Abstract
Fifty percent of the world's population is infected with Helicobacter pylori; however, treatment has been insufficient to eradicate the organisms due to rising antibiotic resistance. Helicobacter infection is characterized by induction of a T helper 1 lymphocyte (Th1) immune response, hypergastrinemia, and suppressed tissue somatostatin (SOM) levels. However, the mechanism by which the immune response regulates acid secretion is not known. We show here that treatment with IFN-γ, a Th1 cytokine, was sufficient to induce gastritis, increase gastrin, and decrease SOM levels within 7 days. In contrast, the T helper 2 lymphocyte cytokine IL-4 increased SOM levels and effectively suppressed gastrin expression and secretion. This result demonstrated reciprocal regulation of acid regulatory peptides by immune modulators. IL-4 pretreatment prevented gastritis in infected wild-type but not in SOM null mice. Thus, the ability of IL-4 to oppose a Th1-mediated infection required SOM. Immunofluorescence was used to document the presence of IL-4 receptors on the gastric SOM-secreting cell (D cell). Moreover, IL-4 stimulated SOM release from primary D cell cultures. Treatment of mice chronically infected with Helicobacter felis for 2 mo with the SOM analogue octreotide resolved the inflammation. Thus, a mechanism by which IL-4 resolves inflammation in the stomach is by stimulating the release of SOM from gastric D cells.
Keywords: gastrin, IFN-γ, inflammation, octreotide, Th2
Chronic inflammation of the gastric mucosa (chronic gastritis) is caused by Helicobacter pylori or by bacterial overgrowth within the hypochlorhydric stomach (1, 2). In some human subjects, chronic gastritis eventually leads to persistent acid hypersecretion and duodenal ulcers (3) or atrophy of the gastric glands (4) subsequent to the appearance of intestine-like cells (intestinal metaplasia) (5). In a small percentage of these infected subjects, the metaplastic changes predispose the stomach to cancer. Chronic gastritis is accompanied by reciprocal changes in the gastric neuroendocrine cell populations regulating acid secretion (6). The number of gastrin-secreting cells (G cells) increases, whereas the somatostatin-(SOM) secreting cells (D cells) decrease. Therefore, Helicobacter-infected subjects become hypergastrinemic (7–10). D cells inhibit gastrin release and parietal cell acid secretion by paracrine-mediated release of the neuropeptide SOM. We have recently shown that SOM is suppressed by high levels of plasma gastrin (11). Due to the hypergastrinemia that develops during infection, the stomach becomes poised to maximize acid secretion by two mechanisms: by gastrin-augmenting parietal cell acid secretion and by gastrin-blocking release of the inhibitory peptide SOM. Although reciprocal changes between gastrin and SOM occur during H. pylori infection, their link to immune regulators is poorly understood. Therefore, the goal of this study was to define the mechanisms by which the immune response generated by Helicobacter infection regulates acid regulatory peptides.
Like humans, mice and primates infected with H. pylori exhibit a T helper 1 lymphocyte (Th1) immune response characterized by recruitment of primarily IFN-γ-expressing T lymphocytes to the stomach and low levels of T helper 2 lymphocytes (Th2) expressing IL-4 (12–14). IFN-γ-null mice do not mount an inflammatory response even after 15 mo of H. pylori infection (15, 16). In contrast, mice deficient in IL-4 exhibit a skewed Th1 immune response to H. pylori colonization (16). Thus, it is believed that IFN-γ plays a pivotal role in orchestrating the mucosal damage observed during gastritis. However, the effect of IFN-γ on secretion of the major acid regulatory peptides has not been examined. It has been established by using primary gastric cultures that SOM inhibits both G cell and parietal cell secretion (17). Moreover, tumor necrosis factor-α inhibits SOM release from isolated D cells (18). IL-1β stimulates gastrin secretion from primary gastric cultures (19). Thus there is collective in vitro evidence suggesting inflammatory cytokine regulation of gastric peptide secretion.
Given the correlation of these Th1 cytokines to the regulation of peptide secretion, we sought to understand whether there is a similar relationship of a Th2 cytokine to the acid secretory inhibitor SOM. The relationship of Th2 cytokines to gastric SOM levels has not been studied. Because SOM levels are decreased in infected patients, establishing a link between SOM and Th2 cytokines would provide an explanation as to why Helicobacter gastritis is a Th1 predominant event. Moreover, because effective adaptive immunity and antibody production are mediated by IL-4 cytokine production, establishing a critical link between SOM and the immune response may facilitate the generation of an effective vaccine. Indeed, dyspeptic subjects who show impaired mucosal immunity and reduced IL-4 secretion fail to eradicate H. pylori (20).
Normalizing the balance between Th1 and -2 immune responses is a mechanism used to resolve tissue damage and associated complications at mucosal surfaces (21). Similar approaches have been proposed to treat the Th1 predominant Helicobacter infection. Coinfection of mice with both Helicobacter and a helminth is sufficient to attenuate the expected Th1-mediated gastritis normally generated by bacteria (22). Parasitic infections activate a Th2 immune response, raising the possibility that counterbalancing a Th1-mediated infection with a Th2 cytokine might be sufficient to prevent Helicobacter gastritis, but this has not been directly tested.
The role of SOM in modulating an immune response has been studied in the spleens of mice infected with Schistosoma mansoni (23). The parasite generates a Th2 immune response by inducing granuloma-based macrophages to secrete IL-4 and SOM (24). IL-4 secreted from granulomas blocks the release of IFN-γ (25). To test whether a similar cytokine circuit exists in the stomach, we examined a role for IL-4 in preventing Helicobacter gastritis and tested whether IL-4 acts through the release of SOM.
Methods
Animal Treatments. To insert the Alzet microosmotic pump (Model 1007D, Durect Corporation, Cupertino, CA) into the peritoneal cavity, wild-type C57BL/6 mice were anaesthetized at 2 mo of age with xylazine (20 mg/ml) and ketamine (100 mg/ml). The pumps delivered PBS or 250 units/kg (15 units per mouse per day) of recombinant mouse IFN-γ (R & D Systems) or IL-4 (R & D Systems) for 7 days before death. Two separate experiments were performed by using four mice per experiment.
SOM null (SOM-/-) mice were bred onto a C57BL/6 congenic background (26). Both wild-type (SOM+/+) and SOM-/- mice were infused with IL-4 for 7 days, as described above, before inoculation with H. felis (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org, for details of bacterial strains, culture conditions and quantification of Helicobacter colonization). For cytokine treatment >7 days, mice were injected with IL-4 (15 units per mouse per day, i.p.) during the 4-week H. felis infection subsequent to the 7-day preinfusion. In a separate series of experiments, SOM+/+ mice infected with H. felis for 2 mo were treated with 30 μg/kg per day i.p. of octreotide (OCT) (Novartis Pharmaceuticals, East Hanover, NJ) for 1 mo. The mice were killed after a total of 3 mo of treatment.
Immunohistochemistry and Immunofluorescence. Methods and reagents used for immunohistochemistry and immunofluorescence are described in Supporting Materials and Methods.
Gastric Acidity and RIAs. Gastric acidity and RIAs were performed as previously published (11, 27). For details, see Supporting Materials and Methods.
Real-Time PCR for IFN-γ Expression. Total RNA was isolated from stomach tissue by using TRIzol Reagent. PCR primers and fluorogenic probes for both IFN-γ and GAPDH genes were designed according to Overbergh et al. (28). Each PCR amplification was performed in triplicate wells in a Bio-Rad I-Cycler. The amount of IFN-γ in each sample was calculated from the standard curve and normalized to the amount of GAPDH. The results were expressed as a ratio of IFN-γ to GAPDH mRNA. For details of primer and probe sequences, PCR conditions and standard curve, refer to Supporting Materials and Methods.
SOM Release from Cultured Canine D Cells. SOM release from cultured canine D cells has been described (29). For a detailed description of the method, refer to Supporting Materials and Methods.
Cell Isolation and Flow Cytometry. Lymphocytes were isolated from the gastric mucosa according to a previously modified method by using dispase (27). For methods and reagents used, refer to Supporting Materials and Methods.
Gastrin and SOM Release from Cultured Mouse Gastric Cells. Refer to Supporting Materials and Methods for details of the method.
Statistical Analysis. The results were analyzed by the unpaired t test by using commercially available software (PRISM, GraphPad, San Diego). P < 0.05 was considered significant.
Results
IFN-γ and IL-4 Induce Reciprocal Changes in the Gastric Peptides Regulating Acid Secretion. H. pylori infection generates Th1 predominant gastric inflammation in human subjects (30). In mice, a comparison between the cytokine profiles of H. pylori- and H. felis-infected mice reveals that both animal models show a Th1 immune response characterized by increased IFN-γ expression (13, 22, 31). IFN-γ is the predominant cytokine generated in a Th1 response, yet its effect on the levels of acid regulatory peptides is unknown. Moreover, regulation of acid secretion by IL-4 or other Th2 cytokines has not been evaluated. To dissect the mechanism by which inflammatory cytokines regulate acid secretion, gastrin and SOM levels were analyzed after IFN-γ or IL-4 infusion into mice. IFN-γ alone was sufficient to recapitulate the mucosal changes observed with Helicobacter infection. Normal mice were infused with 15 units of the cytokine per day for 7 days and compared with the effect of infusing IL-4. Morphometric analysis of immunostained stomach antrums revealed a 2-fold increase in the number of G cells quantified by morphometry (Fig. 1 a and b). Plasma gastrin levels increased 2.5-fold with IFN-γ infusion (Fig. 1b). In contrast, gastrin expression was nearly undetectable after IL-4 infusion (Fig. 1 a and b). Thus, IFN-γ alone was sufficient to stimulate gastrin expression and secretion. IFN-γ infusion suppressed SOM levels (Fig. 1c). Tissue SOM levels in both the fundus and antrum increased in response to IL-4 (Fig. 1c). Thus, IL-4 stimulated gastric SOM expression. As with H. pylori in human subjects (6), we observed the same reciprocal changes in gastrin and SOM in H. felis-infected mice. To establish whether inflammation was present in the mice infused with IFN-γ or IL-4, hematoxylin/eosin-stained sections were examined. Fluorescence-activated cell sorting (FACS) and morphometry were used to quantify the amount of inflammation. After 7 days of IFN-γ infusion, FACS analysis revealed a 3.7-fold increase in the number of immune cells (PBS, 0.8 ± 0.1 × 106 CD3+ cells; IFN-γ, 3.0 ± 0.4 × 106 CD3+ cells, n = 3) consistent with a significant inflammatory response (Fig. 1d). In contrast, IL-4 treatment resulted in a 40% decrease in T cell numbers (IL-4, 0.5 ± 0.1 × 106 CD3+ cells) compared with PBS controls. Morphometric analysis of CD4+ cells correlated with the total gastric CD3 count (PBS, undetectable; IFN-γ, 207 ± 56 CD4+ lymphocytes/100 epithelial cells; IL-4, undetectable). An increase in periodic acid schiff/alcian blue positive mucous cells (mucous gland metaplasia) in the fundus was also observed (Fig. 1d). However, there were no significant histologic changes in the gastric mucosa after 7 days of IL-4 infusion (data not shown).
Fig. 1.
Reciprocal changes in gastrin and SOM by IFN-γ and IL-4. (a) Immunohistochemical staining of G cells after 7 days of IFN-γ or IL-4 infusion. (b) The G cells were quantified by morphometry, and changes in plasma gastrin were determined by RIA. (c) Tissue SOM was quantified by RIA. IFN-γ inhibited, whereas IL-4 stimulated, the expression of tissue SOM. Shown is the mean ± SEM for eight mice from two independent experiments. *, P < 0.05 relative to PBS-treated mice (unpaired t test). (d) Normal gastric glands in control mice (Top). Recruitment of lymphocytes to the gastric mucosa after 7 days of IFN-γ treatment is shown (arrow, Middle). Mucous gland metaplasia is indicated (open arrow, Bottom). Periodic acid Schiff/alcian blue stains of sections from IFN-γ-infused mice showing mucous gland metaplasia are shown (open arrow, Bottom)(×400).
A time course of the reciprocal changes in gastrin and SOM released in response to IFN-γ or IL-4 was studied by using isolated mouse gastric cells from either SOM+/+ or SOM-/- mice (see Fig. 6, which is published as supporting information on the PNAS web site). IFN-γ stimulated the release of gastrin in the presence (SOM+/+) or absence (SOM-/-) of SOM. Thus, relief from SOM inhibition is not the only mechanism that increases gastrin secretion. In contrast, IL-4 suppressed gastrin release while stimulating SOM secretion. Because the inhibition was delayed in the SOM-/- cells, it appears that IL-4 also exhibits a delayed inhibitory effect on the G cell independent of SOM.
IL-4 Infusion Requires SOM to Prevent the Th1 Immune Response Induced by H. felis It has also been shown that H. felis generates a more robust inflammatory response than H. pylori colonization (32). Therefore, we used H. felis in the following animal studies. The changes observed with IL-4 infusion demonstrated that IL-4 stimulates SOM expression in vivo. Although a Th2 immune response can resolve Helicobacter-induced gastritis (22), the mechanism for the resolution is unknown. IL-4 was one of the major Th2 cytokines generated during resolution of the gastritis (22). Moreover, we showed here that IL-4 stimulates gastric SOM expression (Fig. 1c). Therefore, a plausible mechanism for Th2 resolution of Helicobacter gastritis is IL-4-mediated release of SOM. To determine whether IL-4 is sufficient to prevent gastritis, SOM+/+ mice were infused with the cytokine before and during H. felis infection. Histologic examination revealed that H. felis generated a significant inflammatory response within 1 mo and that the gastritis was prevented by IL-4 (Fig. 2a). To quantify the degree of reduced inflammation mediated by IL-4, FACS was used to analyze changes in the number of T cells and confirmed the reduced inflammation (Table 1 and histograms in Fig. 7, which is published as supporting information on the PNAS web site). A decrease in plasma gastrin and increase in antral SOM levels accompanied resolution of the inflammation (Table 1). These results were as predicted based on the cytokine infusions shown in Fig. 1, the effect of helminths on H. felis gastritis (22), and also the knowledge that Th2 immune responses in other tissues are able to counteract Th1-mediated inflammation (21).
Fig. 2.
IL-4 prevention of H. felis gastritis is mediated by SOM. Shown are hematoxylin/eosin stains of SOM+/+ (a) or SOM-/- (b) mice treated with PBS, H. felis, or IL-4 plus H. felis. Quantitative RT-PCR was performed on total stomach RNA. Shown is the ratio of IFN-γ to GAPDH mRNA in SOM+/+ (c) or SOM-/- (d) mice treated with PBS, H. felis, or IL-4 plus H. felis. Data are the mean ± SEM for n = 8 mice, *, P < 0.05 vs. PBS-treated mice (unpaired t test). IL-4 reduces H. felis colonization. Shown is quantitation by qRT-PCR and morphometry of the amount of H. felis colonizing SOM+/+ (e) or SOM-/- (f) mice. The log fluorescent emission measured continuously during PCR per cycle was determined. Early amplification showed increased “starting” fluorescent emission and was used to compute the number of Helicobacter per gram of tissue for each mouse by using a standard curve generated from known quantities of H. felis. Scatter plots showing the bacterial count for each mouse are shown. The mean indicated by a horizontal bar for n = 8 mice is shown. Bacteria per crypt were counted on the basis of the presence of spiral-shaped organisms characteristic of Helicobacter. The mean ± SEM for n = 8 mice is shown in parentheses. *, P < 0.05 vs. PBS mice; #, P < 0.05 vs. H. felis mice (unpaired t test). N/D, nondetectable.
Table 1. T lymphocytes, gastrin, and SOM levels during H. felis infection and IL-4 treatments.
| Treatment | CD3+ cells/stomach (106) | CD4+ cells/100 epithelial cells | Plasma gastrin, pmol/liter | Tissue SOM, pmol/g | |
|---|---|---|---|---|---|
| SOM+/+ | Fundus | Antrum | |||
| PBS | 0.70±0.13 | ND | 14±2 | 1,278±38 | 2,719±108 |
| H. felis | 3.56±1.17* | 192±32* | 30±2* | <2* | 1,696±101* |
| IL-4 + H. felis | 0.86±0.16† | 5±3† | 12±3 | 1,127±71 | 7,096±175*† |
| SOM-/- | |||||
| PBS | 0.79±0.16 | ND | 63±10 | <2 | <2 |
| H. felis | 3.44±0.40* | 200±37* | 94±33 | <2 | <2 |
| IL-4 + H. felis | 4.59±1.42* | 199±26* | 98±38 | <2 | <2 |
ND, not determined.
P < 0.05 vs. PBS control
P < 0.05 vs. H. felis-infected mice
Having established that IL-4 was sufficient to prevent Helicobacter-induced gastritis, SOM-/- mice were used to determine whether this peptide is required to mediate the effect of IL-4. Indeed, we found that IL-4 did not prevent gastritis in the absence of SOM (Fig. 2b). Significant inflammation and metaplasia were observed in the gastric mucosa of the null mice infected with H. felis with or without IL-4 infusion (Fig. 2b). FACS and morphometric analysis of the inflammation showed an increase in the number of T cells in the infected SOM-/- mice that remained elevated with IL-4 treatment (Table 1 and histograms in Fig. 7). PBS-infused SOM-/- mice were hypergastrinemic at baseline and 3-fold higher than PBS-infused SOM+/+ mice. Furthermore, an increase in plasma gastrin with H. felis demonstrated that inflammation stimulated G cells independent of the effect observed without SOM (Table 1). As expected, tissue SOM levels were undetectable in SOM-/- mice (Table 1). Quantitative PCR demonstrated that tissue IFN-γ gene expression was elevated in the infected animals and that IL-4 suppressed IFN-γ expression (Fig. 2c). However, IFN-γ was not suppressed in the SOM-/- mice infected with H. felis and pretreated with IL-4 (Fig. 2d). Thus, we concluded that SOM is required for IL-4 to prevent H. felis-induced gastritis.
IL-4 Prevents H. felis Colonization in SOM+/+ and SOM-/- Mouse Stomach. We considered that resistance to colonization with Helicobacter might account for the reduced gastritis with IL-4 infusion. To examine this possibility, SOM+/+ and SOM-/- infected mice were analyzed for the presence of H. felis by quantitative RT-PCR and morphometric analysis. In PBS-infused mice, all were negative for the presence of H. felis (Fig. 2 e and f). All SOM+/+ and SOM-/- mice infected with H. felis were positive for the organism. In both groups of mice, colonization was reduced significantly with IL-4 treatment despite persistent inflammation in the SOM-/- group (Fig. 2 e and f). This result demonstrated that SOM was required to reduce the inflammation even though IL-4 was sufficient to kill the bacteria. In addition, there was not a direct correlation between the amount of bacteria and the intensity of the inflammation. Because IL-4 was administered 7 days before inoculation, it is possible that the cytokine reduces the initial level of colonization, increases its rate of clearance or both. As previously reported for mouse models (33, 34) and human subjects (35) infected with H. felis or H. pylori, gastric acid levels in infected SOM+/+ mice were lower than in uninfected animals (Fig. 8, which is published as supporting information on the PNAS web site). In contrast, SOM-/- mice exhibited higher gastric acidity. Thus, reduced SOM levels, which remove the inhibition on the G cell, are central to the development of gastric hyperacidity when the stomach is colonized.
IL-4 Directly Stimulates SOM Release from the Gastric D Cell. Because the in vivo results could be explained by cytokine activation of extragastric regulatory circuits, we cocultured primary cells isolated from the gastric mucosa of SOM+/+ and SOM-/- mice with H. felis in the presence or absence of IL-4 or a SOM antagonist. IFN-γ-expressing CD3+ T lymphocytes were quantified by FACS and revealed an increase with H. felis coculture and a decrease with IL-4 treatment (Fig. 3a). The reduction in IFN-γ-expressing cells was blocked by treatment with a SOM receptor antagonist, demonstrating that SOM mediated the IL-4 effect. To determine whether a SOM analogue was as effective as IL-4, the H. felis coculture was treated with OCT (Fig. 3b). We found that OCT treatment was sufficient to reduce the number of IFN-γ-expressing T lymphocytes to the same level as observed with IL-4. The OCT effect was mediated through a SOM receptor because the antagonist blocked the expected reduction (Fig. 3b). In contrast, the number of IFN-γ-expressing T lymphocytes remained elevated in gastric cells isolated from the SOM-/- mouse treated with IL-4 and H. felis (Fig. 3c). However, OCT treatment was able to overcome the lack of inhibition observed with IL-4 in primary cells isolated from the null mice (Fig. 3d). Therefore, the primary cell culture experiments recapitulated the effects of H. felis and IL-4 observed in vivo. The ability of OCT to mimic the effect of IL-4 demonstrated that SOM was not only required but was also sufficient to prevent Helicobacter gastritis. Moreover, the results revealed the presence of a local immunomodulatory circuit involving IL-4 and the SOM-expressing gastric D cell.
Fig. 3.
Inhibition of IFN-γ by IL-4 in CD3+ cells is mediated by SOM. FACS of CD3+ cells expressing IFN-γ was performed on primary cells isolated from SOM+/+ (a and b) and SOM-/- (c and d) mouse stomachs. Primary cultures were exposed to PBS, H. felis, IL-4, or OCT. In addition, cultures were also exposed to H. felis plus IL-4 or OCT with or without SOM antagonist (ANT). Results are expressed as the mean ± SEM for n = 6 experiments performed in triplicate incubations. *, P < 0.05 vs. PBS-treated cultures (unpaired t test).
To determine whether the source of SOM in the mouse stomach after IFN-γ or IL-4 treatment was of epithelial origin rather than immune origin, immunohistochemical staining of the gastric mucosa was performed. The results show that regardless of the type of chronic cytokine treatment, the source of SOM was an epithelial cell (D cell) (Fig. 9, which is published as supporting information on the PNAS web site). To determine whether gastric D cells respond directly to IL-4 treatment, the IL-4 receptor was colocalized to SOM-expressing gastric cells (Fig. 4a). The increase in IL-4 receptors and SOM expression peaked at 2 h after IL-4 treatment (Fig. 4b). Subsequently, SOM and IL-4 receptor expression decreased by 12 and 24 h after treatment. RIA of SOM in the media demonstrated that treatment with 100 nM IL-4 significantly stimulated SOM release within 2 h (Fig. 4c). The amount of total cell SOM content released by IL-4 was 5% and well within the range of levels observed in response to other potent SOM secretagogues (36, 37). After 12 and 24 h of IL-4 incubation, SOM release was undetectable by RIA and correlated with a decrease in IL-4 receptor expression (Fig. 4 b and c). Collectively, these results show that IL-4 stimulates SOM release directly by binding to the IL-4 receptor on the gastric D cell.
Fig. 4.
SOM-secreting D cells express IL-4 receptors. (a Left) Immunofluorescence of isolated canine fundic SOM-secreting D cells (Texas red) that colocalizes with IL-4 receptors (FITC). A cytoplasmic process characteristic of the D cell is shown by the arrow. (b) Summary of FACS of the percent of isolated canine fundic D cells expressing IL-4 receptors after 2, 12, and 24 h of treatment with 100 nM IL-4. (c) SOM release in response to100 nM IL-4 for 2, 12, and 24 h. The results are expressed as the percent of initial cell SOM content (700–1,000 pmol/liter) for three independent experiments. *, P < 0.05 vs. unstimulated (unpaired t test).
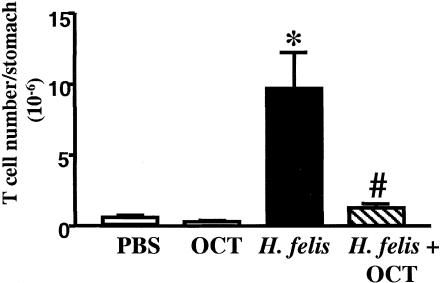
Treatment of Helicobacter-Induced Gastritis with OCT. To demonstrate that SOM alone is sufficient to resolve Helicobacter gastritis, SOM+/+ mice were infected with H. felis for 2 mo before treatment with the SOM analogue, OCT. FACS showed a significant reduction in the total number of T cells after H. felis mice were treated with OCT (Fig. 5). The histochemical stains (hematoxylin/eosin), CD4 immunostaining, and bacterial quantitation correlated with the decrease in the T cells (see Fig. 10, which is published as supporting information on the PNAS web site). IFN-γ expression and plasma gastrin decreased with OCT, and tissue SOM returned to baseline (Table 2, which is published as supporting information on the PNAS web site). Thus, OCT reduced Helicobacter colonization and resolved the Helicobacter-induced gastritis.
Fig. 5.
OCT resolves H. felis gastritis. The number of T cells (CD3+) after a 2-mo H. felis infection was analyzed by FACS. Shown is the mean ± SEM for eight mice. *, P < 0.05 vs. OCT alone-treated mice; #, P < 0.05 vs. H. felis-treated mice (unpaired t test).
Discussion
The findings reported here show that gastrin and SOM are peptides regulated by IFN-γ and IL-4 cytokines (Fig. 11, which is published as supporting information on the PNAS web site). Of therapeutic importance, Helicobacter colonization as well as the resulting gastritis resolved by treating infected mice with IL-4. Moreover, the mechanism of the IL-4 anti-inflammatory effect is through increased expression and release of SOM from the gastric D cell. Furthermore, we showed that gastrin expression is significantly induced by the proinflammatory cytokine IFN-γ. Taken together, these results imply that the reciprocal changes in gastrin and SOM during Helicobacter infection in human subjects may be due to an increase in IFN-γ expression and release during a Th1-mediated gastritis and not due to a direct effect of the organism. A major consequence of persistent hypergastrinemia induced by the inflammation is reduced tissue SOM expression (11). Therefore, any IL-4 that might be generated during the course of the infection would be rendered ineffective due to depressed tissue SOM levels.
In the absence of SOM (SOM-/- mice), basal gastrin concentrations were higher compared with wild-type controls. These results confirm a critical role for SOM in regulating basal levels of gastrin expression (38). The increase in gastrin in SOM-/- mice with Helicobacter infection shows that inflammation is an independent regulator of gastrin, supporting our prior results (11, 27). Thus, gastrin secretion is a component of the gastric innate immune system due to its ability to be regulated by a proinflammatory cytokine. A consequence of inflammation-induced hypergastrinemia is reduced SOM levels and maximal output of the major gastric antimicrobial agent (i.e., acid) from the proximal gut epithelium.
Unlike IFN-γ, we found that IL-4 stimulates the release of SOM directly through IL-4 receptors located on gastric D cells. Thus, SOM is both necessary and sufficient to prevent colonization and the subsequent development of gastritis by Helicobacter. Stimulation of SOM by IL-4 was required to reduce inflammatory T cells recruited during Helicobacter-induced gastritis, suppress IFN-γ expression, and resolve hypergastrinemia. SOM has been shown to be an important modulator of the immune system, down-regulating a number of functions including lymphocyte proliferation, Ig production (39), IFN-γ, tumor necrosis factor α, IL-1β production (40, 41), and granuloma formation (42).
IL-4 inhibited colonization by H. felis in the stomachs of SOM+/+ and SOM-/- mice. Our finding supports a prior study showing that transgenic expression of IL-4 in mice infected with Helicobacter reduces colonization (43). In addition, mice coinfected with H. felis and a helminth produce increased tissue levels of Th2 cytokines, IL-4, IL-10, and TGF-β, with reduced levels of colonization (22). Yamashita et al. (44) have shown that SOM exhibits direct anti-proliferative effects on H. pylori through a cGMP-dependent pathway. Therefore, an explanation for reduced bacterial colonization with IL-4 or OCT treatment may be a direct effect of secreted SOM or its analogue on the organism. In addition, IL-4 appeared to have an inhibitory effect on Helicobacter exclusive of SOM, because there was also decreased colonization in the IL-4-treated SOM-/- mice. However, that IL-4 could reduce colonization but not inflammation in the absence of SOM suggests a more potent effect of SOM on the immune response. The colonization results also confirm a lack of correlation between the quantity of bacteria and the intensity of the immune.
A model response for the immune regulation of gastrin and SOM is depicted (Fig. 11). Gastrin is released in response to IFN-γ (Fig. 11a). IFN-γ removes the inhibitory influence of SOM by suppressing its release from the gastric D cells (Fig. 11). SOM released acutely by IL-4 inhibits gastrin secretion primarily by a direct paracrine effect on the G cell (Fig. 11b). In addition, studies using primary SOM-/- cells uncovered a delayed response by the G cell to IL-4 that is independent of SOM. SOM is also known to act directly on T cells to inhibit IFN-γ production (42). In support of our model, we show here that systemic administration of a SOM analogue is both necessary and sufficient to reestablish the Th1/2 equilibrium and reduce inflammation. Thus, we propose that SOM analogues, e.g., OCT, may be relevant and useful in treating Helicobacter infections in human subjects, preventing further mucosal damage and chronic sequelae and facilitating the generation of a Th2 response with effective antibody production (20).
Supplementary Material
Acknowledgments
This work was supported in part by Public Health Service Grants DK45729, DK61410, and DK62041 (to J.L.M.). Rabbit gastrin antibody no. 1296 and SOM antibody no. 1001 were from the Center for Ulcer Research and Education Digestive Disease Research Center/RIA Core (University of California, Los Angeles) (Grant DK41301). Flow Cytometry was performed by the University of Michigan Cancer Center Core (Grant CA46952), and RIAs were from the University of Michigan Gastrointestinal Peptide Research Center (Grant DK34533).
Abbreviations: SOM, somatostatin; D cell, SOM-secreting cell; G cell, gastrin-secreting cell; SOM-/-, SOM null mice; SOM+/+, wild-type mice; Th1, T helper 1 lymphocyte; Th2, T helper 2 lymphocyte; OCT, octreotide; FACS, fluorescence-activated cell sorting.
References
- 1.Blaser, M. J. & Parsonnet, J. (1994) J. Clin. Invest. 94 4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McColl, K. E., el-Omar, E., Gillen, D. & Banerjee, S. (1997) Semin. Gastrointest. Dis. 8 142-155. [PubMed] [Google Scholar]
- 3.el-Omar, E. M., Penman, I. D., Ardill, J. E., Chittajallu, R. S., Howie, C. & McColl, K. E. (1995) Gastroenterology 109 681-691. [DOI] [PubMed] [Google Scholar]
- 4.Martinez, V., Curi, A. P., Torkian, B., Schaeffer, J. M., Wilkinson, H. A., Walsh, J. H. & Tache, Y. (1998) Gastroenterology 114 1125-1132. [DOI] [PubMed] [Google Scholar]
- 5.Correa, P. (1992) Cancer Res. 52 6735-6740. [PubMed] [Google Scholar]
- 6.Sumii, M., Sumii, K., Tari, A., Kawaguchi, H., Yamamoto, G., Takehara, Y., Fukino, Y., Kamiyasu, T., Hamada, M., Tsuda, T., et al. (1994) Am. J. Gastroenterol. 89 1515-1519. [PubMed] [Google Scholar]
- 7.Peterson, W. L., Barnett, C. C., Evans, D. J., Jr., Feldman, M., Carmody, T., Richardson, C., Walsh, J. & Graham, D. Y. (1993) Am. J. Gastroenterol. 88 2038-2043. [PubMed] [Google Scholar]
- 8.Odum, L., Petersen, H. D., Andersen, I. B., Hansen, B. F. & Rehfeld, J. F. (1994) Gut 35 615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss, S. F., Legon, S., Bishop, A. E., Polak, J. M. & Calam, J. (1992) Lancet 340 930-932. [DOI] [PubMed] [Google Scholar]
- 10.Queiroz, D. M. M., Moura, S. B., Mendes, E. N., Rocha, G. A., Barbosa, A. J. A. & Carvalho, A. S. T. (1994) Lancet 343 1191-1193. [DOI] [PubMed] [Google Scholar]
- 11.Zavros, Y., Rieder, G., Ferguson, A., Samuelson, L. C. & Merchant, J. L. (2002) Am. J. Physiol. Gastrointest. Liver Physiol. 282 G175-G183. [DOI] [PubMed] [Google Scholar]
- 12.Bamford, K. B., Fan, X., Crowe, S. E., Leary, J. F., Gourley, W. K., Luthra, G. K., Brooks, E. G., Graham, D. Y., Reyes, V. E. & Ernst, P. B. (1998) Gastroenterology 114 482-492. [DOI] [PubMed] [Google Scholar]
- 13.Eaton, K. A., Mefford, M. & Thevenot, T. (2001) J. Immunol. 166 7456-7461. [DOI] [PubMed] [Google Scholar]
- 14.Mattapallil, J. J., Dandekar, S., Canfield, D. R. & Solnick, J. V. (2000) Gastroenterology 118 307-315. [DOI] [PubMed] [Google Scholar]
- 15.Sawai, N., Kita, M., Kodama, T., Tanahashi, T., Yamaoka, Y., Tagawa, Y., Iwakura, Y. & Imanishi, J. (1999) Infect. Immun. 67 279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smythies, L. E., Waites, K. B., Lindsey, J. R., Harris, P. R., Ghiara, P. & Smith, P. D. (2000) J. Immunol. 165 1022-1029. [DOI] [PubMed] [Google Scholar]
- 17.Calam, J. (1999) Yale J. Biol. Med. 72 195-202. [PMC free article] [PubMed] [Google Scholar]
- 18.Beales, I., Calam, J., Post, L., Srinivasan, S., Yamada, T. & DelValle, J. (1997) Gastroenterology 112 136-143. [DOI] [PubMed] [Google Scholar]
- 19.Weigert, N., Schaffer, K., Schusdziarra, V., Classen, M. & Schepp, W. (1996) Gastroenterology 110 147-154. [DOI] [PubMed] [Google Scholar]
- 20.Borody, T., Ren, Z., Pang, G. & Clancy, R. (2002) Am. J. Gastroenterol. 97 3032-3037. [DOI] [PubMed] [Google Scholar]
- 21.Neurath, M. F., Finotto, S. & Glimcher, L. H. (2002) Nat. Med. 8 567-573. [DOI] [PubMed] [Google Scholar]
- 22.Fox, J. G., Beck, P., Dangler, C. A., Whary, M. T., Wang, T. C., Shi, H. N. & Nagler-Anderson, C. (2000) Nat. Med. 6 536-542. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock, J. V. & Elliott, D. (2002) Eur. J. Endocrinol. 143 Suppl. 1, S15-S19. [DOI] [PubMed] [Google Scholar]
- 24.Blum, A. M., Elliott, D. E., Metwali, A., Li, J., Qadir, K. & Weinstock, J. V. (1998) J. Immunol. 161 6316-6322. [PubMed] [Google Scholar]
- 25.Blum, A. M., Metwali, A., Mathew, R. C., Cook, G., Elliott, D. & Weinstock, J. V. (1992) J. Immunol. 149 3621-3626. [PubMed] [Google Scholar]
- 26.Low, M. J., Otero-Corchon, V., Parlow, A. F., Ramirez, J. L., Kumar, U., Patel, Y. C. & Rubinstein, M. (2001) J. Clin. Invest. 107 1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zavros, Y., Rieder, G., Ferguson, A., Samuelson, L. C. & Merchant, J. L. (2002) Gastroenterology 122 119-133. [DOI] [PubMed] [Google Scholar]
- 28.Overbergh, L., Valckx, D., Waer, M. & Mathieu, C. (1999) Cytokine 11 305-312. [DOI] [PubMed] [Google Scholar]
- 29.Ayalon, A., Sanders, M. J., Thomas, L. P., Amirian, D. A. & Soll, A. H. (1982) Proc. Natl. Acad. Sci. USA 79 7009-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ernst, P. B. & Gold, B. D. (2000) Annu. Rev. Microbiol. 54 615-640. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi, M., Nedrud, J., Redline, R., Lycke, N. & Czinn, S. J. (1997) Gastroenterology 113 1848-1857. [DOI] [PubMed] [Google Scholar]
- 32.Court, M., Robinson, P. A., Dixon, M. F. & Crabtree, J. E. (2002) J. Infect. Dis. 186 1348-1352. [DOI] [PubMed] [Google Scholar]
- 33.Konturek, P. C., Brzozowski, T., Konturek, S. J., Stachura, J., Karczewska, E., Pajdo, R., Ghiara, P. & Hahn, E. G. (1999) Aliment. Pharmacol. Ther. 13 333-346. [DOI] [PubMed] [Google Scholar]
- 34.Dial, E. J., Hall, L. R., Romero, J. J., Lechago, J., Fox, J. G. & Lichtenberger, L. M. (2000) Dig. Dis. Sci. 45 1308-1314. [DOI] [PubMed] [Google Scholar]
- 35.Yasunaga, Y., Shinomura, Y., Kanayama, S., Higashimoto, Y., Yabu, M., Miyazaki, Y., Murayama, Y., Nishibayashi, H., Kitamura, S. & Matsuzawa, Y. (1997) Aliment. Pharmacol. Ther. 11 801-809. [DOI] [PubMed] [Google Scholar]
- 36.Yamada, T., Soll, A. H., Park, J. & Elashoff, J. (1984) Am. J. Physiol. 247 G567-G573. [DOI] [PubMed] [Google Scholar]
- 37.Soll, A. H., Yamada, T., Park, J. & Thomas, L. P. (1984) Am. J. Physiol. 247 G558-G566. [DOI] [PubMed] [Google Scholar]
- 38.Godley, J. M. & Brand, S. J. (1989) Proc. Natl. Acad. Sci. USA 86 3036-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krantic, S. (2000) Peptides 21 1941-1964. [DOI] [PubMed] [Google Scholar]
- 40.Muscettola, M. & Grasso, G. (1990) Int. J. Neurosci. 51 189-191. [DOI] [PubMed] [Google Scholar]
- 41.Chowers, Y., Cahalon, L., Lahav, M., Schor, H., Tal, R., Bar-Meir, S. & Levite, M. (2000) J. Immunol. 165 2955-2961. [DOI] [PubMed] [Google Scholar]
- 42.Elliott, D. E., Li, J., Blum, A. M., Metwali, A., Patel, Y. C. & Weinstock, J. V. (1999) Eur. J. Immunol. 29 2454-2463. [DOI] [PubMed] [Google Scholar]
- 43.Chen, W., Shu, D. & Chadwick, V. S. (1999) Scand. J. Gastroenterol. 34 987-992. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita, K., Kaneko, H., Yamamoto, S., Konagaya, T., Kusugami, K. & Mitsuma, T. (1998) Gastroenterology 115 1123-1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.