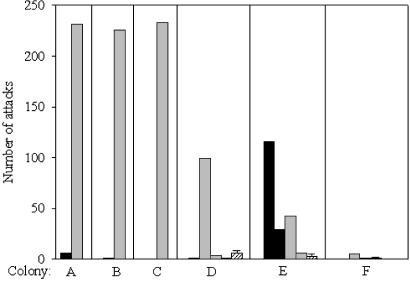
Regulation of Reproduction. Aggressive interactions after reunion. During the separation period, one to three workers (median = 2; Fig. 1) started to lay eggs in the colony parts that lacked an OR. The frequency of aggressive interactions among nestmates was generally low before reunification of parts 1 and 2. However, reuniting the two colony parts led to an immediate increase in the number of attacks directed toward at least one of the NRs in five of six colonies [Wilcoxon matched-pairs test, analyzing the amount of aggression per 10 min: colony A, Z = –3.73 (n = 21); colony B, Z = –3.30 (n = 23); colony C, Z = –4.55 (n = 42); colony D NR1, Z = –4.03 (n = 24); colony E NR1, Z = –3.87 (n = 42), and NR2, Z = –2.12 (n = 42); P < 0.05 in all comparisons]. In colony D, the frequency of aggression increased significantly toward only one of the three NRs, whereas in colony E, both NRs were significantly more frequently attacked than before the reunion (Fig. 1). In colony F, one of the two NRs was attacked five times, but this was not significantly more than before reunion [NR1, Z = –1.89 (n = 42); NR2, Z = –1.00 (n = 42); P > 0.05 in both comparisons].
Fig. 1.
Number of attacks directed toward ORs (▪), NRs ( ), and nonreproductives (
), and nonreproductives ( ; only colonies D–F; mean ± SD) observed after the reunion of two separated colony fragments of P. punctata (observation time: colony A, 210 min; colony B, 230 min; colonies C–F, 420 min; colony D, 240 min). In colonies A–D NRs received more attacks than ORs and nonreproductive workers, suggesting worker policing.
; only colonies D–F; mean ± SD) observed after the reunion of two separated colony fragments of P. punctata (observation time: colony A, 210 min; colony B, 230 min; colonies C–F, 420 min; colony D, 240 min). In colonies A–D NRs received more attacks than ORs and nonreproductive workers, suggesting worker policing.
Whereas the few cases of aggression before reunion were mostly short episodes of antennal boxing, the aggression against NRs after reunion was much more violent. NRs were now often attacked simultaneously by several workers, who, in addition to antennal boxing, dragged them through the nest chamber or foraging arena and immobilized them by biting legs and antennae over several minutes and up to several hours. Furthermore, biting individuals were observed to rub the tip of their gasters against the body, mainly the legs, of the opponent. This behavior was exhibited by both reproductive and nonreproductive workers.
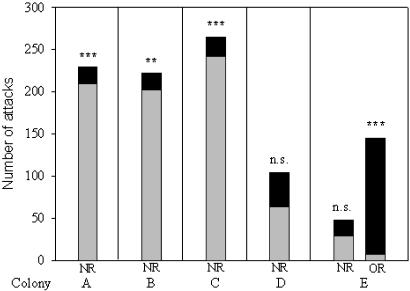
In colonies A, B, and C, NRs were mostly attacked by workers from colony part 1 [colony A, Z = –3.52 (n = 21); colony B, Z = –3.30 (n = 23); colony C, Z = –4.55 (n = 42); P < 0.01 in all comparisons]. In contrast, workers from both parts of colonies D and E were similarly aggressive toward NRs [colony D, Z = –1.69 (n = 24); colony E, Z = –0.64 (n = 42); P > 0.05 in both comparisons; Fig. 2].
Fig. 2.
Number of attacks received by ORs and NRs from nonreproductive workers from colony parts 1 ( ) and 2 (▪) (Wilcoxon matched-pair test; ***, P < 0.001; **, P < 0.01; n.s., not significant; observation time: colony A, 210 min; colony B, 230 min; colonies C and E, 420 min; colony D, 240 min).
) and 2 (▪) (Wilcoxon matched-pair test; ***, P < 0.001; **, P < 0.01; n.s., not significant; observation time: colony A, 210 min; colony B, 230 min; colonies C and E, 420 min; colony D, 240 min).
NRs of colonies A and B and the most fiercely attacked NR of colony D died on day 4 after the reunion; the most frequently attacked NR of colony E died on day 7. In colony C, attacks against the NR continued for more than one week and ceased at ≈10 days after reunion, when the NR was found outside the nest. Dissection revealed that its ovaries no longer contained developing oocytes.
ORs were rarely actively involved in aggressive interactions and also rarely received aggression at any time of the experiment (Fig. 1). Colony E contained two ORs, neither of which were attacked before reunion. However, aggression toward them increased significantly after reunification [OR1, Z = –4.96 (n = 42); OR2, Z = –2.21 (n = 42); P < 0.01 for both comparisons]. OR1 received many more attacks than OR2 [Z =–4.01 (n = 42); P < 0.01]. Attacks against ORs were mainly conducted by those workers that had been separated from the ORs before the reunion, including the NRs [OR1, Z = –4.45 (n = 42), P < 0.01; OR2, Z = –2.21 (n = 42), P < 0.05]. In this colony and in colony F, ORs were occasionally observed attacking NRs.
The variation in the aggressiveness among colonies might in part be explained by the different procedures of reunification. For example, in colonies A–C, workers from part 1 were more aggressive toward the NRs than workers from part 2. However, in colony D, which was treated in the same way as colonies A–C, workers from both parts were equally aggressive toward NRs. Furthermore, the frequency of aggression among nonreproductive workers always remained very low, although in colonies D and E the level was somewhat higher after reunion than before [colony D, Z = –3.11 (n = 42), P = 0.002; colony E, Z = –2.20 (n = 42), P = 0.028; Fig. 1]. Even in these colonies, individual nonreproductive workers were attacked by workers from the other colony part much less than NRs [aggression by colony D workers from part 1, χ2 = 55.54 (P < 0.001); workers from part 2, χ2 = 12.52 (P < 0.001); colony E workers from part 1, χ2 = 13.33 (P < 0.001); workers from part 2, χ2 = 9.8 (P = 0.002)]. This result suggests that the attacks against NRs had nothing to do with nestmate recognition. Furthermore, the outcome of the experiment remained similar regardless of the set-up used: NRs were either rejected or suppressed, independent of how colonies were reunified.
Ovarian development. The results of ovary dissections are given in Table 1. All ORs except OR1 in colony E had fully developed type IV ovaries. Ovarioles of OR1 from colony E were elongated and showed yellow bodies, suggesting that this individual had already produced eggs, although at the time of dissection it contained only one mature and two small oocytes (Table 1). NRs that died during the experiment had considerably less developed or undeveloped ovaries than ORs (types I–III). NRs that survived without receiving any aggression (colony F) or toward which aggression had ceased (colony C) had undeveloped, presumably degenerated type I or II ovaries at the end of the experiment, although they had been seen laying eggs before. One NR in colony D, which had received very few attacks, had type III ovaries and was observed to lay an egg after the reunion in the presence of the OR. Dissection of all individuals from colonies D, E, and F confirmed that all other workers were reproductively inactive and had ovaries of type I or II.
Table 1. Ovarian status of ORs, NRs, and nonreproductives in the ant, Platythyrea punctata.
| Colony |
||||||
|---|---|---|---|---|---|---|
| Group | A | B | C | D | E | F |
| OR | —* | IV | —* | IV | III-IV | IV |
| OR2 | IV | |||||
| NR1 | II† | II† | II | III† | I† | II |
| NR2 | II | II | I | |||
| NR3 | III | |||||
| Nonreproductives | — | — | — | I (14), II (3) (n = 17) | I (4),‡ II (3) (n = 11) | I (28),§ II (2) (n = 34) |
I-IV, ovarian status (number of dissected individuals). For details see text. —, not dissected.
ORs in colonies A and B were not dissected, but their behavior and the frequent observation of egg laying suggests that their ovaries were well developed.
Died during the experiment.
Four individuals could not be dissected.
Four individuals could not be dissected.
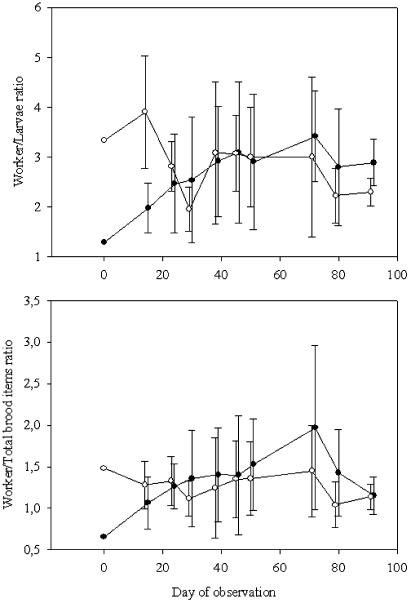
Brood Manipulation. Test colonies, which were supplied with an artificially increased number of larvae and eggs, did not grow faster than control colonies. Fifteen days after the brood was added to the colonies, the test colonies still contained significantly more larvae than control colonies (Mann–Whitney U test, U = 0.5, P = 0.02), however, the number of brood items did not differ between test and control colonies during the other counts (Mann–Whitney U test, P < 0.05 for all comparisons). The ratio of workers to larvae and the ratio of workers to total brood items did not differ between control and test colonies after 24 and 15 days, respectively (Fig. 3). At the end of the experiment, the mean ratio of workers to larvae in test colonies (2.89 ± 0.47) and control colonies (2.30 ± 0.28) was not different from that counted in sixty natural colonies [ref. 18; 2.48 ± 1.48 (ANOVA, n = 69, F = 0.16, df = 2, P > 0.05)]. The same result was found for the mean ratio of workers to the total number of brood items [test colonies, 1.15 ± 0.22; control colonies, 1.14 ± 0.15; natural colonies, 1.18 ± 0.81 (ANOVA, n = 69, F = 0,007, df = 2, P > 0.05)].
Fig. 3.
Mean ratio of the number of workers to the number of larvae (A) and the number of total brood items (cocoons, larvae, and eggs) in test (•) and control colonies (○) (± SD) (B). There was no difference between test and control colonies in the worker to larvae ratio later than 24 days and in the worker to brood ratio later than 15 days after the manipulation (Student's t test, all P > 0.05) of observation.
The decrease in the number of larvae in test colonies probably reflects a high mortality of larvae, because the number of pupae did not increase considerably. The number of eggs never differed between test and control colonies (Mann–Whitney U test, P > 0.05 for all observations), i.e., the extra amount of eggs supplied to test colonies had already disappeared after 15 days, presumably because some had developed to larvae and others had been cannibalized.
Discussion
NR workers of the clonal ant P. punctata that had developed their ovaries during the separation period in an orphaned colony part were fiercely attacked by other workers after reunification with the colony part containing the OR. These attacks resulted in death or loss of reproductive status for NRs and continued monopolization of reproduction for ORs. It is unlikely that this aggression results from a perturbation of dominance hierarchies or changes in colony odor during the separation period. After reunion, NRs were attacked by numerous workers, including some that had never been aggressive toward them before; in the case of hierarchy perturbation we would expect that only a few high-ranking workers attacked NRs. Workers also did not direct their aggression generally toward workers from the other colony part but specifically toward NRs. Thus, aggression does not simply result from odor changes during the separation period. Furthermore, previous observations had suggested the inability of workers of P. punctata to discriminate between nestmates and non-nestmates under laboratory conditions (30).
We therefore conclude that the observed aggression can be considered as worker policing against NRs. Aggression is probably not as strong under natural conditions as in our experiment. The number of egg-layers will typically not be controlled by killing surplus reproductives but by preventing ovary development or at least inducing its reduction. In a previous study on P. punctata (18) it was found that several workers in a colony had degenerated ovaries containing yellow bodies, suggesting that ovaries can degenerate. Aggression toward NRs might finally also result in the foundation of new colonies by budding.
Individual NRs differed considerably in the amount of attacks they received, and in one colony (F), aggression did not increase at all after the reunion. This might result from different ovarian development among NRs. Several studies give evidence that workers are able to assess the degree of ovarian development of nestmates (13–15, 31, 32), probably because of chemical cues correlated with physiological changes. In several ponerine ants, the individual cuticular hydrocarbon profile gradually changes with the degree of ovarian development (33–36). It is possible that the intensity of such cues has to exceed a certain threshold on which workers are able to assess the onset of the ovarian development of another nestmate. Perhaps the pattern in some of the NRs did not exceed this threshold, and they could therefore evade aggressive attacks from nestmates. The high frequency toward one of the ORs in colony E might also be explained by differences in the fertility between the reproductives, because this individual had less developed ovaries than the other OR. This is comparable with the immobilization of mated, OR workers (gamergates) in Gnamptogenys menadensis when their fecundity decreases (13), or with aggression toward less fertile individuals in polygynous colonies (37).
Because of the clonal structure of colonies of P. punctata, policing cannot result from variation in genetic relatedness. The alternative explanation, that policing avoids costs to the colony's overall productivity that arise from excess egg layers, presupposes that colonies with multiple reproductives produce offspring less efficiently than colonies with only a single reproductive (4). Such efficiency costs have been evoked to explain worker policing in the monandrous and monogynous ponerine ant species Diacamma sp. and Harpegnathos saltator (14, 15), as well as in the European hornet, Vespa crabro (11). Similarly, workers selectively destroy worker-laid eggs in the polyandrous wasp, Vespula vulgaris, although they are equally related to the queen's and workers' sons (12), and the relatedness at which policing occurs in the wasp Dolichovespula saxonica is slightly higher than that predicted from relatedness alone (10). The presumed costs associated with worker reproduction have never been directly demonstrated.
In our brood manipulation experiment, the addition of brood from a second reproductive did not lead to increased colony productivity. This might mean that average colonies of P. punctata are incapable of rearing more than the normal amount of brood produced by a single reproductive. The alternative explanation that workers might have recognized and selectively destroyed alien brood is unlikely, because test and control colonies received the same mix of domestic and alien brood, but the number of larvae decreased only in test colonies. Furthermore, ant workers typically accept alien brood without aggression (refs. 38–41; but see refs. 42 and 43), and selective cannibalism or neglect of alien brood is even more unlikely in P. punctata, because this species lacks a clear discrimination between adult nestmates and aliens in the laboratory (30). The number of larvae, but not of eggs, was still higher in test colonies after 15 days. The developmental times from egg to larva and from larva to adult are 2 and 6 weeks, respectively (J.H., unpublished data). We assume that the number of eggs initially declined faster than the number of larvae predominantly because some eggs were destroyed (whereas others developed into larvae) and not because workers policed against eggs from other reproductives.
During the course of our experiment, the average ratio of workers to larvae and that of workers to the total amount of brood in test and control colonies approached values similar to those observed in natural colonies. Our brood manipulation experiment might therefore indicate a strong limitation in the number of brood items that a colony can successfully raise. Worker policing in the P. punctata colonies might therefore maintain an efficient division of labor, with a balanced ratio between a single reproducing individual and a few dozen nonreproducing workers, and keep the social cancer of thelytoky in check.
Worker policing and dominance interactions are energetically costly. In clonal societies, in which restriction of reproduction to a certain number of individuals increases efficiency, selection on the group level should lead to the loss of such behavior and to the evolution of self-restraint over long periods of time (3). In unmanipulated colonies of P. punctata, aggression occurs only when new workers eclose or when a weakening reproductive is replaced. Thus, workers do show self-restraint. At the same time, they are still able to police against other nestmates. Policing behavior might be an atavistic relict from times when sexual reproduction was more common in P. punctata. Thelytoky apparently evolved only recently in this species, because morphologically differentiated queens still occur in some populations of Florida (18). No data are available about the ancestral social structure of P. punctata, and it therefore remains unclear whether worker policing has not yet been replaced by selfpolicing or whether it has evolved only with the development of queenless colonies and thelytokous reproduction.
Acknowledgments
We thank Judith Korb for valuable discussions, three referees for helpful comments, and Departamento de Recursos Naturales y Ambientales for providing a collection permit in Puerto Rico. Field work was supported by Deutscher Akademischer Austausch Dienst.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NR, new reproductive; OR, old reproductive.
References
- 1.Ratnieks, F. L. W. & Reeve, H. K. (1992) J. Theor. Biol. 158 33–65. [Google Scholar]
- 2.Heinze, J., Hölldobler, B. & Peeters, C. (1994) Naturwissenschaften 81 489–497. [Google Scholar]
- 3.Frank, S. A. (1995) Nature 377 520–522. [DOI] [PubMed] [Google Scholar]
- 4.Ratnieks, F. L. W. (1988) Am. Nat. 132 217–236. [Google Scholar]
- 5.Visscher, P. K. (1989) Behav. Ecol. Sociobiol. 25 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visscher, P. K. (1996) Behav. Ecol. Sociobiol. 39 237–244. [Google Scholar]
- 7.Ratnieks, F. L. W. & Visscher, P. K. (1989) Nature 342 796–798. [Google Scholar]
- 8.Halling, L., Oldroyd, B. P., Patimus, B., Wattanachaiyingcharoen, W., Barron, A. B., Nanork, P. & Wongsiri, S. (2001) Behav. Ecol. Sociobiol. 49 509–513. [Google Scholar]
- 9.Oldroyd, B. P., Halling, L. A., Good, G., Wattanachaiyingcharoen, W., Barron, A. B., Nanork, P., Wongsiri, S. & Ratnieks, F. L. W. (2001) Behav. Ecol. Sociobiol. 50 371–377. [Google Scholar]
- 10.Foster, K. & Ratnieks, F. L. W. (2000) Nature 407 692–693. [DOI] [PubMed] [Google Scholar]
- 11.Foster, K. R., Ratnieks, F. L. W. & Raybould, A. F. (2000) Mol. Ecol. 9 735–742. [DOI] [PubMed] [Google Scholar]
- 12.Foster, K. & Ratnieks, F. L. W. (2001) Proc. R. Soc. London Ser. B 268 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobin, B., Billen, J. & Peeters, C. (1999) Anim. Behav. 58 1117–1122. [DOI] [PubMed] [Google Scholar]
- 14.Liebig, J., Peeters, C. & Hölldobler, B. (1999) Proc. R. Soc. London Ser. B 266 1865–1870. [Google Scholar]
- 15.Kikuta, N. & Tsuji, K. (1999) Behav. Ecol. Sociobiol. 46 180–189. [Google Scholar]
- 16.Monnin, T. & Peeters, C. (1997) Naturwissenschaften 84 499–502. [Google Scholar]
- 17.Heinze, J. & Hölldobler, B. (1995) Naturwissenschaften 82 40–41. [Google Scholar]
- 18.Schilder, K., Heinze, J. & Hölldobler, B. (1999) Insectes. Soc. 46 150–158. [Google Scholar]
- 19.Schilder, K., Heinze, J., Gross, R. & Hölldobler, B. (1999) Mol. Ecol. 8 1497–1507. [DOI] [PubMed] [Google Scholar]
- 20.Greef, J. M. (1996) Philos. Trans. R. Soc. London B 351 617–625. [Google Scholar]
- 21.Moritz, R. F. A., Kryger, P. & Allsopp, M. H. (1999) Behavior 136 1079–1092. [Google Scholar]
- 22.Beekman, M., Good, G., Allsopp, M. H., Radloff, S., Pirk, C. W. W. & Ratnieks, F. L. W. (2002) Naturwissenschaften 89 479–482. [DOI] [PubMed] [Google Scholar]
- 23.Pirk, C. W. W., Neumann, P. & Ratnieks, F. L. W. (2003) Behav. Ecol. 14 347–352. [Google Scholar]
- 24.Retana, J. & Cerdá, X. (1990) Ethology 84 105–122. [Google Scholar]
- 25.Martin, S. J., Beekman, M., Wossler, T. C. & Ratnieks, F. L. W. (2002) Nature 415 163–165. [DOI] [PubMed] [Google Scholar]
- 26.Martin, S., Wossler, T. & Kryger, P. (2002) Apidologie 33 215–232. [Google Scholar]
- 27.Allsopp, M. H. & Crewe, R. M. (1993) Am. Bee J. 133 121–123. [Google Scholar]
- 28.Oldroyd, B. P. (2002) Trends Ecol. Evol. 17 249–251. [Google Scholar]
- 29.Hartmann, A. & Heinze, J. (2003) Evolution (Lawrence, Kans.), in press.
- 30.Schilder, K. (1999) Ph.D. thesis (Universität Würzburg, Würzburg, Germany).
- 31.Crosland, M. (1990) Anim. Behav. 39 413–425. [Google Scholar]
- 32.Hölldobler, B. & Carlin, N. F. (1989) Psyche 96 131–151. [Google Scholar]
- 33.Peeters, C., Monnin, T. & Malosse, C. (1999) Proc. R. Soc. London Ser. B 266 1323–1327. [Google Scholar]
- 34.Liebig, J., Peeters, C., Oldham, N. J., Markstädter, C. & Hölldobler, B. (2000) Proc. Natl. Acad. Sci. USA 97 4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinze, J., Stengl, B. & Sledge, M. F. (2002) Behav. Ecol. Sociobiol. 52 59–65. [Google Scholar]
- 36.Cuvillier-Hot, V., Cobb, M., Malosse, C. & Peeters, C. (2001) J. Insect Physiol. 47 485–493. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher, D. J. C. & Blum, M. S. (1983) Science 219 312–314. [DOI] [PubMed] [Google Scholar]
- 38.Carlin, N. F. & Hölldobler, B. (1983) Science 222 1027–1029. [DOI] [PubMed] [Google Scholar]
- 39.Fielde, A. M. (1903) Biol. Bull. (Woods Hole, Mass.) 7 227–250. [Google Scholar]
- 40.Crosland, M. W. J. (1988) Ann. Entomol. Soc. Am. 81 844–850. [Google Scholar]
- 41.Haskins, C. P. & Haskins, E. F. (1950) Anim. Behav. 50 9–14. [Google Scholar]
- 42.Hare, J. F. (1996) Can. J. Zool. 74 2055–2061. [Google Scholar]
- 43.Carlin, N. F. (1988) Advances in Myrmecology, ed. Trager, J. C. (Brill, Leiden, The Netherlands), pp. 267–295.