Abstract
Chemotherapeutic drugs chronically administered to tumor-bearing mice, using a frequent schedule at doses substantially lower than the maximum tolerated dose (MTD) (i.e., metronomic dosing), can cause sustained and potent antiangiogenic effects by targeting the endothelial cells of newly growing tumor blood vessels. These effects appear to occur in the absence of an increase in the severity of side effects caused by destruction of other cell types normally sensitive to MTD chemotherapy, suggesting a marked and selective sensitivity of activated endothelial cells, the basis of which is unknown. Here we report that protracted exposure of endothelial cells in vitro to low concentrations of several different anticancer agents, including microtubule inhibitors and an alkylating agent, caused marked induction of gene and protein expression of TSP-1, a potent and endothelial-specific inhibitor of angiogenesis. Increases in circulating TSP-1 were also detected in the plasma of human tumor-bearing severe combined immunodeficient mice treated with metronomic low-dose cyclophosphamide. Most importantly, the antiangiogenic and antitumor effects of low-dose continuous cyclophosphamide were lost in TSP-1-null C57BL/6 mice, whereas, in contrast, these effects were retained by using a MTD schedule of the same drug. Taken together, the results implicate TSP-1 as a secondary mediator of the antiangiogenic effects of at least some low-dose metronomic chemotherapy regimens.
Keywords: endothelial cells, tumor angiogenesis, endogenous inhibitors, TSP-1-null mice
Conventional cytotoxic chemotherapeutic drugs were designed to treat cancer by directly killing or inhibiting the proliferation of rapidly dividing tumor cells. However, recent studies from a number of laboratories, as summarized in detail by Miller et al. (1), have highlighted the possibility that such drugs may have antitumor effects by an alternative, secondary mechanism, involving inhibition of tumor angiogenesis. The basis for this effect is presumed to be related to the presence of dividing endothelial cells in newly forming tumor blood vessels (2–4). Like other types of normal dividing host cells, such as bone marrow progenitors or hair follicle cells, they would be expected to be sensitive to conventional chemotherapeutic agents, regardless of whether the surrounding tumor cells they are nourishing are resistant to the same drug(s) (5).
Studies by Browder et al. (3) have highlighted the fact that the antiangiogenic effects, and hence some of the antitumor effects, of chemotherapeutic drugs such as cyclophosphamide (CTX) may be nullified by the long break periods between successive cycles of maximum tolerated dose (MTD) chemotherapy, because the damage or loss of activated endothelial cells in tumor vessels may be reversed by various mechanisms. Therefore, Browder et al. proposed a strategy for optimizing the antiangiogenic effects of chemotherapy by chronically administering such drugs on a much more frequent schedule and, hence, at lower doses than the MTD, with no long breaks. This has been termed antiangiogenic chemotherapy or metronomic dosing (6). The potential advantages of giving chemotherapeutic drugs in this manner include the following: (i) it may significantly delay the onset of mutation-dependent mechanisms of acquired drug resistance, because the target of the therapy is presumed to be the genetically stable, host (activated) endothelial cell rather than the genetically unstable and, hence, highly mutable cancer cells; and (ii) it would facilitate long-term integration of chemotherapy drugs with targeted antiangiogenic agents, thus possibly increasing the efficacy and durability of such chemotherapy-based combinations (4).
One of the paradoxical findings concerning the use of low-dose metronomic chemotherapy regimens is the reduction or loss of traditional toxic side effects observed during and after standard MTD chemotherapy. This represents a third advantage. If shortening the breaks between successive exposures of chemotherapy compromises the ability of the host to repair endothelial cell damage, the same might be expected with respect to other types of normal dividing cells, such as bone marrow progenitors. If so, an increase in harmful or undesirable side effects such as myelosuppression and hair loss would be expected. However, this does not seem to be the case, and indeed, such side effects appear to be much less severe or even absent in mice or humans treated with chronic low-dose metronomic chemotherapy regimens (3, 4, 7). This result implies a high degree of selectivity and sensitivity of low-dose continuous chemotherapy for the activated endothelial cells as the cellular target. In this regard, several laboratories, including our own (8–11), have shown that endothelial cells in culture have a striking sensitivity to extremely low concentrations of a chemotherapeutic drug, e.g., in the range of 0.1–50 pM for taxol, taxotere, or vinblastine, compared with most other types of normal cell lines and various tumor cell lines tested. There is also evidence that this selective antiendothelial effect can be enhanced by the more protracted drug exposures in vitro, e.g., 3–6 days, which can also result in eventual apoptosis (9).
The basis for this endothelial cell selectivity and sensitivity is unknown. However, a possible clue was provided on the basis of gene (cDNA microarray) profiling studies recently undertaken in our laboratory of endothelial cells exposed to protracted low-dose chemotherapy drugs in vitro. We observed a marked induction of thrombospondin 1 (TSP-1) (G.B. and R.S.K., unpublished observations), a well known, highly specific, and potent endogenous inhibitor of angiogenesis (12–14). We hypothesized that, in addition to or perhaps even instead of a direct antiproliferative or cytotoxic anti-endothelial cell effect, a secondary effect involving TSP-1 induction might provide an explanation for the specificity of the effects of metronomic chemotherapy treatments, because TSP-1 is thought to mediate its antiproliferative and proapoptotic effects (15, 16) in a highly specific manner on endothelial cells, primarily by binding to the CD36 membrane receptor (17).
We therefore decided to undertake a series of experiments to examine the aforementioned TSP-1 hypothesis. In particular, we wished to assess the antiangiogenic and antitumor effects of MTD versus continuous low-dose chemotherapy regimens in normal versus TSP-1-deficient mice (18), along with a number of other experimental approaches. Here we report evidence that implicates a TSP-1-mediated mechanism in at least some of the antiangiogenic effects of low-dose metronomic chemotherapy. As such, the results establish a linkage between endogenous protein inhibitors of angiogenesis and chemotherapy.
Materials and Methods
Drugs and Cell Lines. Paclitaxel and BMS-275183 were gifts from W. Rose (Bristol-Myers Squibb), epothilone B (EpoB) and 5-methylpyridine EpoB were gifts from K. C. Nicolaou (The Scripps Research Institute, La Jolla, CA), BAL-9504 was obtained from Laboratori Baldacci (Pisa, Italy), 4-hydroperoxycyclophosphamide was provided by S. Ludeman (Duke University, Durham, NC), cyclophosphamide (CTX) was obtained from Carter Horner (Mississauga, ON, Canada), and vinblastine sulfate (VBL) was obtained from Ely Lilly. Human dermal microvascular endothelial cells and human umbilical vein endothelial cells were obtained from Clonetics (San Diego), human cancer cell line MDA-MB-435 and T0.1 were provided by Dalia Cohen (Novartis, Cambridge, MA), and human prostate cancer cell line PC-3 and murine Lewis lung carcinoma (LL/2) cells were obtained from American Type Culture Collection (ATCC). Sterile plastics for cell cultures were purchased from Becton Dickinson. Cells were routinely grown and maintained as described (9, 19). BAL-9504 is a geranylgeranyl transferase inhibitor (20) and was used as a nonchemotherapeutic drug control for the various cytotoxic drugs tested, as explained elsewhere in the article.
Northern Blotting Assay. Human endothelial and tumor cells were continuously treated for 144 h with 100 pM paclitaxel, 100 pM BMS-275183, 100 pM EpoB, 100 pM 5-methylpyridine EpoB, 100 nM 4-hydroperoxycyclophosphamide, and 100 nM BAL-9504, as described (9). At the end of the experiment, both vehicle and drug-treated cells were collected, and total RNA was isolated by using the TRIzol kit (GIBCO) according to the manufacturer's instructions. Northern blotting was carried out as described (21). The human β-actin probe was generated by RT-PCR, and the 1.29-kb TSP-1 probe was prepared from a pGEM2 vector encoding human TSP-1 (ATCC) (22); both were gel-purified, [α-32P]dCTP-labeled, and hybridized to filters that were autoradiographed for at least 2 weeks (TSP-1 probe) or 8 h (β-actin probe). Northern blot images were subjected to densitometric analysis and quantified by using imagequant (Version 4.2, Molecular Dynamics), and the results were shown as a percentage of the TSP-1/β-actin densities ratio.
Western Blotting Assay. Each sample analyzed by Northern blotting was used subsequently for Western blotting. Whole-cell lysates were obtained from both vehicle and drug-treated cells. Protein samples were prepared from TRIzol:chloroform solutions according to the manufacturer's recommendations. Twenty-five micrograms of protein lysate was boiled for 5 min in loading buffer and loaded onto a 7% polyacrylamide/SDS gel. Immobilon-PSQ (Millipore) filters were blotted by using an anti-human TSP-1 antibody (clone B5.2, NeoMarkers, Fremont, CA) or anti-human CRM-1 antibody (sc-7826, Santa Cruz Biotechnology) followed by peroxidase-conjugate immunoglobulins (Promega). For detection, ECL was used (Amersham Biosciences).
Human TSP-1 Detection in Conditioned Medium by Competitive Enzyme Immunoassay. A human TSP-1-competitive enzyme immunoassay (Neogen, Lexington, KY) was used according to the manufacturer's instructions to quantitate secreted TSP-1 in the serum-free conditioned medium obtained from 144-h vehicle and drug-treated human cells. The optical density was determined by using the microplate reader Benchmark Plus (Bio-Rad) set to a wavelength of 490 nm. The TSP-1 concentration was normalized by the total amount of protein of the medium, and the results were shown as the increase percentage of secreted TSP-1 versus vehicle-treated samples. All experiments were repeated two times with at least two replicates per sample.
Endothelial Cell Proliferation Assay. In vitro chemosensitivity testing was performed as described (9) on human dermal microvascular endothelial cells plated in 1% gelatin-coated 96-well plastic plates. Cells were continuously treated for 144 h with 100 pM paclitaxel, 100 pM BMS-275183, 100 pM EpoB, 100 pM 5-methylpyridine EpoB, and 100 nM BAL-9504 alone or in combination with 10 μg/ml A4.1 anti-human TSP-1 (NeoMarkers). To maintain a constant concentration of the drugs during the protracted 144-h period of the experiments, the medium was carefully removed every 24 h, and fresh solutions were added with new medium. At the end of the experiment, cells were pulsed for 6 h with 2 μCi (1 Ci = 37 GBq) of methyl-[3H]thymidine (Amersham Biosciences) per well.
In Vivo Angiogenesis Assessment by Matrigel Plug Perfusion Assay in TSP-1-Null and Wild-Type Mice. To generate TSP-1-null mice, TSP-1-heterozygous mice (18) were backcrossed eight times to wild-type C57BL/6 mice and were then mated to produce TSP-1 knockouts with the C57BL/6 background. The matrigel assay was performed as described (4), with minor modifications. Briefly, 0.5 ml of matrigel (Collaborative Biomedical Products, Bedford, MA) supplemented with 500 ng/ml basic fibroblast growth factor (bFGF) was injected s.c. into both flanks of twelve 6- to 8-week-old female wild-type C57BL/6 mice (The Jackson Laboratory) and of twelve 6- to 8-week-old male/female TSP-1-null C57BL/6 mice. Three of each of the null and wild-type mice were injected with matrigel alone. Mice undergoing treatment were randomized into three groups as follows: group I, saline i.p.; group II, 150 mg/kg CTX i.p. every other day for 5 days (which constitutes one cycle of MTD therapy); and group III, a low-dose metronomic regimen of ≈25 mg/kg CTX orally (p.o.) every day, administered through drinking water, as described (19). At day 10, all 30 mice were injected i.v. with 0.2 ml of 25 mg/ml FITC-dextran (Sigma). Plasma samples were collected, and matrigel plugs were photographed, incubated at 37°C overnight with dispase (Collaborative Research), and homogenized. Fluorescence readings were obtained by using a FL600 Fluorescence Plate Reader (Biotech Instruments, Winooski, Vermont), and angiogenic response was expressed as a ratio of matrigel plug fluorescence/plasma fluorescence.
In Vivo LL/2 Murine Tumor Growth Assessment in TSP-1-Null and Wild-Type Mice. Syngeneic LL/2 cells (0.5 × 106 per 0.2 ml) were injected s.c. into the flanks of 6- to 8-week-old female wild-type C57BL/6 mice and male and female TSP-1-null C57BL/6 mice. When tumors had grown to ≈200 mm3, mice were randomized into groups of five animals. Two independent experiments were performed for wild-type mice and one for TSP-1-null mice, each totaling 20 animals in four groups. The treatment was as follows: group I (control), 0.2 ml of saline i.p. every 3 days; group II, 150 mg/kg CTX i.p. every other day for 5 days (a cycle of MTD therapy), followed by oral administration of saline every day; and group III, 150 mg/kg CTX i.p. every other day for 5 days followed by continuous oral administration of a low-dose metronomic regimen of ≈25 mg/kg CTX every day, as described (19).
Assessment of Plasma TSP-1 Levels in PC-3 Human Prostate Cancer-Bearing Severe Combined Immunodeficient (SCID) Mice. PC-3 human prostate carcinoma cells (2 × 106 per 0.2 ml) were injected s.c. in the right flank of male CB-17 SCID (Charles River Breeding Laboratories). Mice were randomized into groups of five, and when the tumor volume reached 150–200 mm3, therapy was initiated as follows: (i) a low-dose metronomic schedule of continuous p.o. administration of ≈20 mg/kg/day CTX, (ii) a low-dose metronomic schedule of i.p. administration of 0.3 mg/kg per mouse VBL three times weekly, (iii) combination therapy with CTX and VBL, and (iv) consecutive and alternate therapy with CTX and VBL. Control mice were treated with saline o.s. and i.p. After 20 days of treatment, SCID mice were bled from the retroorbital sinus. Plasma samples were immediately frozen and stored at –70°C until assayed. All of the samples were thawed at 4°C only at the time of the TSP-1 competitive enzyme immunoassay. The results were expressed as a ratio between TSP-1 plasma concentration (ng/μl) and correspondent tumor volumes (mm3).
Statistical Analysis. Results are reported as means ± SD or SEM. Statistical significance of differences was assessed by ANOVA, followed by the Student–Newman–Keuls test, using prism (Version 3.0, GraphPad, San Diego). The level of significance was set at P < 0.05.
Results
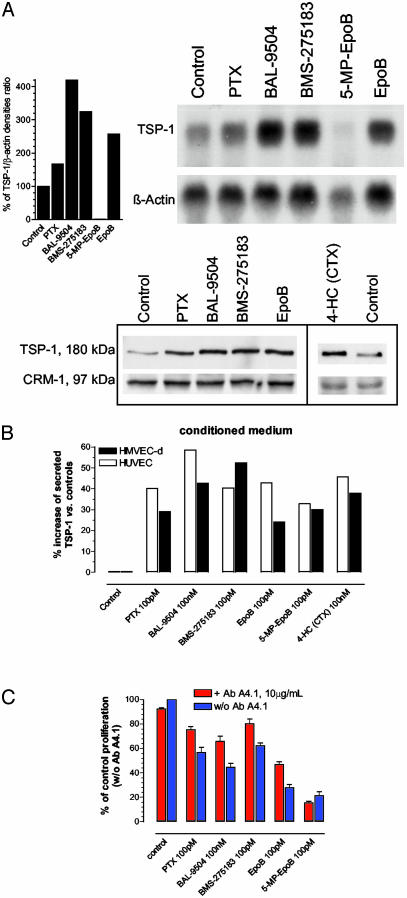
Protracted Low-Dose Drug Treatments in Vitro Increase Human TSP-1 Expression. Our recent results using in vitro, protracted (144 h) low-dose chemotherapy suggested a preferential antiproliferative effect on micro- and macrovascular endothelial cells (9). To investigate possible mechanisms underlying the specificity of this effect, vascular endothelial and human cancer cells were treated for 144 h with drugs successfully used in the previous studies at concentrations near IC50. For example, a cDNA microarray analysis was undertaken of microvascular endothelial cells exposed to the 144-h BAL-9504 treatment to screen and identify differentially expressed genes. This preliminary analysis identified a major change in TSP-1 expression (an increase of >2-fold) that was verified by Northern and Western blotting. An extensive analysis by Northern blotting revealed that human dermal microvascular endothelial (Fig. 1A Upper) and human umbilical vein endothelial cells (data not shown) highly expressed TSP-1 mRNA after protracted low-dose chemotherapy protocols (by 2- to 4-fold as quantified by densitometry) (Fig. 1A Upper Right). Microvascular endothelial cell lysates confirmed a higher expression of TSP-1 protein in treated samples (Fig. 1 A Lower) as well as human umbilical vein endothelial cells (data not shown). Furthermore, TSP-1 was found to be secreted into the medium at higher amounts in treated cultures when compared with controls (Fig. 1B) in both types of endothelial cells tested. To assess whether this secreted TSP-1 was responsible for the antiendothelial effects of the drugs tested by using protracted low-dose schedules, a neutralizing monoclonal antibody (A4.1) that inhibits the binding of TSP-1 to the CD36 receptor (23, 24) was added to the endothelial cultures. Microvascular endothelial cell inhibition was affected by the antibody, resulting in an increase in cell proliferation and survival of drug-treated cells (Fig. 1C), with the only exception being the compound 5-methylpyridine EpoB. Tumor cells (e.g., MDA-MB-435 and TO.1) expressed low levels of TSP-1 and showed only a slight up-regulation at mRNA and secreted protein levels (data not shown). In the aforementioned (and subsequent) experiments, BAL-9504 was used as a noncytotoxic drug control, which might be expected to induce TSP-1 expression given the fact that the activation of Ras-signaling pathways is often associated with down-regulation of TSP-1 (25) and that this geranylgeranyl transferase inhibitor might reverse such effects.
Fig. 1.
(A) Northern blot analysis with its densitometric quantification (Upper Right) and Western blot analysis (Lower Right) of human dermal microvascular endothelial cell samples after 6 days of low-dose treatment with different cytotoxic drugs. β-Actin and CRM-1 were used as loading controls. (B) Human TSP-1 competitive enzyme immunoassay of vascular endothelial cell-conditioned media after 6 days of treatment using low concentrations of different cytotoxic drugs. The results are expressed as the percentage increase of secreted TSP-1 versus control samples and are the mean of two independent experiments with at least two replicates per sample. (C) Effect of protracted (144 h) low-dose cytotoxic drug treatments, in the presence or absence of neutralizing monoclonal antibody A4.1 directed against human TSP-1, on in vitro microvascular endothelial cell proliferation. Shown are mean values ± SEM.
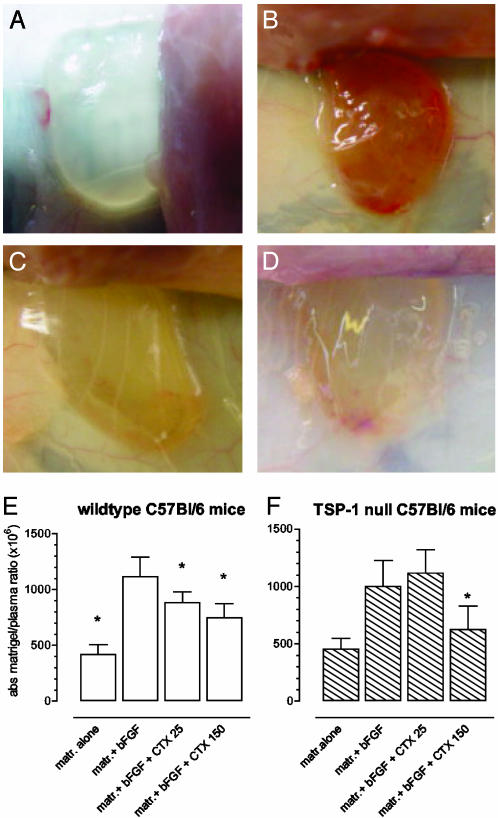
Effects of Chemotherapy Treatments on in Vivo Angiogenesis in TSP-1-Null and Wild-Type Mice. Based on the above in vitro experiments showing TSP-1 induction by protracted low-dose chemotherapeutic treatments, we went on to evaluate the antiangiogenic effects of previously characterized low-dose chemotherapy treatments in wild-type or TSP-1-deficient mice. The direct assessment of angiogenesis inhibition in vivo by using implanted matrigel pellets provided evidence for antiangiogenic activity of both traditional MTD and metronomic low-dose chemotherapeutic regimens, as shown by Browder et al. (3). Moreover, the use of TSP-1-deficient mice allowed us to quantitate and assess the role of this endogenous inhibitor in the antiangiogenic activity of both CTX regimens in a nontumor environment. Four animal groups were treated with a previously used metronomic daily low-dose therapeutic regimen (25 mg/kg CTX per day p.o.) or a MTD schedule of the same drug (150 mg/kg CTX i.p. every other day for 6 days), and the angiogenic activity in s.c. implanted matrigel pellets was quantitatively assessed by measuring the fluorescence of FITC-dextran in wild-type and TSP-1-null mice (Fig. 2 E and F, respectively). Matrigel alone did not induce angiogenesis within the pellet (negative control, Fig. 2 A and E), whereas a conspicuously robust neovascular pattern was induced by adding bFGF (positive control, Fig. 2 B and E). Despite the relatively short period of treatment (7 days), the low-dose CTX regimen significantly inhibited bFGF-induced neovascularization in TSP-1 wild-type mice (Fig. 2 C and E), as did the MTD CTX, as expected, presumably because of its ability to act directly on proliferating and migrating endothelial cells. In sharp contrast, the low-dose CTX regimen was completely ineffective in blocking angiogenesis in TSP-1-null mice (Fig. 2F) when compared with positive control. By contrast, the MTD CTX regimen maintained its antiangiogenic efficacy (Fig. 2F).
Fig. 2.
Inhibition of angiogenesis in vivo by daily low-dose (≈25 mg/kg p.o.) and MTD CTX (150 mg/kg i.v.) in wild-type and TSP-1-null C57BL/6 mice. Angiogenesis was induced in s.c. implanted matrigel (matr.) plugs by bFGF. After 10 days of treatment, mice were injected i.v. with FITC-dextran (4). Macroscopic appearance of representative sample of matrigel alone (negative control) (A), matrigel plus bFGF (positive control) (B), matrigel plus bFGF plus low-dose CTX (C), and matrigel plus bFGF plus MTD CTX (D). Quantification of intravascular FITC content showed a significant inhibition of neovascularization in wild-type mice treated with a low-dose CTX regimen (E) but a complete lack of antiangiogenic activity of the same regimen (CTX 25) in TSP-1-null mice, whereas MTD CTX (CTX 150) retained activity in these mice (F). Shown are mean values ± SD (*, P < 0.05).
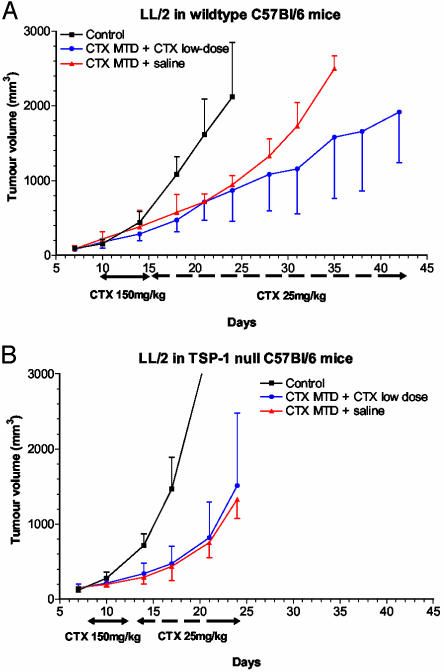
Effects of MTD Versus Metronomic Chemotherapy on in Vivo Murine LL/2 Tumor Growth in TSP-1-Null and Wild-Type Mice. We next tested and compared the effects of MTD versus metronomic low-dose CTX regimens on the growth of LL/2 in syngeneic TSP-1-deficient and wild-type mice. As expected, the growth pattern of tumor cells was different in the two strains of mice: LL/2 tumors grew faster in TSP-1-null mice when compared with the wild-type controls, consistent with published studies (12–14). After one upfront cycle of MTD CTX to slow the growth of these very rapidly growing tumors (and to use as an internal control for the subsequent metronomic low-dose schedule of the same drug in the same mice), the mice were switched to the oral low-dose regimen administered through drinking water or normal saline. In the wild-type mice, the brief MTD regimen followed by normal saline had an initial antitumor effect, compared with the untreated controls, but tumors started to grow exponentially after switching to normal saline, whereas the MTD schedule followed by the low-dose regimen slowed tumor growth (Fig. 3A). In sharp contrast, the MTD-CTX-plus-saline and the MTD-plus-low-dose-treatment groups both showed an initial response but with a complete overlapping profile of tumor growth in the TSP-1-null mice (Fig. 3B). These results clearly showed that the effects of oral low-dose metronomic CTX, but not those of MTD CTX, were blocked in TSP-1-null mice (Fig. 3B). Thus, it appears that the efficacy of the metronomic part of the therapy is lost in the absence of host TSP-1, in contrast to the MTD of the same drug, and that the MTD CTX was the sole cause of suppressed tumor growth in the treated TSP-1-null mice.
Fig. 3.
Effects of MTD CTX (150 mg/kg i.v.), which was then switched to low-dose CTX (25 mg/kg p.o.) or normal saline on LL/2 tumor in syngeneic C57BL/6 mice. The two regimens were compared in wild-type (A) and TSP-1-null (B) mice. Results showed a complete loss of antitumor activity after a continuous low-dose CTX in TSP-1-null C57BL/6 mice (B) compared with wild-type controls (A), whereas the MTD regimen suppressed tumor growth to an equivalent extent in both types of mice.
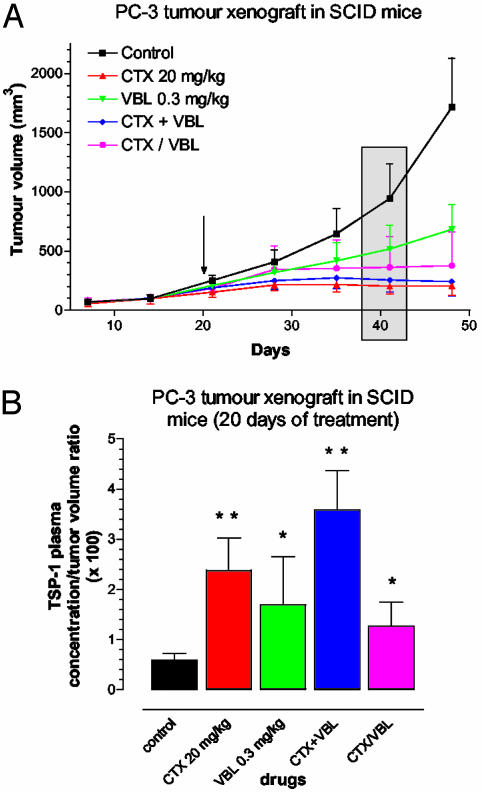
TSP-1 Plasma Levels Are Increased in PC-3 Tumor-Bearing Mice Treated with Low-Dose Metronomic Chemotherapy Regimens. In an attempt to correlate previously established metronomic chemotherapy treatments with systemic TSP-1 expression in vivo (4, 19), we undertook measurements of plasma TSP-1 levels in human tumor-bearing SCID mice exposed to various and known-to-be-effective continuous low-dose chemotherapy protocols. Such experiments could also provide an indication of the possibility of exploiting circulating TSP-1 levels as a surrogate marker for metronomic chemotherapy regimens. Around day 20 of treatment (Fig. 4A, gray rectangle), all of the regimens showed the anticipated antitumor effect versus the control group, particularly the low-dose CTX and CTX-plus-VBL combined schedules. Moreover, the corresponding plasma TSP-1/tumor volume ratio, at the same day of treatment, significantly increased by 2- to 6-fold (Fig. 4B). The significant increase in TSP-1 concentration, normalized per tumor volume, in responder mice suggests a strong and (given our aforementioned results) possibly causative relationship between this endogenous inhibitor of angiogenesis and the antitumor effects of metronomic chemotherapy treatments on PC-3 xenografts, similar to the results in the syngeneic C57BL/6 mice.
Fig. 4.
(A) Effect of two different metronomic chemotherapy regimens (continuous administration of CTX 20 mg/kg p.o. or VBL 0.3 mg/kg administered three times a week) and their combination at the same doses (CTX + VBL) or consecutively and alternatively (CTX/VBL) in human PC-3 tumor xenografts. At day 20, treatment was started (arrow), and at days 40 and 41, mice were bled from the retroorbital sinus, and tumor volumes were measured, respectively (gray rectangle). (B) The TSP-1-competitive enzyme immunoassay was performed on those blood samples, and the results are given as the ratio of TSP-1 plasma concentration to tumor volume. The ratio significantly increased in all treated groups and strongly correlated with the response to the low-dose metronomic chemotherapy regimens. Shown are mean values ± SD (*, P < 0.05; **, P < 0.005).
Discussion
Our results, in addition to establishing a link between the endogenous inhibitor of angiogenesis, TSP-1, and frequent low-dose metronomic chemotherapy, provide an explanation for the relative lack of traditional side effects, or a theoretically expected increase in such effects, normally associated with MTD chemotherapy when using metronomic regimens of the same drug, because induction of TSP-1 would not be expected to have harmful effects on CD36-negative cells such as bone marrow progenitors, hair follicle cells, etc. Previously, it was assumed that activated endothelial cells of tumor vessels were directly killed by metronomic chemotherapy regimens (3, 4). However, although this may be the case when using standard MTDs of chemotherapy, our results suggest the possibility of a secondary, i.e., indirect, mechanism that is highly specific to endothelial cells, namely, TSP-1-mediated growth arrest and apoptosis, when low-dose nontoxic metronomic schedules are used. Five lines of evidence were obtained to support this conclusion, the most important and striking of which was the virtually complete loss of antiangiogenic and antitumor activity when using daily low-dose cyclophosphamide administered through drinking water in C57BL/6 TSP-1–/– mice, in contrast to the retention of these activities when using the MTD of the same drug. These in vivo observations might seem to be inconsistent with the modest activity of the anti-TSP-1 antibody in blocking the antiproliferative effects of protracted exposure of endothelial cells to various low-dose chemotherapeutic drugs in vitro. However, this antibody exerts its activity by interfering with the binding of TSP-1 to CD36 receptors, and it is known that endothelial cells variably express CD36 and can even lose CD36 expression on short-term culture (17, 24, 26). Indeed, some of the blocking effects of TSP-1 on endothelial cell migration and proliferation, as well as some of the proapoptotic effects, may be mediated in a CD36-independent manner (12–14).
An important point raised by our results is the signaling mechanism by which TSP-1 is induced by the various low-dose drug treatments. In this regard, it is well known that treatment with chemotherapeutic drugs can trigger signal transduction pathways, such as stress-activated kinases, leading to the induction of mediators of cell-cycle arrest and apoptosis (including p53) and many other genes/proteins (27, 28). A recent gene-profiling study by Yoo et al. (29) in which cDNA microarrays showed that docetaxel (taxotere) induced extensive changes in global gene expression in treated head and neck squamous-cell carcinoma; interestingly, one of these changes was induction of TSP-1. In this regard, induction/up-regulation of the p53 suppressor gene by DNA-damaging activities of CTX may in turn cause enhanced expression of TSP-1, because p53 is known to induce TSP-1 expression (30).
Another issue our results raise is how extensive the repertoire of cells is that show TSP-1 induction in response to metronomic low-dose chemotherapy. Is this phenomenon largely restricted to endothelial cells or can many other cells, including tumor cells, respond in this manner? The increase in circulating TSP-1 detected in the plasma of metronomic chemotherapy-treated mice we detected would suggest that other cell types besides endothelial cells may also show TSP-1 induction, although as yet we have no evidence for this possibility. Also important to consider is the question of whether all of the antiangiogenic effects of low-dose CTX are mediated by TSP-1 or whether they are the product of an interaction between low-grade direct effects of CTX on endothelial cells and TSP-1 induction.
An additional way in which low-dose chemotherapy regimens could induce an antiangiogenic effect is by decreasing the mobilization and/or viability of circulating bone marrow-derived endothelial precursor cells (CEPs), which can contribute to tumor angiogenesis (31, 32), similar to certain other antiangiogenic agents, including endogenous inhibitors such as endostatin and angiostatin (33, 34). It is conceivable that such an effect may also be mediated by increases in circulating TSP-1. Bertolini et al. (35) have recently obtained evidence that metronomic low-dose CTX regimens can cause a sustained drop in the levels and viability of CEPs, whereas MTD regimens of the same drug did not do so and in fact were associated with a rapid rebound of such cells shortly after completion of a MTD therapy cycle. This result may help explain the robust repair to damaged tumor endothelia during the long breaks between successive cycles of MTD CTX therapy but not during metronomic dosing and scheduling of the same drug (3).
Finally, our results raise the possibility that in addition to low-dose chemotherapy, some other types of drugs that inhibit angiogenesis may conceivably operate through a similar type of mechanism, especially when used in a more frequent schedule of administration involving relatively low doses, such as IFN-α (36, 37). Thus, the geranylgeranyl transferase inhibitor we tested, BAL-9504, was found to induce TSP-1 expression in vitro in endothelial cells after a protracted low-dose regimen was used. This result may be related to the fact that activation of Ras-related signaling pathways can decrease TSP-1 expression (25). Hence, drugs that target elements of such pathways may cause an increase in TSP-1 expression. Of interest as well would be to test the effects of antiangiogenic TSP-1 peptides (38) in conjunction with metronomic chemotherapy regimens, especially after such metronomic regimens begin to lose their efficacy and relapses become apparent (19).
In summary, our results highlight a previously undescribed function for an endogenous inhibitor of angiogenesis, TSP-1. It will be of interest to determine whether other drugs that inhibit angiogenesis mediate their effects through a similar type of mechanism and, if so, whether induction of alternative endogenous inhibitors of angiogenesis such as IFN-α, angiostatin, etc. might be involved in mediating antiangiogenic effects in a manner similar to TSP-1. In addition, our results suggest that it might be possible to exploit circulating TSP-1 levels in plasma as surrogate markers to guide optimal dosing for metronomic chemotherapy regimens as well as to monitor the activity of such therapies.
Acknowledgments
We thank Cassandra Cheng for excellent secretarial assistance, Dr. Alicia Viloria-Petit for critical review of the manuscript, and Crocetta Accardi, Karoline Fisher, and Jennifer Donabie-Haines of Comparative Research for excellent technical assistance. G.B. was supported by the Sunnybrook Trust for Medical Research, and R.S.K. holds a Tier I Canada Research Chair. This work was supported by National Institutes of Health Grants CA41223 (to R.S.K.), HL68003, and CA92644 (to J.L.) and grants from the Canadian Institutes for Health Research and the National Cancer Institute of Canada (to R.S.K.).
Abbreviations: bFGF, basic fibroblast growth factor; CTX, cyclophosphamide; EpoB, epothilone B; LL/2, Lewis lung carcinoma; MTD, maximum tolerated dose; p.o., orally; SCID, severe combined immunodeficient; TSP-1, thrombospondin 1; VBL, vinblastine sulfate.
References
- 1.Miller, K. D., Sweeney, C. J. & Sledge, G. W., Jr. (2001) J. Clin. Oncol. 19 1195–1206. [DOI] [PubMed] [Google Scholar]
- 2.Eberhard, A., Kahlert, S., Goede, V., Hemmerlein, B., Plate, K. H. & Augustin, H. G. (2000) Cancer Res. 60 1388–1393. [PubMed] [Google Scholar]
- 3.Browder, T., Butterfield, C. E., Kraling, B. M., Marshall, B., O'Reilly, M. S. & Folkman, J. (2000) Cancer Res. 60 1878–1886. [PubMed] [Google Scholar]
- 4.Klement, G., Baruchel, S., Rak, J., Man, S., Clark, K., Hicklin, D., Bohlen, P. & Kerbel, R. S. (2000) J. Clin. Invest. 105 R15–R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerbel, R. S. (1991) BioEssays 13 31–36. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan, D., Bergers, G. & Bergsland, E. (2000) J. Clin. Invest. 105 1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colleoni, M., Rocca, A., Sandri, M. T., Zorzino, L., Masci, G., Nole, F., Peruzzotti, G., Robertson, C., Orlando, L., Cinieri, S., et al. (2002) Ann. Oncol. 13 73–80. [DOI] [PubMed] [Google Scholar]
- 8.Vacca, A., Iurlaro, M., Ribatti, D., Minischetti, M., Nico, B., Ria, R., Pellegrino, A. & Dammacco, F. (1999) Blood 94 4143–4155. [PubMed] [Google Scholar]
- 9.Bocci, G., Nicolaou, K. C. & Kerbel, R. S. (2002) Cancer Res. 62 6938–6943. [PubMed] [Google Scholar]
- 10.Wang, J., Lou, P., Lesniewski, R. & Henkin, J. (2003) Anticancer Drugs 14 13–19. [DOI] [PubMed] [Google Scholar]
- 11.Grant, D. S., Williams, T. L., Zahaczewsky, M. & Dicker, A. P. (2003) Int. J. Cancer 104 121–129. [DOI] [PubMed] [Google Scholar]
- 12.Chen, H., Herndon, M. E. & Lawler, J. (2000) Matrix Biol. 19 597–614. [DOI] [PubMed] [Google Scholar]
- 13.de Fraipont, F., Nicholson, A. C., Feige, J. J. & Van Meir, E. G. (2001) Trends Mol. Med. 7 401–407. [DOI] [PubMed] [Google Scholar]
- 14.Lawler, J. (2002) J. Cell. Mol. Med. 6 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff, J. E., Molenkamp, G., Hotfilder, M. & Laterra, J. (1997) Klin. Padiatr. 209 275–277. [DOI] [PubMed] [Google Scholar]
- 16.Guo, N., Krutzsch, H. C., Inman, J. K. & Roberts, D. D. (1997) Cancer Res. 57 1735–1742. [PubMed] [Google Scholar]
- 17.Dawson, D. W., Pearce, S. F., Zhong, R., Silverstein, R. L., Frazier, W. A. & Bouck, N. P. (1997) J. Cell Biol. 138 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawler, J., Sunday, M., Thibert, V., Duquette, M., George, E. L., Rayburn, H. & Hynes, R. O. (1998) J. Clin. Invest. 101 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man, S., Bocci, G., Francia, G., Green, S., Jothy, S., Bergers, G., Hanahan, D., Bohlen, P., Hicklin, D. J. & Kerbel, R. S. (2002) Cancer Res. 62 2731–2735. [PubMed] [Google Scholar]
- 20.Macchia, M., Jannitti, N., Gervasi, G. & Danesi, R. (1996) J. Med. Chem. 39 1352–1356. [DOI] [PubMed] [Google Scholar]
- 21.Francia, G., Mitchell, S. D., Moss, S. E., Hanby, A. M., Marshall, J. F. & Hart, I. R. (1996) Cancer Res. 56 3855–3858. [PubMed] [Google Scholar]
- 22.Rak, J., Mitsuhashi, Y., Sheehan, C., Tamir, A., Viloria-Petit, A. M., Filmus, J., Mansour, S. J., Ahn, N. G. & Kerbel, R. S. (2000) Cancer Res. 60 490–498. [PubMed] [Google Scholar]
- 23.Volpert, O. V., Lawler, J. & Bouck, N. P. (1998) Proc. Natl. Acad. Sci. USA 95 6343–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez, B., Volpert, O. V., Crawford, S. E., Febbraio, M., Silverstein, R. L. & Bouck, N. (2000) Nat. Med. 6 41–48. [DOI] [PubMed] [Google Scholar]
- 25.Watnick, R. S., Cheng, Y. N., Rangarajan, A., Ince, T. A. & Weinberg, R. A. (2003) Cancer Cell 3 219–231. [DOI] [PubMed] [Google Scholar]
- 26.Swerlick, R. A., Lee, K. H., Wick, T. M. & Lawley, T. J. (1992) J. Immunol. 148 78–83. [PubMed] [Google Scholar]
- 27.Stadheim, T. A., Xiao, H. & Eastman, A. (2001) Cancer Res. 61 1533–1540. [PubMed] [Google Scholar]
- 28.Boldt, S., Weidle, U. H. & Kolch, W. (2002) Carcinogenesis 23 1831–1838. [DOI] [PubMed] [Google Scholar]
- 29.Yoo, G. H., Piechocki, M. P., Ensley, J. F., Nguyen, T., Oliver, J., Meng, H., Kewson, D., Shibuya, T. Y., Lonardo, F. & Tainsky, M. A. (2002) Clin. Cancer Res. 8 3910–3921. [PubMed] [Google Scholar]
- 30.Dameron, K. M., Volpert, O. V., Tainsky, M. A. & Bouck, N. (1994) Science 265 1582–1584. [DOI] [PubMed] [Google Scholar]
- 31.Lyden, D., Hattori, K., Dias, S., Costa, C., Blaikie, P., Butros, L., Chadburn, A., Heissig, B., Marks, W., Witte, L., et al. (2001) Nat. Med. 7 1194–1201. [DOI] [PubMed] [Google Scholar]
- 32.Monestiroli, S., Mancuso, P., Burlini, A., Pruneri, G., Dell'Agnola, C., Gobbi, A., Martinelli, G. & Bertolini, F. (2001) Cancer Res. 61 4341–4344. [PubMed] [Google Scholar]
- 33.Ito, H., Rovira, I. I., Bloom, M. L., Takeda, K., Ferrans, V. J., Quyyumi, A. A. & Finkel, T. (1999) Cancer Res. 59 5875–5877. [PubMed] [Google Scholar]
- 34.Capillo, M., Mancuso, P., Gobbi, A., Monestiroli, S., Pruneri, G., Dell'Agnola, C., Martinelli, G., Shultz, L. & Bertolini, F. (2003) Clin. Cancer Res. 9 377–382. [PubMed] [Google Scholar]
- 35.Bertolini, F., Paul, S., Mancuso, P., Monestiroli, S., Gobbi, A., Shaked, Y. & Kerbel, R. S. (2003) Cancer Res. 63 4342–4346. [PubMed] [Google Scholar]
- 36.Ezekowitz, R. A., Mulliken, J. B. & Folkman, J. (1992) N. Engl. J. Med. 326 1456–1463. [DOI] [PubMed] [Google Scholar]
- 37.Slaton, J. W., Perrotte, P., Inoue, K., Dinney, C. P. & Fidler, I. J. (1999) Clin. Cancer Res. 5 2726–2734. [PubMed] [Google Scholar]
- 38.Reiher, F. K., Volpert, O. V., Jimenez, B., Crawford, S. E., Dinney, C. P., Henkin, J., Haviv, F., Bouck, N. P. & Campbell, S. C. (2002) Int. J. Cancer 98 682–689. [DOI] [PubMed] [Google Scholar]