Abstract
Cytokines and inflammation have been implicated in the pathogenesis of heart failure. For example, IL-6 family cytokines and the gp130 receptor play important roles in cardiac myocyte survival and hypertrophy. Signal transducer and activator of transcription 3 (STAT3) is a major signaling protein that is activated through gp130. We have created mice with a cardiomyocyte-restricted deletion of STAT3. As measured by serial echocardiograms, mice with cardiac specific deletion of STAT3 are significantly more susceptible to cardiac injury after doxorubicin treatment than age-matched controls. Intriguingly, STAT3 appears to have a critical role in protection of inflammation-induced heart damage. STAT3-deficient mice treated with lipopolysaccharide demonstrated significantly more apoptosis than their WT counterparts. At the cellular level, cardiomyocytes with STAT3 deleted secrete significantly more tumor necrosis factor α in response to lipopolysaccharide than those with WT STAT3. Furthermore, histologic examination of the cardiomyocyte-restricted STAT3-deficient mice reveals a dramatic increase in cardiac fibrosis in aged mice. Although no overt signs of heart failure are present in young STAT3-deficient mice, they spontaneously develop heart dysfunction with advancing age. These results indicate the crucial functions of STAT3 in cardiomyocyte resistance to inflammation and other acute injury and in pathogenesis of age-related heart failure.
Inflammatory cytokines have been proposed to play an important role in the genesis and progression of many types of heart failure. The recent vesnorinone trial demonstrated a clear inverse relationship between circulating levels of IL-6 and cumulative survival in patients with advanced heart failure (1). However, neither a causal relationship between inflammatory cytokines and clinical progression of heart failure nor the molecular pathogenesis caused by inflammatory cytokines in cardiac tissue has been established.
The receptor for IL-6 is formed by a heterodimer between the ligand binding subunit and a signal transduction subunit, gp130. Signaling through this protein can be activated by other cytokines whose ligand binding subunits also form heterodimers with gp130 (2). Interruption of gp130 signaling in pan-knockout (all cell types affected) mice results in embryonic lethality with multiple organ defects, including thin-walled and dilated cardiac ventricles (3). Although mice with cardiac myocyte-restricted deletion of gp130 have apparently normal hearts at 2 months of age, after proximal aortic banding, these animals fail to develop compensatory hypertrophy and rapidly progress to cardiac dilation and failure. Histologic examination of these hearts shows massive cardiac myocyte apoptosis (4). These findings support the idea that gp130 signaling is critical for both the hypertrophic stress response of cardiac myocytes to pressure overload and the prevention of heart failure secondary to apoptosis.
Activation of gp130 leads to downstream activation of at least three different signaling pathways, the Ras mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol 3-kinase-dependent pathway, and the Janus kinase–signal transducer and activator of transcription (STAT) pathway (5). Several studies have implicated that one member of the STAT family of transcription factors, STAT3, is essential for gp130-mediated hypertrophy and cytoprotection in the heart. STAT3 is activated under a variety of stress conditions, such as pressure overload, hypoxia, and acute myocardial infarction (6–8). The expression of several genes involved in hypertrophy (e.g., c-fos, ANP), cytoprotection (Bcl-xL, MnSOD), and angiogenesis (vascular endothelial growth factor) are also regulated by STAT3 (9–12). The observation that transgenic mice with cardiac-specific overexpression of STAT3 show not only myocardial hypertrophy but are also protected against doxorubicin-induced apoptosis provides evidence that STAT3 can protect the heart from stress-induced injury in vivo (13). Other investigators have shown, using cultured cardiomyocytes, that blockade of MAPK activation can prevent the antiapoptotic effect of gp130 activation in response to cardiotrophin-1 (14). Knockout of individual MAPKs does not produce a known cardiac defect in mice, likely because of redundancies in the MAPK signaling system. Therefore, assessing the relative importance of MAPKs by using this strategy is difficult.
Conventional knockout of the STAT3 gene leads to embryonic lethality at embryonic day 6.5 (15). Therefore, we chose to use the Cre-loxP system to ablate STAT3 specifically in cardiac myocytes. This strategy proved successful in allowing embryonic survival of animals whose cardiomyocytes do not express STAT3. Here, we report the characterization of these animals. We found that cardiac-restricted deletion of STAT3 leads to increased susceptibility to doxorubicin-induced heart failure. Treatment with the classic proinflammatory molecule lipopolysaccharide (LPS) resulted in increased apoptosis and elevated cardiomyocyte elaboration of tumor necrosis factor α (TNF-α). Histologic examination of failing STAT3 knockout mice demonstrates marked fibrosis and increased apoptosis, possibly as a result of an enhanced inflammatory response to this stress. Finally, these mice spontaneously develop cardiac dysfunction with age in the absence of other added stressors. These findings suggest that STAT3 can regulate inflammatory responses of cardiomyocytes and is an important factor for protection and survival of cardiomyocytes in vivo.
Methods
Generation Mice with Cardiac-Specific Deletion of STAT3. Mice homozygous for a STAT3 allele with two loxP sites in introns 17 and 20 were crossed with heterozygous mice that express α-myosin heavy chain (α-MHC)-Cre transgene (16). Three different primer pairs were routinely used to detect the STAT3flox and STAT3D alleles and the inserted Cre gene by PCR analysis. The STAT3flox allele was detected by using primers 1 (5′-ATT GGA ACC TGG GAC CAA GTG G) and 2 (5′-ACA TGT ACT TAC AGG GTG TGT GC), which amplified a 520-bp fragment, whereas the STAT3 WT allele gave a 480-bp fragment. The STAT3D allele was detected as a 480-bp fragment with primers 1 and 3 (5′-GCT GGC TCA TAG GCA AAA ACA C). The α-MHC-Cre transgene was detected by using the primers 5′-CCA GCT AAA CAT GCT TCA TCG TCG TC and 5′-ATT CTC CCA CCG TCA GTA CGT GAG, which amplified a 300-bp fragment.
Cell Extraction and Immunoblotting. Tissues were homogenized in whole-cell extraction buffer [400 mM KC1/10 mM NaHPO4/1 mM EDTA/1 mM DTT/10% (wt/vol) glycerol/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml pepstatin/1 mM PMSF/5 mM NaF/1 mM Na3VO4]. After centrifugation at 14,000 rpm, the supernatant was collected, and protein concentration was quantified (BCA kit, Pierce). Twenty micrograms of protein was separated by SDS/PAGE and then transferred to Immuno-Blot poly(vinylidene difluoride) membrane (Bio-Rad). After blocking with 5% nonfat milk in washing buffer [10 mM Tris·H·HC1/150 mM NaC1/1 mM EDTA/0.1% (wt/vol) Triton X-100, pH 8.0], the membranes were soaked in α-STAT3 antibody (C20; Santa Cruz Biotechnology), followed by soaking in anti-rabbit IgG coupled with horseradish peroxidase and visualized by using SuperSignal Chemiluminescent Substrate (Pierce).
Echocardiographic Assessment. All animals were treated in accordance with the institutional animal care guidelines of Yale Medical School. Animals were lightly anesthetized with inhaled isofluorane. 2D M-mode echocardiography was performed by using a Hewlett–Packard model Sonos 5500 with a 12-MHz probe. The short axis measurements were taken at the level of the midpapillary muscle. The long axis measurements were made in a plane containing the aortic and mitral valves. Three measurements were taken at end-systole and end-diastole in both views. These measurements were averaged to calculate an overall fractional shortening.
Histological Examination. Hearts were rapidly excised from fully anesthetized mice at the indicated ages and washed in PBS. The hearts were fixed in 4% paraformaldehyde at 4°C for 24 h and embedded in paraffin. Sections were stained with hematoxylin/eosin. Masson's trichrome staining was used to detect fibrosis in heart sections. In situ labeling of apoptotic cells was performed on tissue sections by using the ApopTag Peroxidase Kit (Intergen, Purchase, NY), according to the manufacturer's instruction.
Cardiomyocyte Culture. Cardiomyocytes of neonatal mouse were prepared and cultured as described (17) with some modifications. Dissociated cardiac cells were plated on a tissue culture dish for 2 h until most of the fibroblasts were attached on the dish. Floating cells (cardiomyocytes) were collected and plated at a density of 2 × 106 cells per ml in a 48-well tissue culture dish coated with 1% gelatin.
LPS Treatment of Cardiac-Specific STAT3 Knockout Mice and Cardiomycytes and TNF-α Measurement. Adult mice (>8 weeks old) were treated with a single dose of 5 mg/kg LPS, i.p. Cardiomyocytes were plated on a culture dish for 36 h, and then the medium was changed to serum-free M199 media. After 12 h, cells were treated with LPS (Sigma L8274, 100 μg/ml in M199). The culture media from treated or control cells were collected after 2, 6, 12, and 24 h. Concentrations of TNF-α were measured with an R & D Systems mouse TNF-α DuoSet kit.
Results
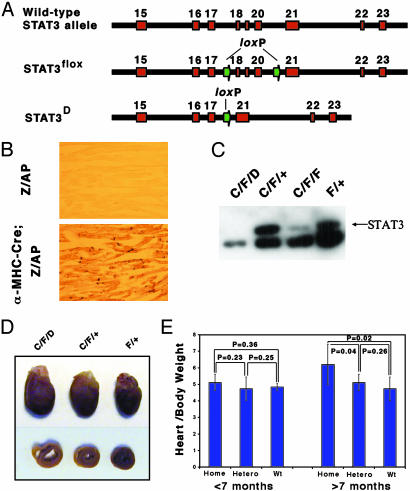
Generation of Mice Lacking STAT3 Expression in Cardiac Myocytes. Mice lacking STAT3 expression in all tissues die early during embryonic development (15). To study the biological function of STAT3 in the heart we generated cardiomyocyte-specific STAT3 knockout mice. For this purpose we first created a conditional STAT3 gene allele (STAT3flox) in embryonic stem cells containing three loxP sequences by gene targeting (Fig. 1). Two loxP sequences flank exons 18, 19, and 20 of the murine STAT3 allele. Because exons 19 and 20 harbor the coding region for the Src homology 2 domain, Cre recombinase activity produces mutant STAT3 protein lacking this essential domain for STAT3 function (Fig. 1 A, STAT3D). Details of the generation of STAT3 flox mice will be described elsewhere (G.-X.C., Y.I., and X.-Y.F., unpublished work).
Fig. 1.
Generation of cardiomyocyte-specific STAT3-deficient mice. (A) Schematic representation of STAT3 alleles. (B) Detection of Cre recombinase in cardiomyocytes using Z/AP double reporter mice (see text for details). (C) Ablation of STAT3 protein in cardiac myocytes. Twenty micrograms of protein extract was used for Western blot analysis. (D) Dilated hearts in cardiac-specific STAT3 knockout mice. The hearts from 9-month-old WT (+/+), heterozygous (C/F/+), and STAT3-deficient mice (C/F/D) were excised (Upper). Cross sections through the same hearts are shown (Lower). (E) Heart (g)/body (kg) weight ratio was calculated in all genotypes mice grouped by age (<7 months and ≥7 months) and t test was performed. Difference was shown between the homozygous and heterozygous (P = 0.04) and homozygous and WT (P = 0.02) mice older than 7 months.
To obtain cardiac-specific deletion of STAT3 in mice we used transgenic mice expressing Cre recombinase under the control of the α-MHC promoter (16). To confirm the cardiac-specific expression we first crossed these mice with Z/alkaline phosphatase (AP) double reporter mice (18). In Z/AP mice β-galactosidase is expressed ubiquitously under the control of the chicken β-actin promoter. The transgene is flanked by loxP sites and followed by a sequence encoding human AP. AP is not expressed until after Cre recombinase-mediated excision of the lacZ gene. This system allows for the detection of Cre recombinase activity in tissue samples by using AP staining. Heart sections for Z/AP α-MHC-Cre mice showed a strong expression of human AP in cardiac myocytes (≈90%), whereas in Z/AP mice without the α-MHC-Cre transgene human AP expression could not be detected (Fig. 1B).
Next, α-MHC-Cre transgenic mice were crossed with heterozygous STAT3D/+ (D/+) or STAT3flox/+ (C/F/+) mice to generate Cre+ STAT3D/+ (C/D/+) or Cre+ STAT3flox/+ (C/F/+) mice. The mice were bred with homozygous STAT3flox/flox (F/F) mice to generate mice with cardiac-specific STAT3 deletion [Cre+ STAT3flox/D (C/F/D) and Cre+ STAT3flox/flox (C/F/F), respectively]. To provide biochemical evidence that STAT3 expression is indeed abolished in the heart we performed Western blot analysis. As shown in Fig. 1C, STAT3 expression was almost not detectable in the heart from STAT3 knockout (C/F/D) mice, whereas WT STAT3+/+ and heterozygous Cre+ STAT3flox/+ (C/F/+) mice retained similar levels of STAT3 protein. The weak band observed in the protein extract from a Cre+ STAT3flox/flox (C/F/F) mouse is likely caused by contamination with noncardiomyocytes, such as blood cells or endothelial cells. To exclude the possibility that Cre+ STAT3flox/flox (C/F/F) mice may have an incomplete deletion of STAT3 in cardiomyocytes we used Cre+ STAT3flox/D (C/F/D) knockout mice throughout the study.
C/F/D mice were born with the expected Mendelian frequency. Because the α-MHC gene is expressed mainly after birth and only weakly during embryogenesis we cannot exclude that STAT3 may have a critical function in the early development of the heart. All mice reached adulthood and showed no obvious phenotype up to 6 months of age. However, mice older than 6 months gradually demonstrated signs of heart failure (including development of ascites, dyspnea, and cachexia). In these mice the heart was enlarged, primarily because of dilation. A typical example is shown in Fig. 1 D and E.
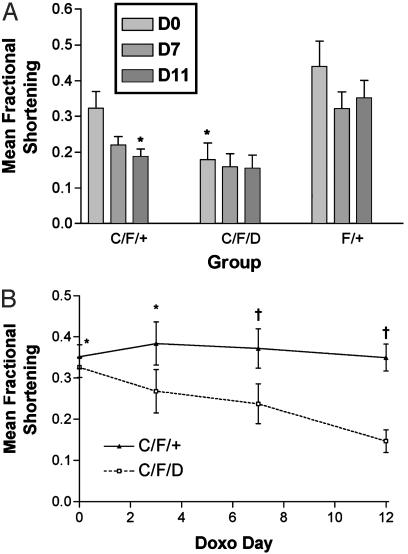
Effect of Doxorubicin Treatment on Mice Lacking Cardiac STAT3 Expression. To evaluate whether STAT3 signaling is important for protection of cardiomyocytes against proapoptotic injury, we treated animals with doxorubicin and followed their cardiac function with serial echocardiograms. Mice (9 months of age) received a single dose of 10 mg/kg i.p. on day 0. Those animals showing significant signs of morbidity were killed. Fig. 2A shows the results from a group of mice we analyzed. There was a significant difference in the baseline function of the knockout animals compared with the controls. The heterozygotes (C/F/+) tended to have baseline function that was worse than the WT (F/+) controls, but better than the knockouts (C/F/D), although this difference might not achieve statistical significance because of the insufficient number of animals we had. Although all three groups showed a decline in function over time after the doxorubicin treatment, the most significant decline was observed in the heterozygotes. We reasoned that it might be difficult to detect a decline in function in the knockout animals at this age (9 months) because of their significant baseline dysfunction. In fact, of the 17 animals treated, 3 either expired spontaneously or were killed because of signs of significant congestive heart failure (ascites and respiratory distress). Of these three, two were knockout animals and one was a heterozygote. To better address whether the knockouts are more susceptible to doxorubicin-induced heart failure, we therefore chose younger knockouts and heterozygotes (aged 6–7 months) with similar baseline function and repeated the experiment. As shown in Fig. 2B, the knockout animals showed a much more significant decrease in cardiac systolic function after doxorubicin treatment (P < 0.01 for C/F/+ vs. C/F/D at days 7 and 12). This finding supports the hypothesis that STAT3 provides an important function in protection of cardiomyocytes against doxorubicin-induced injury.
Fig. 2.
Effect of doxorubicin treatment on mice lacking STAT3 expression in cardiomyocytes. (A) Left ventricular fractional shortening was measured as described at baseline (D0) and on days 7 (D7) and 11 (D11) after doxorubicin treatment. Summary of mean fractional shortening is by group. *, P < 0.05 vs. F+ on D0. (B) Analysis of fractional shortening in knockout (C/F/D) and heterozygote (C/F/+) animals initially matched for left ventricular function over a 12-day time course after injection of doxorubicin. *, P = not significant for C/F/D vs. C/F/+ on days 0 and 3; †, P < 0.01 for C/F/D vs. C/F/+ on days 7 and 12.
Mice Lacking Cardiac STAT3 Expression Show Significantly More Myocyte Apoptosis than Controls in Response to LPS. Inflammation has recently emerged as a major factor in the development and progression of heart failure. LPS (endotoxin) is an important factor causing myocardial dysfunction during inflammation and sepsis. We next examined the effect of LPS treatment on cardiomyocyte survival.
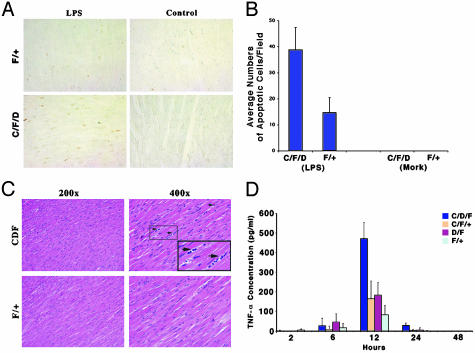
Mice were treated with LPS, and cardiac tissues were harvested 39 h later. Hearts from these mice demonstrated significantly more apoptosis than those from controls in response to LPS (Fig. 3A). No terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling-positive nuclei were seen in hearts from either controls or knockouts treated with vehicle. The results are quantitated as shown in Fig. 3B. The enhanced degree of apoptosis seen in the knockouts implies that the heart tissues from STAT3 null animals may have a hyperactive response to inflammatory stimuli. Consistent with this idea, we observed that after LPS treatment, mononuclear (likely inflammatory) cells appeared in the cardiac tissues of the knockout mice (Fig. 3C Upper Right, mononuclear cells are indicated by the arrows). These results suggest that the absence of functional STAT3 in cardiomyocytes may trigger stronger inflammatory responses to endotoxin, resulting in elevated endotoxin-induced apoptosis in heart.
Fig. 3.
Endotoxin-induced cardiac apoptosis and possible inflammatory responses in knockout and control mice. (A) Cardiac tissue samples from representative animals after 39 h of LPS treatment were examined by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (dark brown nuclei are positive). F/+, WT; C/F/D, knockout mice. (B) Bar graph showing average (n = 8) numbers of TUNEL-positive staining cells. (C) Hematoxylin/eosin staining of heart sections obtained 39 h after LPS treatment. (Upper) Possible inflammatory cells (arrow) throughout heart sections with low (Left, ×200) and high (Right, ×400) magnification [knockout (C/F/D) or WT (F/+) animals]. Mononuclear infiltrating cells are indicated by arrows. (D) Secreted TNF-α levels in isolated cardiomyocyte cultures from neonatal mice with the indicated genotypes. Cultures were incubated with or without LPS for 2, 6, 12, and 24 h.
Isolated Cardiac Myocytes Lacking STAT3 Expression Elaborate Higher Levels of TNF-α than Controls in Response to LPS. To elucidate the mechanism by which cardiac myocyte STAT3 deletion renders the heart more susceptible to proinflammatory stimuli and apotosis, we wanted to identify possible inflammatory mediators that might be generated in response to LPS. TNF-α has a major role in inflammatory responses; however, we have failed technically to identify possible presence of TNF-α in mouse cardiomyocytes in vivo, because there is no anti-mouse TNF-α antibody commercially available for tissue detection. As an alternative, we isolated cardiac myocytes from STAT3 null mice, cultured them in vitro, and treated them with LPS. The amounts of TNF-α secreted into the media were examined over 24 h. As shown in Fig. 3D, STAT3-deleted myocytes secreted significantly more TNF-α after LPS treatment than myocytes with WT STAT3. The heterozygous (C/F/+ and F/D) cardiac myocytes also generated higher amount of TNF-α than those of WT, but less than knockout cells. Enhanced induction of TNF-α in STAT3 null cells could contribute to stronger inflammatory responses with recruitment of inflammatory cells leading to increase cardiac myocyte apoptosis and tissue damage.
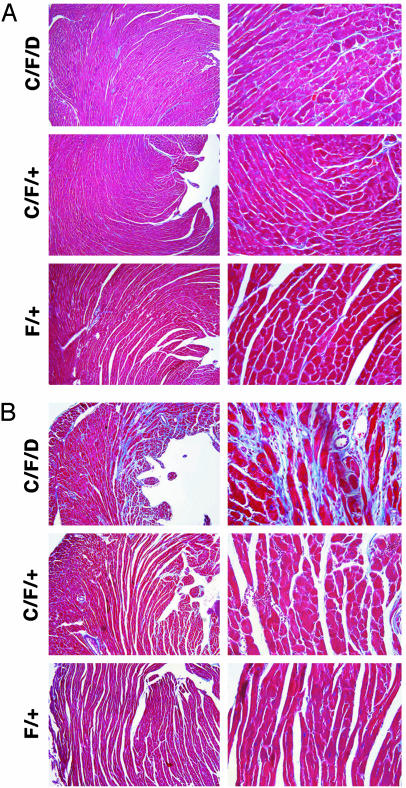
Mice Lacking Cardiac STAT3 Expression Develop Cardiac Fibrosis with Advanced Age. Although mice with myocytes lacking functional STAT3 develop normally, we suspected that the intrinsic defect that causes higher sensitivity to oxidant stress (doxorubicin) and might gradually lead to maladaptive cardiac remodeling with aging. Cardiac tissue samples from animals with age-matched transgenic animals were stained by using trichrome blue to evaluate the degree of fibrosis. As shown in Fig. 4, tissues from 6-month-old mice were first examined. At this age, a minor difference was observed: mice with STAT3 null cardiomyocytes had mild perivascular fibrosis, whereas WT STAT3 mice had no significant fibrosis (Fig. 4A). At 9 months of age, however, a dramatic difference was observed: STAT3 null mice developed severe fibrosis, whereas WT mice still had no significant fibrosis (Fig. 4B).
Fig. 4.
Mice lacking cardiac STAT3 develop severe cardiac fibrosis with advanced age. (A) Cardiac tissues from 6-month-old mice were examined by trichrome blue staining. Tissue from cardiac STAT3 null mice (C/F/D) (Top) and control mice [heterozygous C/F/+ (Middle) or WT F/+ mice (Bottom)]. (B) Similar staining was done with 9-month-old mice. (Magnification: ×20.)
Furthermore, cardiac tissue samples from groups of mice treated with doxorubicin for 7–11 days were also examined to evaluate the degree of fibrosis. These samples were evaluated in a blinded manner by a single observer and ranked on a scale from 0 to 4. The results are shown in Table 1 along with the definitions used to establish each graded result. Animals in the knockout group demonstrated significantly more fibrosis than controls. At the age of 9 months, heterozygous mice also developed severe fibrosis comparing with controls, indicating that loss of only one allele of STAT3 caused increased probability to develop fibrosis.
Table 1. Trichrome staining of cardiac samples.
| Fibrosis score | C/F/D, no. of animals | F/+, no. of animals |
|---|---|---|
| Grade 0 | 0 | 3 |
| Grade 1 | 2 | 2 |
| Grade 2 | 1 | 0 |
| Grade 3 | 2 | 0 |
| Grade 4 | 2 | 0 |
0 = no fibrosis; 1 = localized small amount of fibrosis; 2 = mild patchy fibrosis; 3 = moderate, diffuse fibrosis; 4 = severe, diffuse fibrosis. P < 0.01 for trend to worse fibrosis in C/F/D vs. F/+ animals by χ2 analysis.
Because TNF-α is a critical factor in the induction of cardiac fibrosis (19), it is possible that the potential of generation of a higher level of TNF-α and increased sensitivity to inflammation observed in STAT3 knockout mice may contribute to pathogenesis of the heart of these mice.
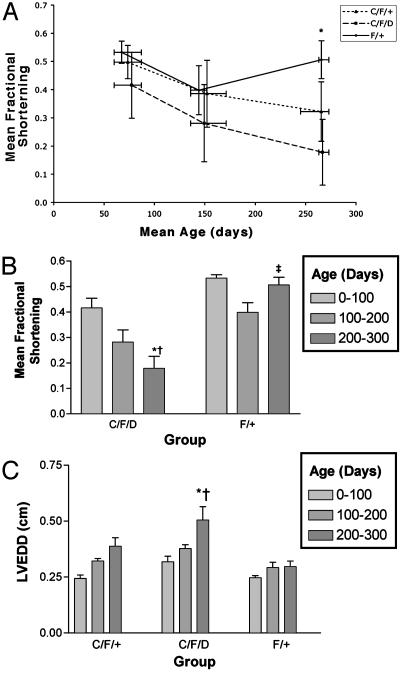
Decreased Cardiac Function with Age in Mice with STAT3 Null Cardiomyocytes. Based on the pathological differences described above, we next examined whether there are alterations in the left ventricular systolic function of aged mice. We measured left ventricular fractional shortening in 64 animals ranging from 60 to 273 days in age. As shown in Fig. 5A, there was a significant difference in the cardiac function between knockout animals older than 200 days and age-matched controls. The difference between fractional shortening in the animals <100 days was not statistically significant. The decline in systolic function was significantly greater for the knockout animals compared with controls (Fig. 5B, P < 0.001 for C/F/D vs. F/+ at >200 days of age). The heterozygotes appear to have a phenotype that is somewhere between that of the controls and the knockouts, although this difference did not reach statistical significance over the time period studied (P = not significant for C/F/+ vs. C/F/D or F/+ at all time points). In addition, the knockout animals demonstrated a significant increase in left ventricular chamber size, consistent with maladaptive cardiac remodeling (Fig. 5C). These results may suggest that STAT3 is essential for preservation of cardiac function over time. This conclusion is consistent with the observation that STAT3-deficient mice develop progressive cardiac fibrosis (as shown above).
Fig. 5.
Effect of age on mice lacking cardiac STAT3 expression. Fractional shortening was measured in 64 untreated animals. (A) Animals were grouped by age at intervals of 100 days. Graph shows mean fractional shortening for each group ± SEM. *, P < 0.01 compared with C/F/D age 1–100 days; †, P < 0.001 compared with F/+ age 200–300 days; ‡,P = not significant compared with F/+ age 0–100 days. (B) Scatter plot showing mean fractional shortening (± SD) vs. age (error bars show age range for each group studied). *, P < 0.01 for difference in fractional shortening between C/F/D vs. F/+ at age >200 days; P = not significant for C/F/+ vs. C/F/DorF/+ (data not shown). (C) Effect of age on left ventricular end diastolic diameter (measurements made in short axis). *, P < 0.001 compared with C/F/D 0–100 days; †, P < 0.001 compared with F/+ 200–300 days.
Discussion
To discern whether circulating cytokines play a role in the progression of human heart failure or are simply a marker of more severe disease, it is necessary to establish model systems in which molecular and cellular mechanisms of cytokine function and its role in heart failure can be further investigated.
The finding that multiple members of the IL-6 cytokine family (e.g., cardiotrophin-1 and leukemia inhibitory factor) can also elicit a hypertrophic response in cardiomyocytes is attributable to the fact that the receptors for all three of these cytokines share the gp130 subunit. The IL-6 cytokine family has been shown to protect cardiomyocytes from apoptotic cell death in response to serum starvation or ischemia (14, 20). Signaling through gp130 results in activation of both the MAPK and Janus kinase/STAT pathways. The relative contribution of each of these pathways to the hypertrophic and cytoprotective functions of IL-6 family cytokines is not known. Signaling through both pathways has been implicated in the hypertrophic response (9). The antiapoptotic effects of gp130 signaling may occur through upregulation of Bcl-xL (21), which occurs via gp130 activation of STAT1 in some tissues. In other tissues, this pathway does not appear to be involved, and antiapoptosis is mediated by other, as yet undefined pathways (22).
In the current study, we first demonstrate that animals lacking cardiomyocyte expression of STAT3 are more susceptible to doxorubicin-induced cardiac injury and development of heart failure (Fig. 2). More significantly, however, we found that cardiac deletion of STAT3 may lead to higher sensitivity to endotoxin-induced cell death and inflammation, possibly resulting from increased production of TNF-α (Fig. 3). Thus, it is likely that the intrinsic defect that leads to higher inflammatory responses to environmental factors causes cumulative heart damage in STAT3 null mice, which eventually leads to severe pathogenesis of cardiac system of these mice with advanced age. This hypothesis is supported by the observation that severe cardiac fibrosis develops with age or after oxidative stress in the STAT3-deficient mice (Fig. 4 and Table 1). The increased production of TNF-α might directly contribute to the development of the pathogenesis by causing interstitial inflammation, fibrosis, and age-related heart failure (19), which mimics the pattern seen in our STAT3 cardiomyocyte knockout mice. Furthermore, STAT3 has been shown as a survival factor for cardiomyocytes, so loss of STAT3 may result in a higher rate of apoptosis, which also contributes to observed fibrosis and pathogenesis in heart. Finally, this model may provide an explanation for the unexpected finding that the cardiac STAT3-deficient animals develop myocardial dysfunction in an age-related manner in the absence of additional cardiac insult (Fig. 5).
Whether the gp130 cardiac-specific knockout mice will develop heart failure with advanced age has not been described. These mice have been reported to have normal cardiac function and histology at 8 weeks (4), which is similar to our observations in STAT3 cardiac knockouts <100 days (Fig. 5). Animals with a global deletion of STAT3 die in utero (15), as do those with global deletion of gp130 (3), and both of these genotypes result in embryonic defects that include cardiac abnormalities. Interestingly, both cardiac-specific deletion of gp130 and STAT3 results in animals who not only survive, but whose cardiac structure and function is apparently normal at a young age, suggesting that signaling through the gp130/STAT3 pathway in some cell type other than the cardiomyocyte itself is required for normal cardiac development or that the α-MHC-Cre-loxP strategy used in these mice allows cardiomyocyte expression of STAT3 during critical events early in embryogenesis.
The mechanism of doxorubicin-induced heart failure is likely to involve generation of significant free radical myocyte injury. In light of the STAT3 knockout animals' increased response to this type of injury, it is interesting to speculate whether age-related accumulation of oxidant stress in the untreated knockout animals is a cause for their observed decline in function over time. Our data support the idea that both cardiac myocyte apoptosis and fibrosis may contribute to the observed decline in function. Reactive oxygen species have clearly been shown to cause myocardial fibrosis with subsequent development of heart failure (for a review, see ref. 23). Age-related increases in cardiac apoptosis and fibrosis have also been hypothesized to account for decreases in cardiac function with age in humans (for a review, see ref. 24). Activation of STAT3 signaling may play an important role in delaying this process by protecting the heart from oxidative stress.
To achieve the maximum reduction in STAT3 protein expression, we (like many other investigators) used α-MHC Cre transgenic mice in whom a single STAT3 allele was deleted in all tissues and crossed these with STAT3 loxP animals. It is therefore important to exclude the possibility that removal of the single STAT3 allele from all tissues alone contributes to the phenotype we observed in the C/F/D animals. To test this, we also evaluated animals in whom one allele of STAT3 was deleted in all tissues and the other allele was flanked by loxP site but no Cre recombinase was expressed (F/D). It is important to note that these animals did not demonstrate an age-related decline in cardiac function that differed significantly from the C/F/+ animals (P > 0.05 for fractional shortening of F/D vs. C/F/+ animals up to 300 days of age). These results also indicate that the deletion of one allele of STAT3 rather than the expression of Cre recombinase is responsible for the intermediate phenotype of heterozygous mice. Further, although these animals appear to have normal cardiac function at a young age, our evaluation of their cardiac function was restricted to that obtained by echocardiography at rest. It is possible that more invasive hemodynamic measurements, perhaps with the addition of increased cardiac workload, might uncover more subtle defects in their cardiac function at a younger age.
In summary, we believe that this study provides crucial information for understanding the importance of STAT3 signaling in the preservation of cardiac myocyte integrity and function. More importantly, our findings suggest an important mechanism by which this pathway is required for regulation of inflammatory responses of cardiomyocytes, which may contribute to development of cardiac fibrosis and cause heart dysfunction.
Acknowledgments
We thank Dr. Frank Giordano for advice and help in initiating this project and Michael Wolfgang for reading the manuscript and providing comments. X.-Y.F. was a recipient of a Career Development Award from the National Institutes of Health. J.J.J. was an Anna Fuller Fellow. This work is supported by National Institutes of Health Grants AI34522 and AR44906 (to X.-Y.F.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: STAT, signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; α-MHC, α-myosin heavy chain; AP, alkaline phosphatase.
References
- 1.Deswal, A., Petersen, N. J., Feldman, A. M., Young, J. B., White, B. G. & Mann, D. L. (2001) Circulation 103 2055-2059. [DOI] [PubMed] [Google Scholar]
- 2.Taga, T. & Kishimoto, T. (1997) Annu. Rev. Immunol. 15 797-819. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida, K., Taga, T., Saito, M., Suematsu, S., Kumanogoh, A., Tanaka, T., Fujiwara, H., Hirata, M., Yamagami, T., Nakahata, T., et al. (1996) Proc. Natl. Acad. Sci. USA 93 407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota, H., Chen, J., Betz, U. A., Rajewsky, K., Gu, Y., Ross, J., Jr., Muller, W. & Chien, K. R. (1999) Cell 97 189-198. [DOI] [PubMed] [Google Scholar]
- 5.Hirano, T., Nakajima, K. & Hibi, M. (1997) Cytokine Growth Factor Rev. 8 241-252. [DOI] [PubMed] [Google Scholar]
- 6.Pan, J., Fukuda, K., Kodama, H., Makino, S., Takahashi, T., Sano, M., Hori, S. & Ogawa, S. (1997) Circ. Res. 81 611-617. [DOI] [PubMed] [Google Scholar]
- 7.Hishinuma, S., Funamoto, M., Fujio, Y., Kunisada, K. & Yamauchi-Takihara, K. (1999) Biochem. Biophys. Res. Commun. 264 436-440. [DOI] [PubMed] [Google Scholar]
- 8.Negoro, S., Kunisada, K., Tone, E., Funamoto, M., Oh, H., Kishimoto, T. & Yamauchi-Takihara, K. (2000) Cardiovasc. Res. 47 797-805. [DOI] [PubMed] [Google Scholar]
- 9.Kunisada, K., Tone, E., Fujio, Y., Matsui, H., Yamauchi-Takihara, K. & Kishimoto, T. (1998) Circulation 98 346-352. [DOI] [PubMed] [Google Scholar]
- 10.Negoro, S., Kunisada, K., Fujio, Y., Funamoto, M., Darville, M. I., Eizirik, D. L., Osugi, T., Izumi, M., Oshima, Y., Nakaoka, Y., et al. (2001) Circulation 104 979-981. [DOI] [PubMed] [Google Scholar]
- 11.Funamoto, M., Fujio, Y., Kunisada, K., Negoro, S., Tone, E., Osugi, T., Hirota, H., Izumi, M., Yoshizaki, K., Walsh, K., et al. (2000) J. Biol. Chem. 275 10561-10566. [DOI] [PubMed] [Google Scholar]
- 12.Osugi, T., Oshima, Y., Fujio, Y., Funamoto, M., Yamashita, A., Negoro, S., Kunisada, K., Izumi, M., Nakaoka, Y., Hirota, H., et al. (2002) J. Biol. Chem. 277 6676-6681. [DOI] [PubMed] [Google Scholar]
- 13.Kunisada, K., Kumanogoh, A., Negoro, S., Funamoto, M., Osugi, T., Kishimoto, T. & Yamauchi-Takihara, K. (2000) Cytokine 12 1512-1518. [DOI] [PubMed] [Google Scholar]
- 14.Sheng, Z., Knowlton, K., Chen, J., Hoshijima, M., Brown, J. & Chien, K. (1997) J. Biol. Chem. 272 5783-5791. [DOI] [PubMed] [Google Scholar]
- 15.Takeda, K., Noguchi, K., Shi, W., Tanaka, T., Matsumoto, M., Yoshida, N., Kishimoto, T. & Akira, S. (1997) Proc. Natl. Acad. Sci. USA 94 3801-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agah, R., Frenkel, P. A., French, B. A., Michael, L. H., Overbeek, P. A. & Schneider, M. D. (1997) J. Clin. Invest. 100 169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson, P., McGrath A. & Savion, S. (1982) Circ. Res. 51 787-801. [DOI] [PubMed] [Google Scholar]
- 18.Lobe, C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. & Nagy, A. (1999) Dev. Biol. 208 281-292. [DOI] [PubMed] [Google Scholar]
- 19.Kubota, T., McTiernan, C., Frye, C., Slawson, S., Lemster, B., Koretsky, A., Demetris, A. & Feldman, A. (1997) Circ. Res. 81 627-635. [DOI] [PubMed] [Google Scholar]
- 20.Stephanou, A., Brar, B., Heads, R., Knight, R. D., Marber, M. S., Pennica, D. & Latchman, D. S. (1998) J. Mol. Cell. Cardiol. 30 849-855. [DOI] [PubMed] [Google Scholar]
- 21.Fujio, Y., Kunisada, K., Hirota, H., Yamauchi-Takihara, K. & Kishimoto, T. (1997) J. Clin. Invest. 99 2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akira, S. (2000) Oncogene 19 2607-2611. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai, T., Davis, J. G., Horie, T., O'Rourke, D. M. & Greene, M. I. (2001) Proc. Natl. Acad. Sci. USA 98 5526-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, A. S., Sane, D. C., Wannenburg, T. & Sonntag, W. E. (2002) Cardiovasc. Res. 54 25-35. [DOI] [PubMed] [Google Scholar]