Abstract
Our studies of mice deficient for the E2F1 and E2F2 transcription factors have revealed essential roles for these proteins in the cell cycle control of pancreatic exocrine cells and the regulation of pancreatic beta cell maintenance. Pancreatic exocrine cells in E2f1/E2f2 mutant mice become increasingly polyploid with age, coinciding with severe exocrine atrophy. Furthermore, mice deficient for both E2F1 and E2F2 develop nonautoimmune, insulin-dependent diabetes with high penetrance. Surprisingly, transplantation of wild-type bone marrow can prevent or rescue diabetes in E2f1-/-E2f2-/- mice. We hypothesize that exocrine degeneration results in a destructive environment for beta cells, which can be alleviated by restoration of the hematopoietic system that is also defective in E2f1-/-E2f2-/- mice. The demonstration that beta cell maintenance under conditions of stress is influenced by bone marrow-derived cells may provide important insight into the design of therapies to boost islet mass and function in diabetic patients.
Homeostatic control of normal glucose levels is achieved by coordinate secretion of insulin and glucagon, produced by beta and alpha cells in pancreatic islets, respectively (1). Type I insulin-dependent diabetes mellitus (IDDM) is a familial disorder with primarily juvenile onset that is characterized by the autoimmune destruction of beta cells in the pancreas, resulting in insufficient insulin secretion and hyperglycemia. Type II or non-IDDM (NIDDM) is the most common form of diabetes, resulting from both insulin resistance in target organs and insufficient insulin production from beta cells (2). The exocrine pancreas expands dramatically in the few weeks following birth in the mouse. Exocrine cells produce digestive enzymes that are secreted into a ductal network that joins a common duct emptying into the duodenum. Chronic pancreatitis is characterized by progressive and irreversible loss of pancreatic exocrine and endocrine function (3, 4). In the majority of patients suffering from chronic pancreatitis, endocrine pancreatic insufficiency is correlated with exocrine dysfunction (3, 4). In addition, pancreatic exocrine dysfunction has been described frequently in IDDM and NIDDM patients (5).
The mechanisms regulating the postnatal proliferative expansion of the exocrine and endocrine pancreas, as well as the maintenance of the pancreas in adults, have not been well established. E2F activity controls the transcription of a group of genes that encode proteins important for cell cycle progression (6). E2F transcriptional activity is critical for the regulation of cell cycle progression and is composed of heterodimers formed by the association of one of six E2F family members with one of three DP proteins. The combined and individual disruption of E2f genes in mice has revealed both distinct and overlapping roles for E2Fs in vivo (6). Our recent studies have shown essential roles for E2F2 in S phase progression and hematopoiesis (7). In this study, we characterized the development of severe pancreatic degeneration and insulin-dependent diabetes in E2f1-/-E2f2-/- mice, revealing essential roles for E2F1 and E2F2 in postnatal pancreas development and homeostatic maintenance. Our findings provide connections between cell cycle defects resulting from E2F1/2 loss, pancreatic degeneration, and diabetes. In addition, a surprising supportive role for bone marrow (BM)-derived cells in maintaining beta cell mass in the face of exocrine degeneration may have important implications for pancreatitis associated diabetes in humans.
Materials and Methods
Mice. The maintenance and genotyping of E2f1, E2f2, and Rag2 mutant and CAR transgenic mice has been described (8, 9). A drop of peripheral blood from the tail tip was used to measure the nonfasting blood sugar level by using MediSense Precision QID test strips and reader (generally measured between 1 and 2 p.m.). For glucose tolerance tests, nondiabetic female double knockout (DKO) and control mice of similar weights were s.c. injected at 0.6 g/kg (body weight) by using a 50% glucose stock solution, and blood sugar levels were monitored. For insulin rescue experiments, diabetic male DKO mice were s.c. injected with human recombinant insulin (gift of Ronald Gill, Barbara Davis Center for Childhood Diabetes). Different doses of insulin were administrated according to blood glucose levels (1 unit of insulin for 10–15 mM, 2 units for 15–25 mM, and 3 units for 25 mM and higher). For BM transplants (BMTs), 1 × 107 nucleated BM cells from donor mice were transplanted into DKO or control mice. Recipient mice were given either a half-lethal (450-rad) or lethal (900-rad) dose of γ radiation and transferred via subocular injection. The University of Colorado Health Sciences Center Animal Care and Use Committee approved all mouse experiments.
Histological Analysis and Fluorescence in Situ Hybridization (FISH). Tissue fixation, immunohistochemistry, and immunofluorescence (IF) were performed as described (10). Antibodies used were anti-insulin, anti-glucagon, and anti-amylase (1:5,000 each; I2018, G2654, and A8273 from Sigma, respectively), except that anti-insulin from Dako (A0564) was used for IF experiments. Secondary antibodies were from Jackson ImmunoResearch. Morphometric determination of beta, alpha, and acinar cell area and mass was performed by using an Olympus BX51 microscope and Pixera CL600 camera to capture consecutive images of triple IF stained (Fig. 2 and see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org) random pancreatic sections. The area covered by cells staining with the indicated fluorescent antibody was then determined for all pictures covering each section by using IMAGE PRO 4.1 software (Media Cybernetics, Silver Spring, MD) (confirmed by visual inspection to eliminate artificially stained areas).
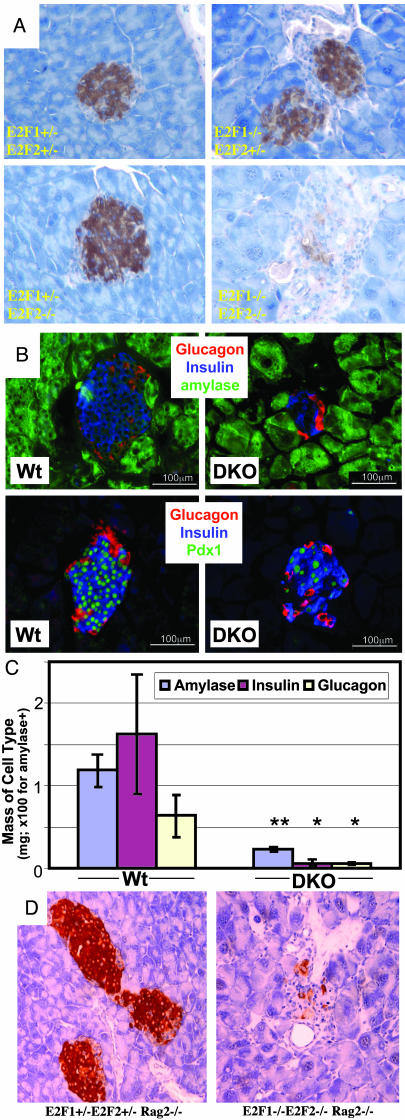
Fig. 2.
Age-dependent loss of beta cells in DKO pancreata. Pancreas sections from 2-mo-old (A and D) or 3- to 4-mo-old (B) male mice of the indicated genotypes (note Rag2-/- in D). In A and D, insulin (brown) was detected by immunohistochemistry. In B and C, amylase, insulin, glucagons, and Pdx1 were detected by IF. The same magnification was used within each set. (C) From IF experiments as shown in B (three mice per group), the mass occupied by cells expressing the indicated proteins was determined by morphometric analysis of sections immunostained for insulin, glucagon, and amylase (see Materials and Methods). The percentage of the tissue area occupied by each cell type was multiplied by the total weight of the pancreas to obtain the mass of that cell type per pancreas.
FISH was performed as described (11) with biotin-labeled mouse chromosome 2-specific point probe 98 cM (Applied Genetics Laboratories) followed by streptavidin–FITC detection of the human CAR transgene (9) labeled with Spectrum red. Analysis was performed on an Olympus BX60 epifluorescence microscope using single interference filter sets of blue (4′,6-diamidino-2-phenylindole), green (FITC), and red (Texas red), as well as dual (green/red) and triple (blue/green/red) band pass filters.
Results
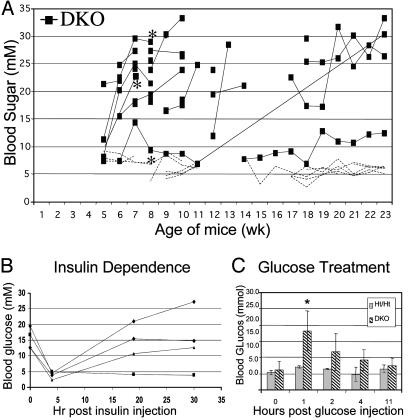
DKO Mice Develop Nonautoimmune, Insulin-Dependent Diabetes. Mouse genotypes are denoted as E2f1 genotype/E2f2 genotype, with genotypes indicated by wild type (WT), heterozygous (Ht), and mutant (Mt), with E2f1-/-E2f2-/- mice referred to as DKO. For example, Mt/Ht mice are E2f1-/-E2f2+/-. Dramatically, all DKO male mice (22 of 22) developed diabetes (nonfasting blood sugar ≥10 mM consecutively measured at least 1 week apart) between 1 and 4 months of age (Fig. 1A). In fact, 12 of 13 mice monitored weekly became diabetic within 8 weeks of age. Injection of insulin into diabetic DKO males temporarily restored normal blood glucose levels, indicating that DKO mice are insulin responsive (Fig. 1B). We only observed diabetes in mice lacking all four E2f1 and E2f2 alleles, and islet cell mass was not substantially reduced in mice lacking three of four E2f1/E2f2 alleles (data not shown). Interestingly, the development of diabetes in DKO females (5 of 6; average 6.7 mo) was markedly delayed relative to males, although prediabetic females exhibited substantially reduced beta cell numbers (data not shown). Accordingly, nondiabetic DKO females were glucose intolerant, indicative of insufficient insulin production, because injection of glucose into DKO females resulted in extended hyperglycemia lasting up to 11 hr, whereas control littermates restored normal blood glucose levels within 30 min of injection (Fig. 1C). Female hormones may contribute to delaying diabetes, as progesterone increases beta cell mass during pregnancy (12). Because beta cell reductions precede hyperglycemia in female DKO mice, beta cell loss does not appear to result from hyperglycemia.
Fig. 1.
DKO mice develop nonautoimmune insulin-deficient diabetes. (A) Blood glucose levels of DKO males (black squares) and littermates of other E2f1/2 genotypes (dashed lines with no symbols; two Mt/WT, six Ht/Mt, one WT/Ht, and six Ht/Ht) at different ages. A continuous line represents multiple measurements from a single mouse. Mice designated with an asterisk were also Rag2-/-. (B) Blood glucose levels of four diabetic DKO males at the indicated times after injection of insulin. (C) Blood glucose levels of five nondiabetic DKO females and five Ht/Ht females at the indicated times after injection of glucose. Error bars indicate standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for all figures.
In prediabetic 24-day-old male DKO mice, comparable insulin expression in islets was observed, indicating that embryonic beta cell development appears relatively normal (Fig. 6A). Notably, even in these young DKO mice, DKO islet architecture and cellular morphology appeared less uniform. In older (>1 mo) DKOs, we observed selective loss of the beta cells in the pancreatic islets (Figs. 2 A–C and 6B). Glucagon-expressing alpha cell numbers are also reduced, although to a lesser extent relative to beta cells. The expression of Pdx1, which is specifically expressed in beta cells and is required for beta cell development and function (13), decreases in DKO pancreata coincident with decreased insulin expressing cell numbers (Fig. 2B). Pdx1Pos/InsulinNeg cells were not observed, indicating that reduced numbers of insulinPos cells is not due to reduced insulin expression or degranulation, but to the loss of beta cells. Thus, beta cell mass in DKO mice deteriorates with age.
We did not observe lymphocytic infiltration in or around islets of prediabetic and diabetic DKO mice, as determined by anti-CD4 and anti-CD8 immunohistochemistry for the detection of T cells (data not shown). Mature lymphocytes are not required for the development of diabetes, because immunodeficient triple mutant male mice lacking E2F1, E2F2, and RAG2 developed diabetes and islet degeneration in a manner indistinguishable from immunocompetent DKO males (Figs. 1 A and 2D). Because these mice had no mature lymphocytes, beta cell loss does not result from autoimmune destruction.
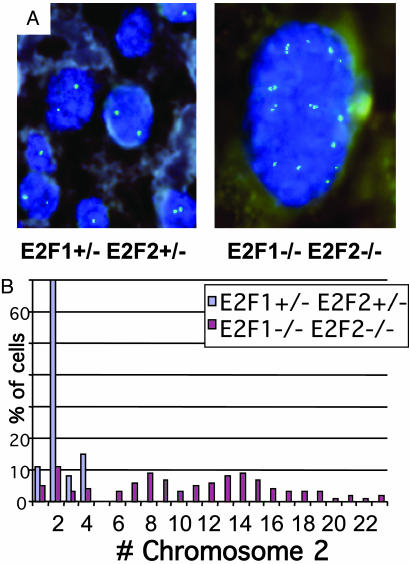
Pancreatic Exocrine Cells of E2f1/E2f2 Mutant Mice Become Highly Polyploid. Pancreas exocrine cells and nuclei from Mt/Ht and DKO mice are substantially larger (Fig. 2), as has been noted in E2f1-/- mice (14). By FISH using specific probes mapped to chromosomes 2 and 5, striking polyploidy was observed in the exocrine pancreas of E2f1/2 mutant mice. In the WT and Ht/Ht samples, exocrine cells had normal sized nuclei with around two copies of chromosome 2-specific FISH signals in each cell. In sharp contrast, DKO exocrine cells had much larger nuclei and exhibited an average of 5–10 FISH signals per cell (Fig. 3 and Table 1, which is published as supporting information on the PNAS web site). Mt/Ht exocrine cells exhibited similar polyploidy to DKO cells, and Ht/Mt cells exhibited a more modestly increased ploidy. This polyploidy was observed per nucleus, and the cells were not generally multinucleate. These data actually underrepresent the extent of polyploidy because they are based on the analysis of 5-μm sections, roughly the diameter of a WT exocrine nucleus but significantly smaller than the diameter of a DKO exocrine nucleus. Increased ploidy occurs mostly during postnatal exocrine expansion and correlates with the temporal pattern and severity of exocrine degeneration in DKO mice. In the exocrine pancreata of 4- and 11-day-old DKO pups, we observed only a small fraction of polyploid cells (Table 1), and pancreas structure was not visibly perturbed (data not shown). We also observed polyploidy in the liver and salivary glands of E2f1/2 mutant mice, whereas normal ploidy was evident in pancreatic endocrine islets, intestines, kidney, skin, brain, and various hematopoietic progenitors (data not shown).
Fig. 3.
Exocrine pancreas cells in DKO mice are highly polyploid. (A) FISH analysis of pancreatic exocrine cells from 10-week-old male Ht/Ht and DKO littermates. Chromosome 2-specific signals are in green, and 4′,6-diamidino-2-phenylindole (DAPI) staining of DNA is shown in blue. The same magnifi-cation was used for both pictures (original magnification, ×100). (B) Quantitation of chromosome 2 signals per nuclei from the pancreas sections shown in A. The y axis represents the percentage of cells (of 100 counted) with the indicated number of chromosome 2 signals per nucleus.
Mt/Ht and DKO Mice Develop Progressive Pancreatic Degeneration. We noted a substantial reduction of pancreata size in E2f1/2 mutant mice. Whole pancreata were removed from mice of different E2f genotypes and weighed. Mice were divided into three groups according to their ages. We did not observe differences between males and females of any genotype, so data include both sexes. Pancreata from Mt/Ht and DKO mice at all ages were significantly smaller than Ht/Ht mice (Fig. 4A), whose pancreata are indistinguishable from WT mice (data not shown). Notably, WT/Mt and Ht/Mt mice had normal pancreas weight compared with Ht/Ht mice. Older DKO mice (>100 d) had more severe pancreas mass loss than Mt/Ht mice, which in turn showed more substantial pancreas mass reduction relative to Mt/WT mice. No beta cells could be discerned in older DKO pancreata and the few remaining exocrine cells were large, dysplastic with hyperchromatic pleomorphic nucleoli (Fig. 4B). These exocrine cells were separated from each other by what appear to be fibroblast-like cells and frequent ductal remnants. Although terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assays did reveal increased apoptosis in DKO pancreata (0.3% of DKO exocrine cells vs. 0% of Ht/Ht exocrine cells; significant numbers of TUNEL-positive cells were not evident in islets; data not shown), we are not confident that this apoptosis fully accounts for exocrine atrophy. Morbid Mt/Ht mice also showed similar exocrine pancreatic atrophy, but in older mice (mostly >1 yr), probably accounting for the premature morbidity of many of these mice (8).
Fig. 4.
Exocrine degeneration in E2f1/2 mutant mice. (A) Pancreata dissected from mice of the indicated E2f1/E2f2 genotypes were weighed, and pancreas weights are divided into three age groups. Within each age group, statistical significance of differences was determined by comparing to the pancreata weights of Ht/Ht mice. In the second and third age groups, DKO mice also show highly significant pancreata weight decreases compared with Mt/Ht mice (P < 0.001 for each). For the 50-day group, the average ages for Ht/Ht, Mt/Ht, Ht/Mt, and DKO mice were 46.8, 52.3, 51.5, and 47.4 days, respectively. Similarly, the average ages for the 100- and 200-day groups were 162.4, 155.3, 146, and 134.4 days and 270.8, 280.7, 279.8, and 272.3 days, respectively. Age ranges were similar (≈35–75 days, 100–190 days, and 210–405 days) for the three groups, because littermates were generally compared. For Mt/WT and WT/Mt mice in the 200-day group, average ages were 491 (range, 440–570 days) and 463 (range, 440–485 days) days. The average pancreas weight for WT littermates of an average age of 455 days (range, 440–485 days) was 0.23 g. (B) Pancreas sections from a sick 4-mo-old male DKO mouse (Right) and a control littermate (Left) immunostained for insulin (brown). The same magnification was used for both pictures (original magnification, ×20). (C) Pancreas sections from morbid 13-mo-old (Left) and 12-mo-old (Right) male Mt/Ht mice stained with H&E (Left) or immunostained for insulin (Right; dark brown indicates positive cells). Two islets in each picture are indicated with arrows. The same magnification was used for both pictures (original magnification, ×4).
Although the exocrine pancreata in older Mt/Ht mice was virtually completely degenerated, islets containing apparently healthy beta cells remained (Fig. 4C). A survey of 14 Mt/Ht mice aged 6 mo to 1 yr old revealed normal blood sugar levels in all mice. Also note that although pancreas mass did not decrease as dramatically in older Mt/Ht mice relative to DKO mice (Fig. 4A), eventual exocrine cell loss was similar, with other cell types (including cells that morphologically appear to be adipocytes) accounting for the greater mass in Mt/Ht pancreata. Thus, exocrine degeneration does not necessarily lead to beta cell loss. Finally, we frequently observed hugely distended intestines with large quantities of undigested material in morbid DKO and some Mt/Ht mice (Fig. 6C). We speculate that this phenotype partly results from pancreatic atrophy and the consequent loss of appropriate digestive enzymes and neutralizing bases normally secreted into the intestine. In summary, from these observations we conclude that E2F1 seems to be the major E2F family member that regulates exocrine pancreas expansion and/or maintenance in mice, with some functional redundancy with E2F2.
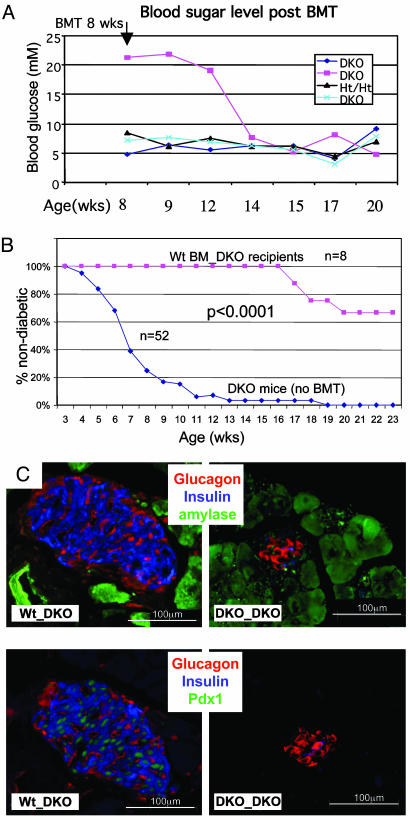
Islet Degeneration and Diabetes in DKO Mice Can Be Prevented or Rescued by BMT Using WT Donors. We previously showed that E2f2 mutant and DKO mice display dramatic cell autonomous defects in hematopoiesis, with severe monocyte/macrophage, lymphocyte, and erythrocyte deficiencies (7). We performed BMT with WT BM into either prediabetic or diabetic irradiated DKO males to address whether hematopoietic deficiencies contribute to diabetes development. Strikingly, WT BMT could either prevent diabetes development or even stably restore normal blood glucose levels in previously diabetic mice (Fig. 5 A and B). We here define diabetes prevention as maintenance of normal blood glucose levels (<10 mM) at least 6 weeks past the age (8 weeks) when >90% of unmanipulated DKO males have developed diabetes. Although the degeneration of pancreas exocrine cells was still apparent, substantial improvements (average of three to five times) in the numbers of Pdx1Pos and InsulinPos islet cells were evident after WT BMT, but not DKO BMT (Figs. 5C and 6 D and F). Note that although the number of islets was greatly increased over control DKO mice (although the extent of this increase is variable), the number of beta cells per islet was not restored to the levels found in Ht/Ht or WT mice. In fact, we frequently observed isolated individual beta cells in DKO recipients of WT BM (data not shown), which are rarely found in control Ht/Ht and WT mice. Interestingly, although glucagon-producing alpha cells localize to the periphery of the islets (Fig. 2B) in WT pancreata, we consistently observe alpha cells scattered throughout the islets of DKO recipients of WT BM (Fig. 5C). Notably, WT BMT into DKO mice did not improve the numbers of amylase-expressing acinar cells (Fig. 6D).
Fig. 5.
Prevention or correction of diabetes in DKO mice by BMT. (A) Eight-week-old male mice of the indicated E2f1/E2f2 genotypes were sublethally irradiated and transplanted with WT BM cells (one experiment is shown). Nonfasting blood sugar levels were monitored as shown. (B) Transplantation of WT BM into prediabetic DKO males effectively prevents the development of diabetes. The percentages of DKO that are nondiabetic are plotted as a function of age. DKO males were either untransplanted (blue diamonds) or transplanted with WT BM (pink squares). Two of the DKO recipients of WT BM died at 20 and 26 weeks of unknown causes, but with normal blood sugar levels. Others were healthy at time of death at 16, 18, 23, and 26 weeks. The average age of death (or killing when clearly morbid) of untransplanted DKO males is 15.4 weeks. Five DKO mice received lethal irradiation before BMT, and the other three recipients received sublethal irradiation. Transplanted WT BM was also contained a GFP transgene (7), and >95% of peripheral nucleated blood cells in recipient mice were GFP+ after 1 mo posttransplantation. (C) Anti-insulin, glucagon, amylase, and Pdx1 IF (as indicated) on sections from male DKO recipients of either DKO or WT BM (4 mo old; 2.5 mo after BMT). See Fig. 6D for morphometric quantitations.
In all, WT BMT prevented or corrected diabetes in 8 of 8 and 2 of 12 DKO recipients, respectively. Importantly, transplantation of DKO BM into DKO recipients (0 of 8) or sublethal irradiation without BMT (0 of 3) neither prevented nor rescued diabetes (data not shown). Thus, the procedure itself was not responsible for the rescue of diabetes by WT BM, and DKO BM is clearly deficient in some cell type that can support islet maintenance. Our data indicate that prevention of diabetes in DKO recipients of WT BM is more efficient than rescue, probably because of irreversible islet loss in diabetic DKOs and extensive pancreatic degeneration that cannot provide a platform for islet restoration. In addition, restoration of hematopoiesis is more difficult in sick mice that exhibit pancreatitis and diabetes. The ability to restore normal peripheral leukocyte numbers in recipient DKO mice, which was most evident after transplantation into prediabetic DKO males, correlated with the prevention or rescue of diabetes (Fig. 6E).
We considered the possibility that BM-derived stem cells were differentiating into beta cells in the recipient DKO mice, restoring glucose homeostasis. However, beta cells in the pancreata of DKO recipients of WT BM were of recipient, not donor, origin as determined by FISH analysis using a donor specific probe (Fig. 6G). Thus, the rescue and prevention in DKO mice by BMT experiments suggest that cells or factors derived from the BM play important supportive roles in modifying the viability of endocrine islet cells. Finally, transplantation of DKO BM into WT recipients did not result in diabetes or obvious islet/exocrine degeneration (data not shown). Thus, DKO BM-derived cells do not appear to confer a dominant, destructive effect on islet maintenance.
Discussion
The disruption of E2f1 and E2f2 results in dramatic cell cycle perturbations during hematopoiesis and during the postnatal expansion of the exocrine pancreas. Our data suggest that the resulting BM deficiencies and exocrine degeneration in combination lead to beta cell destruction and diabetes. These results not only shed important light on functions for specific E2Fs in cell cycle control, but reveal an unexpected supportive role for BM-derived cells in protecting islets from a destructive environment, which may be particularly relevant for pancreatitis-induced diabetes.
We do not currently understand how the loss of E2F1 and E2F2 results in repeated S phases without intervening mitosis, and why polyploidy is limited to particular tissues. E2F1/2-dependent regulation of the expression of targets required to generate Cyclin A- or B-dependent kinase activity may be required for appropriate entry into mitosis before reentry into a new G1 and S phase. In fact, E2F-dependent regulation of Cyclin A has been shown to be required for mitosis, via Cyclin A-dependent inhibition of anaphase promoting complex activity (15). In Schizosaccharomyces pombe, mutations in B type cyclins that prevent mitotic Cdk activation after S phase result in endoreduplication (16). Alternatively, E2F activity has also been demonstrated to control the expression of multiple components of the spindle assembly checkpoint (17), the disruption of which can allow reentry into S phase without mitosis.
Our data show that exocrine atrophy is progressive after birth, and is correlated with the level of endoreduplication within the exocrine compartment. As exocrine degeneration worsens, pancreatic interstitial cells increase in number and fibrotic deposits become pronounced. In the pancreata of morbid DKO and Mt/Ht mice, areas are occupied by adipocytes and the pancreas is strongly fibrotic. These changes reflect the morphological changes also observed during chronic pancreatitis in humans (3, 4), as well as in postmortem samples of type 2 diabetic human pancreata (18). In both cases, deterioration of islet function is often associated, and we are therefore not surprised to observe this occurring in our mouse model. However, given our observations that WT BM is capable of preventing or rescuing exocrine atrophy-induced diabetes, an unexpected link between tissue restoration and the BM exists.
Our hypothesis is that pancreatic degeneration in DKO mice creates a destructive environment for islets. Islets can be protected from pancreatic atrophy-induced destruction by BM-derived cells or their secreted factors. Given that DKO mice exhibit severe hematopoietic deficiencies, we speculate that these supportive cells are hematopoietic. Islet loss in DKO mice may be due to the decreased presence of cells that scavenge oxygen radicals, other waste products, or apoptotic cells. Indeed, beta cells are particularly sensitive to oxidative stress (19), which might result from exocrine degeneration. Alternatively, anemia and the resulting hypoxia in DKO mice may contribute, together with pancreatic degeneration, to islet destruction. Interestingly, impaired beta cell function in patients with thalassemia is improved after BMT (20). Given that WT BM can, albeit with reduced frequency, restore glucose homeostasis to already diabetic DKO mice, WT BM-derived cells might also somehow contribute to beta cell neogenesis.
Other untested possibilities are that WT BMT results in improvements in either the vasculature that supports islets or in exocrine cell health/organization. In fact, BM-derived stem cells have recently been shown to support beta cell proliferation and regeneration after streptozotocin-induced damage, coinciding with improved BM-derived vasculature (21). Although we find nonislet cells of donor origin in the vicinity of islets in DKO recipients of WT BMT (data not shown), we have not determined their cell type or how they might contribute to islet maintenance. However, our preliminary experiments examining platelet-endothelial cell adhesion molecule 1 (PECAM-1)-positive vascular cells in and around islets have not revealed obvious differences between WT, DKO, and DKO BMT recipient pancreata (data not shown). Finally, the facts that E2f1-/-E2f2-/-Rag2-/- mice develop diabetes and that transplantation of DKO BM cells into WT mice does not result in islet destruction, and the lack of detectable inflammatory infiltration of mononuclear cells during islet degeneration in DKO mice, argue against a model whereby diabetes results from increased levels of hematopoietic-derived destructive factors or leukocyte-mediated destruction of beta cells.
Although we cannot rule out some islet autonomous effects of E2f1/2 loss on the development of diabetes, it is interesting that diabetes in DKO mice may result from the combination of distinct effects of the loss of these E2Fs in different tissues, but apparently largely independent of the cell autonomous loss of E2F1 and E2F2 in islet cells. Thus, islet damage caused by exocrine cell degeneration (primarily resulting from E2f1 mutation) is not properly alleviated because of severe hematopoietic defects (resulting primarily from E2f2 mutation). Note that DKO mice transplanted with WT BM and old Mt/Ht mice display a similar pancreatic phenotype: the maintenance of beta cells in the face of severe exocrine degeneration (Figs. 4C and 6F). These data suggest that because hematopoiesis is relatively normal in Mt/Ht mice (7), exocrine atrophy does not lead to islet destruction and diabetes. Nonetheless, functional redundancy between E2F1 and E2F2 still contributes to both phenotypes, as the combined loss of all E2F1 and E2F2 results in the most severe phenotype in each case.
Our analyses of E2f1/2 mutant mice have uncovered unexpected contributions of BM cells to the survival and/or proliferation of beta cells under conditions of stress, with important potential implications for human diabetes. Our data suggest that BM-derived cells may limit islet destruction and diabetes resulting from pancreatitis. Although speculative, altered function of BM-derived cells may also contribute to type II diabetes, by failing to properly support beta cell survival in response to hyperglycemia-induced oxidative stress. The future identification of the BM-derived cells or factors that can protect islet cells from a destructive environment may provide new therapeutic tools for patients with pancreatitis-induced diabetes or Type II diabetes.
Supplementary Material
Acknowledgments
We thank R. Gill for critical review of this manuscript, and L. Bloomquist (Barbara Davis Center for Childhood Diabetes) for tissue processing, which was supported by Diabetes Endocrinology Research Center (DERC) Grant P30 DK 57516. J.D. is supported by National Institutes of Health Grants CA77314 and DK063299, a Pilot and Feasibility grant from DERC (Grant P30 DK 57516), and a Scholar Award from the Leukemia and Lymphoma Society. J.S.T. is supported by National Institutes of Health Predoctoral Training Grant T32-GM08730. C.J.H. is supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant 2-RO1 HL61382-04. M.V.-G. and the Cytogenetics Core are supported by National Institutes of Health Grant P30 CA46934.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BM, bone marrow; BMT, BM transplantation; IF, immunofluorescence; FISH, fluorescence in situ hybridization; DKO, double knockout; Ht, heterozygous; Mt, mutant.
References
- 1.Kim, S. K. & Hebrok, M. (2001) Genes Dev. 15 111-127. [DOI] [PubMed] [Google Scholar]
- 2.Rane, S. G. & Reddy, E. P. (2000) Front. Biosci. 5 D1-D19. [DOI] [PubMed] [Google Scholar]
- 3.Larsen, S. (1993) Dan. Med. Bull. 40 153-162. [PubMed] [Google Scholar]
- 4.Raue, G. & Keim, V. Z. (1999) Gastroenterology 117 Suppl. 1, 4-9. [Google Scholar]
- 5.Hardt, P. D., Killinger, A., Nalop, J., Schnell-Kretschmer, H., Zekorn, T. & Klor, H. U. (2002) Pancreatology 2 30-33. [DOI] [PubMed] [Google Scholar]
- 6.DeGregori, J. (2002) Biochim. Biophys. Acta 1602 131-150. [DOI] [PubMed] [Google Scholar]
- 7.Li, F. X., Zhu, J. W., Hogan, C. J. & DeGregori, J. (2003) Mol. Cell. Biol. 23 3607-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu, J. W., Field, S. J., Gore, L., Thompson, M., Yang, H., Fujiwara, Y., Cardiff, R. D., Greenberg, M., Orkin, S. H. & DeGregori, J. (2001) Mol. Cell. Biol. 21 8547-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan, Y. Y., Leon, R. P., Marks, R., Cham, C. M., Schaack, J., Gajewski, T. F. & DeGregori, J. (2000) Proc. Natl. Acad. Sci. USA 97 13784-13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRyckere, D. & DeGregori, J. (2002) Methods 26 57-75. [DOI] [PubMed] [Google Scholar]
- 11.Varella-Garcia, M., Gemmill, R. M., Rabenhorst, S. H., Lotto, A., Drabkin, H. A., Archer, P. A. & Franklin, W. A. (1998) Cancer Res. 58 4701-4707. [PubMed] [Google Scholar]
- 12.Nieuwenhuizen, A. G., Schuiling, G. A., Liem, S. M., Moes, H., Koiter, T. R. & Uilenbroek, J. T. (1999) Eur. J. Endocrinol. 140 256-263. [DOI] [PubMed] [Google Scholar]
- 13.Kim, S. K. & MacDonald, R. J. (2002) Curr. Opin. Genet. Dev. 12 540-547. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki, L., Jacks, T., Bronson, R., Goillot, E., Harlow, E. & Dyson, N. J. (1996) Cell 85 537-548. [DOI] [PubMed] [Google Scholar]
- 15.Lukas, C., Sorensen, C. S., Kramer, E., Santoni-Rugiu, E., Lindeneg, C., Peters, J. M., Bartek, J. & Lukas, J. (1999) Nature 401 815-818. [DOI] [PubMed] [Google Scholar]
- 16.Broek, D., Bartlett, R., Crawford, K. & Nurse, P. (1991) Nature 349 388-393. [DOI] [PubMed] [Google Scholar]
- 17.Ren, B., Cam, H., Takahashi, Y., Volkert, T., Terragni, J., Young, R. A. & Dynlacht, B. D. (2002) Genes Dev. 16 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu, M., Hayashi, T., Saitoh, Y. & Itoh, H. (1989) Am. J. Clin. Pathol. 91 531-534. [DOI] [PubMed] [Google Scholar]
- 19.Benoist, C. & Mathis, D. (1997) Cell 89 1-3. [DOI] [PubMed] [Google Scholar]
- 20.Galimberti, M., De Sanctis, V., Lucarelli, G., Polchi, P., Angelucci, E., Baronciani, D., Giardini, C., Erer, B., Gaziev, J., Balducci, R., et al. (1993) Bone Marrow Transplant. 12 Suppl. 1, 102-103. [PubMed] [Google Scholar]
- 21.Hess, D., Li, L., Martin, M., Sakano, S., Hill, D., Strutt, B., Thyssen, S., Gray, D. A. & Bhatia, M. (2003) Nat. Biotechnol. 21 763-770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.