Abstract
Eradication of HIV infection depends on the elimination of a small, but stable population of latently infected T cells. After the discontinuation of therapy, activation of latent virus can rekindle infection. To purge this reservoir, it is necessary to define cellular signaling pathways that lead to activation of latent HIV. We used the SCID-hu (Thy/Liv) mouse model of HIV latency to analyze a broad array of T cell-signaling pathways and show in primary, quiescent cells that viral induction depends on the activation of two primary intracellular signaling pathways, protein kinase C or nuclear factor of activated T cells (NF-AT). In contrast, inhibition or activation of other important T cell stimulatory pathways (such as mitogen-activated protein kinase, calcium flux, or histone deacetylation) do not significantly induce virus expression. We found that the activation of NF-κB is critical to viral reactivation; however, all pathways that stimulate NF-κBdonot reactivate latent virus. Our studies further show that inhibition of NF-κB does not prevent activation of HIV by NF-AT, indicating that these pathways can function independently to activate the HIV LTR. Thus, we define several molecular pathways that trigger HIV reactivation from latency and provide evidence that latent HIV infection is maintained by the functional lack of particular transcription factors in quiescent cells.
Complete eradication of HIV has largely been prevented by the presence of a long-lived, stable population of latently infected, quiescent T cells (reviewed in refs. 1–5). Highly active antiretroviral therapy has been successful in substantially reducing viral loads; however, after the cessation of therapy, virus derived from the latent reservoir is able to rekindle infection (6–12). The generation of latency occurs after HIV infection of a transcriptionally active cell, which predominantly results in productive infection and cell death. However, if cellular transcription ceases before either viral or immunologic cytopathic effects, the virus can become dormant (13). The decline in HIV expression is tightly linked to decreased cellular activation; however, it is currently unclear what factors and signaling pathways subsequently reactivate virus expression in primary cells. Because viral reactivation is required for recognition by antiviral agents (14), these pathways could ultimately serve as targets for future therapies. Consequently, it is important to identify the molecular events in T cells that regulate the transition from latent to productive infection.
Activation of a T cell is initiated when its T cell receptor (TcR) interacts with an antigen-presenting cell (APC) displaying a foreign peptide in the context of MHC. This interaction sends a signal via the TcR-associated protein CD3 to promote cell activation. However, productive T cell activation requires secondary stimulatory signals, such as through CD28 (reviewed in ref. 15). Together, these costimulatory signals result in activation of transcription factors that regulate cellular activation, entrance into the cell cycle, proliferation, and differentiation into an effector cell. Crucial to the initial activation is the binding of the CD4 molecule on the surface of the T cell to MHC II on the APC. This interaction places the src-family kinase Lck in proximity with the TcR complex, initiating the phosphorylation of CD3-associated proteins and promoting the first steps in TcR signaling, including an increase in intracellular calcium concentration and activation of PKC (reviewed in ref. 15). An important role of these pathways and others triggered by TcR stimulation is to activate the transcription factors nuclear factor of activated T cells (NF-AT) and NF-κB. Both NF-AT and NF-κB are present in unstimulated cells in an inactive form (16, 17). Cellular stimulation then allows their immediate activation and translocation to the nucleus whereby, in collaboration with other factors, they induce the expression of a variety of genes that promote T cell activation. Although relatively poorly defined, it seems that the primary role of CD28 is to stimulate signals via the phosphatidylinositol 3-kinase (PI3-K) pathways to induce IL-2 production, which is critical for T cell proliferation and survival.
The HIV LTR contains multiple regulatory elements that bind to cellular transcription factors and control viral transcription (18). However, the scarcity of latently infected cells in vivo (19) and the coculture conditions necessary for their analysis have largely prevented a detailed examination of the molecular pathways and factors that regulate latent virus reactivation in primary cells. Various chronically/latently infected cell lines have shown that signaling events that activate NF-κB translocation into the nucleus are particularly important (ref. 20 and reviewed in ref. 21). Stimulation of latently infected cell lines with the cytokine tumor necrosis factor (TNF) α activates viral expression in an NF-κB-dependent fashion (22–24) whereas deletion of the κB sites in the viral LTR dramatically diminishes viral expression (23, 25). After antigenic stimulation of T cells, stimulation of PKC induces NF-κB activation (26, 27). Direct activation of PKC by phorbol esters induces HIV expression in latently infected cell lines (28–30) by facilitating the binding of the NF-κB and AP1 transcription factors to the viral LTR (31, 32). Importantly, we and others have shown that phorbol esters potently induce expression of latent virus in peripheral blood mononuclear cells and primary, quiescent T cells (33, 34). Induction of other transcription factors in these cell lines, such as AP-1 by the mitogen-activated protein kinase pathway or Sp1, also activate HIV LTR activity however, their action is largely enhanced via interaction with NF-κB (35–37). Like NF-κB, activation of the transcription factor NF-AT is induced early after T cell stimulation and is critical to T cell activation and proliferation (16). Although it is unclear how the transcription factor NF-AT affects latent HIV, there are NF-AT-binding sites in the viral LTR (18), and stable expression of an active form of NF-AT in primary T cells dramatically enhances HIV replication during initial infection (38, 39).
There is also evidence in cell lines that viral latency is maintained by active repression of the viral LTR. The site of proviral integration, local chromatin structure, and DNA methylation all could affect replication because integration of the virus into a transcriptionally inactive chromosomal site might impede viral transcription (40). Accordingly, incubation of some chronically/latently infected cell lines with a histone deacetylase (HDAC) inhibitor such as trichostatin A (TsA), to induce nucleosomal remodeling and over-ride chromosomal repression, reactivates latent HIV expression (40, 41). Similarly, the presence of binding sites for the HDAC-sequestering transcription factors YYI and LSF on the LTR might actively maintain the packed chromatin structure (42).
The relationship of cell line models to latent infection in primary, quiescent cells still remains unclear. It has been extensively shown that the quiescent state is associated with latency in primary cells (5). Therefore, the finding that latency is maintained in proliferating cell lines may indicate an unusual mechanism for the formation of latency, such as the TAR and tat mutations that produce the latent infection of the ACH-2 and U1 cell lines, respectively (43, 44), or selection and outgrowth of a very few integration events into a transcriptionally dormant chromosomal location (40). Moreover, studies using these cell lines to analyze specific signaling pathways may be confounded by the existence of activated factors that are not present in quiescent cells. Thus, it is important to determine the pathways that maintain latency and regulate viral reactivation in primary, quiescent cells.
To analyze the molecular events that regulate latent infection, we used the SCID-hu (Thy/Liv) model of HIV latency (13). The SCID-hu (Thy/Liv) mouse contains coimplanted human fetal liver, as a source of human hematopoietic progenitor cells, and human fetal thymus to provide a microenvironment for human T lymphopoiesis (45). Functionally and phenotypically normal human T cells are generated in this system for over 1 yr (46). This model has been used extensively to study HIV pathogenesis and to model therapeutic approaches to infection (reviewed in refs. 47 and 48). Using HIV-infected SCID-hu (Thy/Liv) mice, we have previously established that the decrease in cellular RNA transcription as thymocytes mature can result in the generation of a stable and abundant source of cells latently infected with HIV (13). This system provides an opportunity to quantitate viral reactivation at the single cell level and determine the effects of various stimuli on latent infection. In the present study, we illustrate the importance of PKC and NF-AT activity in viral reactivation from latency. We show that induction of these factors alone, in the absence of other signaling pathways that are normally activated during T cell stimulation, is able to activate latent virus. Furthermore, viral reactivation is facilitated by NF-κB activity although not all pathways that activate NF-κB reactivate latent virus.
Materials and Methods
Infection of SCID-hu Mice. SCID-hu mice were prepared by implantation of human fetal liver and thymus (Advanced Bioscience Resource, Alameda, CA) as described (13). Thy/Liv implants were mock-infected with medium, with HIVNL-r-HSAS,orwithHIVJR-CSF by direct injection (13). p24 gag protein production during culture was assessed by ELISA (Coulter).
Cell Isolation, Culture, and Treatment. Thy/Liv implants were harvested 5 weeks postinfection, and single cell suspensions of thymocytes were immediately pooled in the presence of 100 ng/ml Indinavir (Merck) and 10 μM 3′-azido-3′-deoxythymidine (AZT, Sigma). Thymocytes from mock-infected implants were isolated and cultured in parallel for each condition. To enrich for CD4 single positive (SP) latently infected thymocytes, total thymocytes were stained with antibodies to human CD8 (Miltenyi Biotec, Auburn, CA) and negatively selected by using the autoMacs magnetic cell sorter (Miltenyi Biotec). These cells were then stained with a rat antibody to murine CD24 (muCD24; Pharmingen) and depleted of productively infected cells by panning in flasks coated with rabbit anti-rat antibody (Sigma). Purity was assessed by flow cytometry using different antibody clones to avoid epitope masking (Coulter). All cells were cultured in RPMI medium 1640 supplemented with 2% human AB serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 100 nM Indinavir, and 10 μM AZT.
Thymocytes were costimulated with plate-bound anti-CD3 and soluble anti-CD28 as described (13). For inhibition studies, isolated thymocytes were preincubated in the indicated inhibitor for 1 hr before costimulation. The concentrations of each inhibitor or activator are based on previously published concentrations and are as follows: 4 mM N-acetyl butyric acid (Sigma), 50 nM wortmannin (Calbiochem), 10 μM PP2, 1 μM bisindolmaleimide II (Calbiochem), 1 μM H-89 (Calbiochem), 500 ng/ml cyclosporin A (Sigma), 10 μM PD98059 (Calbiochem), 1 μM ionomycin (Sigma), 50 nM thapsagargin (Sigma), 200 nM TsA (Sigma), 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma), 1 μM prostratin, 2 μg/ml phytohemagglutinin (PHA), 1.5 μM gliotoxin (Sigma), 100 ng/ml cycloheximide (Sigma), 500 ng/ml IL-16, and 100 units/ml TNFα (R & D Systems).
Flow Cytometry. Cells from cultures were stained with mAb specific for human CD8, CD4, CD45, and muCD24 directly conjugated to peridinin chlorophyll protein (PerCP; Becton Dickinson), phycoerythrin, allophycocyanin, or FITC (Coulter), respectively. Samples were analyzed on a FACSCalibur flow cytometer using the CELL QUEST program (Becton Dickinson). Forward vs. side-scatter profiles were used to define the live population. These cells were further gated on the human CD45-positive population to exclude murine cells. Quadrants for the assessment of HIV reporter expression were set such that uninfected cells treated in parallel had <1% muCD24 expression. Cell cycle analysis of CD4 SP thymocytes from the Thy/Liv implant was performed by flow cytometry as described (49).
RNA Analysis. CD4 SP,muCD24-negative thymocytes were obtained, and total RNA was isolated with the RNeasy extraction kit (Qiagen, Chatsworth, California). Reverse transcription and PCR amplification were performed for viral LTR/gag and cellular β2-microglobulin (β2m) RNA sequences with the Qiagen OneStep RT-PCR kit by using the manufacturer's protocol. To quantitate the number of LTR/gag or β2m transcripts, in vitro transcribed standards were generated (Promega). Serial dilutions of each in vitro transcript were amplified in parallel. The in vitro transcripts were detectable to below 10 RNA copies. HIV LTR/gag and β2m primers have been described (14). All RT-PCR amplifications were performed on the ABI7700 (Applied Biosystems) as described (14). Potential DNA contamination was excluded with a no reverse transcription reaction.
Results
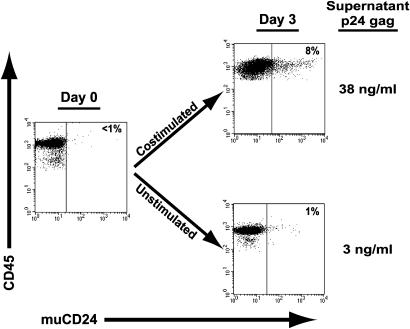
Influence of Different T Cell Stimulatory Pathways on Latent HIV Expression. To determine the molecular pathways specifically responsible for initiating HIV reactivation from latency, we infected SCID-hu (Thy/Liv) mice with the pathogenic, CXC chemokine receptor 4 (CXCR4)-tropic reporter virus HIVNL-r-HSAS (50) in which the cDNA sequence for muCD24 is inserted in the vpr gene of HIVNL4–3. Productive infection then directs the expression of muCD24 on the surface of infected cells, allowing quantitation by flow cytometry. We have previously shown that deletion of the vpr gene does not alter the pathogenesis of HIV in the thymus (51). Five weeks after infection, mature CD4 SP,muCD24-negative thymocytes were isolated by negative selection, resulting in a population of cells that expressed <1% CD8 or muCD24 (Fig. 1). Cells were continually cultured in the presence of AZT and a viral protease inhibitor to prevent further reverse transcription or in vitro virus spread. Costimulation of the cells with antibodies to CD3 and CD28 quantitatively reactivated latent virus whereas cells cultured without stimulation remained latently infected (Fig. 1), demonstrating that signal transduction pathways activated via stimulation of the TcR and coreceptor complex induce latent virus expression.
Fig. 1.
Costimulation induces latent HIV expression. HIVNL-r-HSAS-infected CD4 SP, muCD24-negative thymocytes (day 0) were cultured without stimulation or costimulated with antibodies to CD3 and CD28 for 3 days (day 3) and assessed for human CD45 and HIV (muCD24) expression by flow cytometry. The percentage of muCD24-positive cells is indicated in each panel. The concentration of viral p24 gag in the supernatant is shown next to each panel.
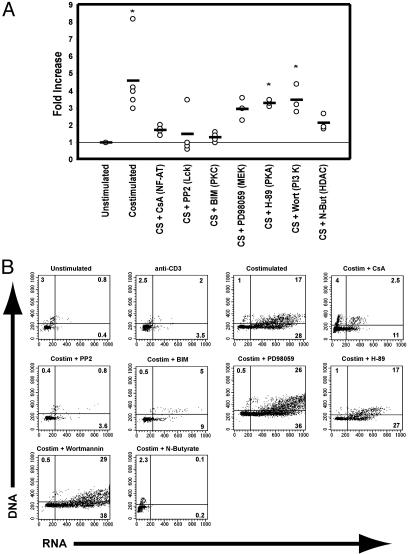
The requirement for in vivo replication to generate sufficient amounts of latent infection in this system prohibits the use of viral strains containing LTR mutations. To further define the signal transduction pathways involved in activating latent virus expression, the population containing the latently infected cells was costimulated in the presence of pharmacologic inhibitors of specific pathways involved in T cell activation (Fig. 2 A). Incubation of the cells with an inhibitor of PKC (bisindolmaleimide II), Lck (PP2), or NF-AT [cyclosporin A (CsA)] function before costimulation, almost completely abrogated HIV reactivation from latency. Inhibition of histone deacetylase activity (N-butyrate), which blocks cells at the G1a phase of the cell cycle and prevents de novo infection (49), caused a 50% reduction in HIV reactivation, demonstrating the dichotomy that exists between factors needed during initial infection and those required for activation of integrated provirus. Alternatively, blockade of the PKA or PI3-K pathways (with H-89 or wortmannin, respectively) had only minor effects on viral reactivation (Fig. 2A), suggesting that only certain pathways involved in T cell activation are needed to increase HIV LTR activity. After inhibition of the mitogen-activated protein kinase kinase (MEK)/mitogen-activated protein kinase (MAPK) pathway with PD98059, viral expression was not significantly different from cells cultured without stimulation (P = 0.08; Fig. 2A) although the value approached significance. On the other hand, viral expression was not significantly distinct from costimulated cells (P = 0.21), suggesting that blockade of that pathway does not inhibit viral reactivation. Similar results were observed with these inhibitors after costimulation of cells latently infected with the wild-type, CC chemokine receptor 5 (CCR5)-tropic HIVJR-CSF (not shown), indicating that deletion of the vpr gene is not influencing these results and that CCR5 and CXCR4 viruses respond similarly to activation-inducing signals. Interestingly, the same pathways responsible for virus reactivation after costimulation were also needed for cell cycle progression. Inhibition of PKC, NF-AT, or Lck activity blocked cell cycling in response to costimulation whereas others did not (Fig. 2B), initially suggesting a tight relationship between cellular activation and HIV transcription.
Fig. 2.
Signal transduction pathways involved in reactivating latent virus after costimulation. (A) CD4SP, muCD24-negative thymocytes from HIVNL-r-HSAS-infected Thy/Liv implants were either cultured without stimulation, costimulated, or costimulated in the presence of the indicated inhibitor for 2 days, and HIV reactivation (muCD24 expression) was quantitated by flow cytometry. Cells from many experiments costimulated (CS) in the presence of the indicated inhibitor are shown. Primary data can be viewed in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org. The graph displays the fold increase (as assessed by flow cytometry) in HIV reporter expression for each condition compared with the unstimulated control, which is set at 1. The protein or pathway inhibited by each compound is provided within the parentheses. Each point represents results from a single experiment, and the horizontal lines indicate mean values. Use of each inhibitor was associated with minimal toxicity (90–100% viability compared with cells costimulated without any inhibitors) except for N-butyrate (50% viability compared with costimulated cells). *, P = <0.05 compared with unstimulated cells by Dunnett's two-tailed multiple comparisons test. (B) Effect on cell cycling by each inhibitor after costimulation. Uninfected CD4SP thymocytes from Thy/Liv implants were cultured as indicated for 2 days and stained for RNA and DNA content. The lower left quadrants of each panel indicate cells in the G0 or G1a phases of the cell cycle whereas the lower and upper right quadrants represent cells in the G1b and S through M phases, respectively. The percentage of cells in each quadrant is given. Events in the upper left quadrants are likely bald nuclei or doublets formed during the staining procedure.
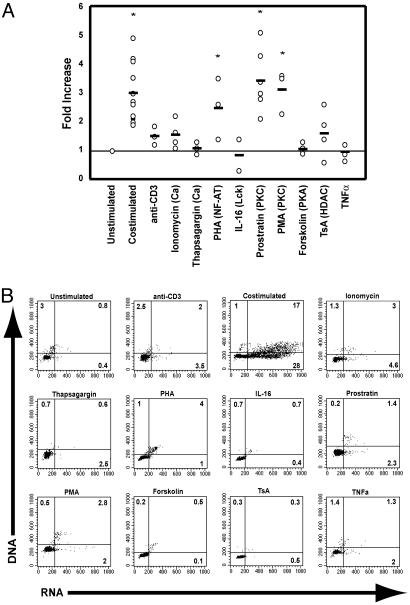
Activation of Specific Molecular Pathways Differentially Affects Viral Reactivation and Cell Cycle Progression. Although entrance into the cell cycle is required for de novo HIV infection (49), we have previously shown that cell cycling is not necessarily required to reactivate latent HIV expression (49, 52). However, the tested agents above that blocked HIV reactivation after costimulation also inhibited cell cycle progression. Therefore, we sought to determine which T cell signaling pathways specifically triggered the viral LTR, independent of cell cycling. To address this issue, latently infected cells were incubated with agents that activate defined pathways involved in T cell activation. Activators of the PKC pathway (the phorbol esters phorbol 12-myristate 13-acetate and prostratin) induced a substantial increase in virus expression as measured by the frequency of cells expressing the virus-encoded reporter (Fig. 3A), without inducing cell cycle progression (Fig. 3B). This finding illustrates that factors induced by PKC are specifically able to reactivate latent virus. Stimulation of the PKC pathway is known to induce NF-κB activity. Interestingly, however, not all pathways that lead to NF-κB activation reactivate latent virus because TNFα did not induce viral expression (Fig. 3A) although the TNF receptors are expressed on mature thymocytes (53, 54) and exogenous addition of TNF to in vitro thymocyte cultures has been shown to stimulate infection (54). Consistent with the lack of HIV expression in cells costimulated in the presence of CsA (Fig. 2A), incubation of latently infected cells with an activator of NF-AT (PHA) induced expression of latent virus (Figs. 3A and 4C), but not to the same extent as after activation of PKC.
Fig. 3.
Reactivation of latent HIV by different signal transduction pathways. (A) CD4SP,muCD24-negative thymocytes were cultured in the presence of the indicated agent for 2 days and analyzed as in Fig. 2A. The protein or pathway inhibited by each compound is stated in parentheses. Primary data can be viewed in Fig. 6, which is published as supporting information on the PNAS web site. Use of each agent was associated with minimal toxicity (90–100% viability compared with cells costimulated without any inhibitors) except for TsA and PHA (50% viability compared with costimulated cells). (B) Effect of each agent on cell cycle progression. Uninfected CD4SP thymocytes from Thy/Liv implants were cultured as in Fig. 2B.
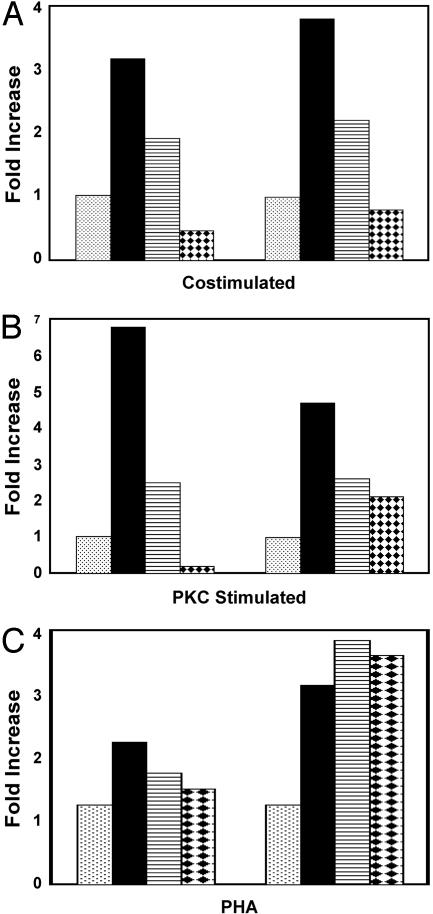
Fig. 4.
NF-κB is required for HIV reactivation whereas de novo protein synthesis is not. (A) RNA was isolated from HIVNL-r-HSAS-infected CD4SP,muCD24-negative thymocytes after7hof culture without stimulation (stippled bars), after costimulation (filled bars), or after costimulation in the presence of cycloheximide (striped bars) or gliotoxin (diamond bars). For each condition, real-time RT-PCR was performed to quantify the levels of LTR/gag RNA (normalized to the number of β2m transcripts). The graph shows the fold increase in LTR/gag transcripts for each condition compared with the unstimulated control, which is set at 1. Each group of bars represents a separate experiment. (B) CD4SP,muCD24-negative thymocytes were incubated without stimulation (stippled bars), with the PKC activator prostratin (filled bars), or with prostratin plus cycloheximide (striped bars) or prostratin plus gliotoxin (diamond bars). RNA was isolated after 7 h of culture and quantitated as in A. (C) CD4SP,muCD24-negative thymocytes were incubated without stimulation (stippled bars), with PHA (filled bars), with PHA plus cycloheximide (striped bars), or with PHA plus gliotoxin (diamond bars). RNA was isolated and quantitated after 7 h of culture.
Anti-CD3 stimulation alone or pharmacologic agents that increase intracellular calcium levels had minimal effects on virus reactivation (Fig. 3 A) or cell cycle progression (Fig. 3B). Relaxation of chromatin structure with the histone deactylase inhibitor TsA, which has been shown to reactivate virus replication in latently infected cell lines, did not substantially reactivate latent virus (Fig. 3A) or move the cells into cycle (Fig. 3B), indicating that, for the most part, chromatin structure alone does not account for the maintenance of latency. Furthermore, although inhibition of Lck activity during costimulation blocks viral reactivation (Fig. 2A), activation of Lck with IL-16 (55) was not sufficient to induce virus expression (Fig. 3A). In agreement with the inhibition studies, activation of PKA by forskolin (Fig. 3B) or PI3-K with antibodies to CD28 (not shown; ref. 13) did not induce expression of latent virus. Induction of latent virus by IL-7 (14, 52), which signals via both the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and PI3-K pathways, is similarly not inhibited by wortmannin (not shown).
NF-κB Directly Activates Latent HIV Expression in the Absence of New Protein Synthesis. Previous studies in cell line models of HIV latency have shown that signal transduction pathways culminating in NF-κB activation can efficiently induce expression of latent HIV. We have shown that two such pathways, PKC and Lck, are both required to reactivate latent virus in response to costimulation. Therefore, to elucidate the importance of NF-κB on viral reactivation from latency, we compared the induction of RNA transcription from latent virus in costimulated cells vs. cells costimulated in the presence of the NF-κB inhibitor gliotoxin (56). Assessment of reporter expression was not possible because inhibition of NF-κB activity in this manner causes apoptosis within 24 h after costimulation (56). Costimulation of latently infected cells for 7 h, however, does not result in apoptosis and induced a 3- to 4-fold increase in viral LTR/gag HIV transcripts that was blocked when NF-κB activity was inhibited (Fig. 4A). Similarly, activation of the PKC pathway induced a >5-fold increase in LTR/gag RNA expression in latently infected cells. Stimulation of the viral LTR by the PKC pathway was largely dependent on NF-κB activation because incubation with gliotoxin substantially blocked viral reactivation (Fig. 4B). Consistent with the specific activation of NF-AT by PHA, inhibition of NF-κB activity after treatment of latently infected cells with PHA did not affect viral RNA induction (Fig. 4C), thus illustrating both the relative specificity of the inhibitor and more importantly, the ability of different transcription factors, NF-κB and NF-AT, to individually induce latent HIV replication.
Transcription factors such as NF-κB and NF-AT are present in an inactive form in quiescent T lymphocytes and have pleiotropic effects during T cell activation. Therefore, it was of interest to determine whether cellular stimulation induced HIV expression by increasing the activity of preexisting transcription factors or secondarily reactivated HIV by inducing other proteins. Thus, we determined whether de novo protein synthesis was required for viral reactivation. Costimulation of the latently infected cells in the presence of the protein synthesis inhibitor cycloheximide decreased viral RNA synthesis ≈60% compared with cells that were costimulated without the inhibitor (Fig. 4A). However, there was still a 2-fold increase in viral transcripts compared with cells left unstimulated (Fig. 4A), indicating that presynthesized transcription factors (such as NF-κB and NF-AT) are sufficient to initially induce viral reactivation. Activation of the viral LTR by the PKC pathway was similarly decreased by incubation with cycloheximide, but, as seen with costimulation, induction of viral transcription was not completely blocked (Fig. 4B). Likewise, inhibition of protein synthesis did not completely block reactivation of viral transcription after incubation with PHA (Fig. 4C). Cycloheximide, however, completely inhibited viral p24 protein production as determined by ELISA (not shown), indicating that the inhibitor was sufficient to stop protein synthesis but not viral gene expression. Thus, de novo cellular protein production is not required for the initial reactivation of virus from latency; however, it does seem to be necessary to induce optimal or sustained viral replication.
Discussion
Our studies show in quiescent primary cells that the NF-AT and PKC pathways are central inducers of latent HIV replication and illustrate that multiple cellular pathways are able to independently reactivate latent HIV expression. Inhibition of the PKC or NF-AT pathways almost completely abrogates viral reactivation whereas their specific activation, in the absence of other signals, is able to stimulate virus expression. Importantly, we show that the cellular protein NF-κB is essential to viral reactivation from latency in primary cells because inhibition of NF-κB activity potently blocked viral transcription in response to PKC activation or costimulation. Although inhibition of the signaling events originating from Lck similarly block HIV reactivation, it seems that they do so by inhibiting other regulatory factors induced after T cell stimulation because agents that activate Lck [IL-16 (55)] alone do not reactivate latent virus. The same is likely true for histone deacetylase activity because its inhibition only moderately blocks HIV reactivation after costimulation whereas TsA alone does not reactivate virus expression from the majority of cells harboring latent viral genomes. Although pharmacolgic agents can produce some background inhibition, the different abilities of the agents used herein to induce latent virus expression indicate their capability to identify the signaling pathways involved in viral reactivation. For example, the NF-κB inhibitor gliotoxin blocks viral RNA production in response to phorbol esters (which signal largely through NF-κB), but not PHA (which signals primarily through NF-AT), indicating the relative specificity of this agent. Similarly, the opposing impact on viral expression seen by using inhibitors vs. activators of individual pathways confirms the general specificity of the pathway analyzed. For example, inhibition of PKC activity blocked viral reactivation whereas activation of that pathway induced viral expression. Conversely, neither activation nor inhibition of the PKA pathway affected viral reactivation.
Our data further illustrate the dichotomy that exists between HIV latency in primary quiescent cells and in cell lines. Although phorbol esters, TsA, and TNFα induce latent virus in cell lines (22–24, 28–30, 40), neither TsA nor TNFα substantially reactivate virus expression in our system from quiescent primary cells. Interestingly, both the PKC pathway and TNFα induce NF-κB expression, but only PKC activates latent virus in quiescent cells. Previous studies have shown that tat-driven expression of the HIV LTR depends on PKC activity (29), presumably via NF-κB activation; yet, the binding of NF-κB alone to the LTR is not sufficient to activate viral transcription (57). Because tat is absent during latency in quiescent cells (14, 58), the activation of NF-κB by TNFα is not sufficient to activate the LTR. In addition to NF-κB, PKC may induce other required factors, which TNFα does not, that stabilize the viral RNA and allow for the initial stages of transcription and production of tat (32). The finding that presynthesized factors activated immediately after T cell stimulation potently induce viral reactivation may explain why viral expression can be observed before expression of cell surface activation markers (59–61). Because NF-κB and NF-AT are present in quiescent cells in an inactive form, cellular stimulation quickly induces their activity and allows for the initial induction of viral transcription.
αCD3 stimulation alone, although to a lower level than costimulation, can induce HIV expression, while initiating only minimal changes in cellular RNA expression (13). Thus, TcR signals that alone do not trigger cellular proliferation and in some cases induce anergy (62) can lead to viral reactivation from latency. These data illustrate both the minimal requirements for LTR activation and the dichotomy that exists whereby signals can differentially affect viral and cellular processes. Although calcium is required for PKC activity (15), exogenously increasing intracellular calcium levels with ionomycin or thapsagargin only minimally activated expression of latent virus. It is interesting to note that, although activation via CD3 also triggers PKC and NF-AT activity, it does not induce virus replication as efficiently as the pharmacologic activators of PKC and NF-AT. This result may be due to the strength of signal sent in each case.
It is unclear to what extent the latently infected cells generated in the SCID-hu system represent the CD4+ memory T cell that is the predominant source of latent infection in humans, because naive T cells (which the mature thymocytes in our system closely resemble) have a higher threshold for activation than do memory cells (15). Accordingly, our data correlate well with what is known from human studies suggesting that viral reactivation from resting CD4+ T cells can be stimulated with antibodies to CD3, phorbol esters, or the combination of PHA with IL-2 (33, 63, 64). Moreover, the ability of the corticosteroid dexamethasone to inhibit viral reactivation from patient cells, although it has pleiotropic cellular effects, suggests a role for NF-κB (64), thereby further indicating that the molecular events regulating latency in the SCID-hu system are similar to memory cells. Our studies may not address the latency generated by viral integration into transcriptionally inactive chromosomal regions (40), which may have different requirements for activation. Moreover, other viral reservoirs allowing persistence during highly active antiretroviral therapy, such as macrophages (65, 66), may also contribute to viral rebound after the withdrawal of therapy, and it is unclear how the data obtained from these studies would relate to those reservoirs.
Previous studies have indicated that cell cycle progression is critical for reverse transcription, nuclear import, and integration (49, 67, 68). Other studies have shown that, after proviral integration, the activation of early signal transduction pathways and transcription factors, independent of cell cycle progression, controls viral replication (34, 52). For therapeutic strategies, it is important to note that, although the pathways that most efficiently induce latent virus are necessary for T cell activation, they do not alone fully activate the cell to replicate. Our data show that many of the pathways critical to T cell activation and function are dispensable for viral gene expression. Likewise, many of the T cell signal transduction pathways that are thought to augment viral infection [PKA (69), mitogen-activated protein kinase (70), and PI3-K (71)] alone do not increase replication from latently integrated proviruses. These data indicate that maintenance of latency results from the functional lack of specific transcription factors required for viral gene expression. Once these factors are activated, transcriptional latency is broken, regardless of subsequent cell cycle progression or cellular protein synthesis. By using factors induced via multiple pathways, HIV has successfully parasitized T lymphocytes and ensured optimal replication immediately after cellular stimulation.
Supplementary Material
Acknowledgments
We thank Genhong Cheng, Steve Cole, and Scott Kitchen for critical review of the manuscript and R. Cortado, A. Kacena, and N. Jones for technical assistance. This work was supported by National Institutes of Health Grants AI36059 and AI36554, the American Foundation for AIDS Research, the Pendleton Foundation, the University of California Universitywide AIDS Research Program, and the University of California at Los Angeles Centers for AIDS Research. D.G.B. is the recipient of a Janis V. Giorgi Memorial Fellowship from the University of California at Los Angeles AIDS Institute.
Abbreviations: TcR, T cell receptor; NF-AT, nuclear factor of activated T cells; PI3-K, phosphatidylinositol 3-kinase; TNF, tumor necrosis factor; TsA, trichostatin A; PHA, phytohemagglutinin; muCD24, murine CD24; SP, single positive; β2m, β2-microglobulin; CsA, cyclosporin A.
References
- 1.Ho, D. D. (1998) Science 280, 1866-1867. [DOI] [PubMed] [Google Scholar]
- 2.Chun, T. W. & Fauci, A. S. (1999) Proc. Natl. Acad. Sci. USA 96, 10958-10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butera, S. T. (2000) Antiviral Res. 48, 143-176. [DOI] [PubMed] [Google Scholar]
- 4.Pomerantz, R. J. (2002) Clin. Infect. Dis. 34, 91-97. [DOI] [PubMed] [Google Scholar]
- 5.Blankson, J. N., Persaud, D. & Siliciano, R. F. (2002) Annu. Rev. Med. 53, 557-593. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., Stuyver, L., Mizell, S. B., Ehler, L. A., Mican, J. A., Baseler, M., Lloyd, A. L., Nowak, M. A. & Fauci, A. S. (1997) Proc. Natl. Acad. Sci. USA 94, 13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finzi, D., Hermankova, M., Pierson, T., Carruth, L. M., Buck, C., Chaisson, R. E., Quinn, T. C., Chadwick, K., Margolick, J., Brookmeyer, R., et al. (1997) Science 278, 1295-1300. [DOI] [PubMed] [Google Scholar]
- 8.Wong, J. K., Hezareh, M., Gunthard, H. F., Havlir, D. V., Ignacio, C. C., Spina, C. A. & Richman, D. D. (1997) Science 278, 1291-1295. [DOI] [PubMed] [Google Scholar]
- 9.Zhang, L., Chung, C., Hu, B. S., He, T., Guo, Y., Kim, A. J., Skulsky, E., Jin, X., Hurley, A., Ramratnam, B., et al. (2000) J. Clin. Invest. 106, 839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun, T. W., Davey, R. T., Jr., Ostrowski, M., Shawn Justement, J., Engel, D., Mullins, J. I. & Fauci, A. S. (2000) Nat. Med. 6, 757-761. [DOI] [PubMed] [Google Scholar]
- 11.Finzi, D., Blankson, J., Siliciano, J. D., Margolick, J. B., Chadwick, K., Pierson, T., Smith, K., Lisziewicz, J., Lori, F., Flexner, C., et al. (1999) Nat. Med. 5, 512-517. [DOI] [PubMed] [Google Scholar]
- 12.Ramratnam, B., Mittler, J. E., Zhang, L., Boden, D., Hurley, A., Fang, F., Macken, C. A., Perelson, A. S., Markowitz, M. & Ho, D. D. (2000) Nat. Med. 6, 82-85. [DOI] [PubMed] [Google Scholar]
- 13.Brooks, D. G., Kitchen, S. G., Kitchen, C. M., Scripture-Adams, D. D. & Zack, J. A. (2001) Nat. Med. 7, 459-464. [DOI] [PubMed] [Google Scholar]
- 14.Brooks, D. G., Hamer, D. H., Arlen, P. A., Gao, L., Bristol, G., Kitchen, C. M. R., Berger, E. A. & Zack, J. A. (2003) Immunity 19, 413-423. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins, M. K., Khoruts, A., Ingulli, E., Mueller, D. L., McSorley, S. J., Reinhardt, R. L., Itano, A. & Pape, K. A. (2001) Annu. Rev. Immunol. 19, 23-45. [DOI] [PubMed] [Google Scholar]
- 16.Rao, A., Luo, C. & Hogan, P. G. (1997) Annu. Rev. Immunol. 15, 707-747. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., May, M. J. & Kopp, E. B. (1998) Annu. Rev. Immunol. 16, 225-260. [DOI] [PubMed] [Google Scholar]
- 18.Pereira, L. A., Bentley, K., Peeters, A., Churchill, M. J. & Deacon, N. J. (2000) Nucleic Acids Res. 28, 663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun, T. W., Carruth, L., Finzi, D., Shen, X., DiGiuseppe, J. A., Taylor, H., Hermankova, M., Chadwick, K., Margolick, J., Quinn, T. C., et al. (1997) Nature 387, 183-188. [DOI] [PubMed] [Google Scholar]
- 20.Nabel, G. & Baltimore, D. (1987) Nature 326, 711-713. [DOI] [PubMed] [Google Scholar]
- 21.Rabson, A. B. & Lin, H. C. (2000) Adv. Pharmacol. 48, 161-207. [DOI] [PubMed] [Google Scholar]
- 22.Folks, T. M., Clouse, K. A., Justement, J., Rabson, A., Duh, E., Kehrl, J. H. & Fauci, A. S. (1989) Proc. Natl. Acad. Sci. USA 86, 2365-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duh, E. J., Maury, W. J., Folks, T. M., Fauci, A. S. & Rabson, A. B. (1989) Proc. Natl. Acad. Sci. USA 86, 5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborn, L., Kunkel, S. & Nabel, G. J. (1989) Proc. Natl. Acad. Sci. USA 86, 2336-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, B. K., Feinberg, M. B. & Baltimore, D. (1997) J. Virol. 71, 5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson, C., McCaffrey, P. G., Rao, A. & Sen, R. (1991) J. Immunol. 147, 416-420. [PubMed] [Google Scholar]
- 27.Sun, Z., Arendt, C. W., Ellmeier, W., Schaeffer, E. M., Sunshine, M. J., Gandhi, L., Annes, J., Petrzilka, D., Kupfer, A., Schwartzberg, P. L. & Littman, D. R. (2000) Nature 404, 402-407. [DOI] [PubMed] [Google Scholar]
- 28.Kinter, A. L., Poli, G., Maury, W., Folks, T. M. & Fauci, A. S. (1990) J. Virol. 64, 4306-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakobovits, A., Rosenthal, A. & Capon, D. J. (1990) EMBO J. 9, 1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folks, T. M., Justement, J., Kinter, A., Schnittman, S., Orenstein, J., Poli, G. & Fauci, A. S. (1988) J. Immunol. 140, 1117-1122. [PubMed] [Google Scholar]
- 31.Rabbi, M. F., al-Harthi, L., Saifuddin, M. & Roebuck, K. A. (1998) Virology 245, 257-269. [DOI] [PubMed] [Google Scholar]
- 32.West, M. J., Lowe, A. D. & Karn, J. (2001) J. Virol. 75, 8524-8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkosky, J., Culnan, D. M., Roman, J., Dornadula, G., Schnell, M., Boyd, M. R. & Pomerantz, R. J. (2001) Blood 98, 3006-3015. [DOI] [PubMed] [Google Scholar]
- 34.Korin, Y. D., Brooks, D. G., Brown, S., Korotzer, A. & Zack, J. A. (2002) J. Virol. 76, 8118-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang, X., Chen, Y. & Gabuzda, D. (1999) J. Biol. Chem. 274, 27981-27988. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, N. D., Edwards, N. L., Duckett, C. S., Agranoff, A. B., Schmid, R. M. & Nabel, G. J. (1993) EMBO J. 12, 3551-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roebuck, K. A., Gu, D. S. & Kagnoff, M. F. (1996) AIDS 10, 819-826. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita, S., Su, L., Amano, M., Timmerman, L. A., Kaneshima, H. & Nolan, G. P. (1997) Immunity 6, 235-244. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita, S., Chen, B. K., Kaneshima, H. & Nolan, G. P. (1998) Cell 95, 595-604. [DOI] [PubMed] [Google Scholar]
- 40.Jordan, A., Defechereux, P. & Verdin, E. (2001) EMBO J. 20, 1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laughlin, M. A., Chang, G. Y., Oakes, J. W., Gonzalez-Scarano, F. & Pomerantz, R. J. (1995) J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 9, 332-339. [PubMed] [Google Scholar]
- 42.Coull, J. J., Romerio, F., Sun, J. M., Volker, J. L., Galvin, K. M., Davie, J. R., Shi, Y., Hansen, U. & Margolis, D. M. (2000) J. Virol. 74, 6790-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emiliani, S., Van Lint, C., Fischle, W., Paras, P., Jr., Ott, M., Brady, J. & Verdin, E. (1996) Proc. Natl. Acad. Sci. USA 93, 6377-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emiliani, S., Fischle, W., Ott, M., Van Lint, C., Amella, C. A. & Verdin, E. (1998) J. Virol. 72, 1666-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCune, J. M., Namikawa, R., Kaneshima, H., Shultz, L. D., Lieberman, M. & Weissman, I. L. (1988) Science 241, 1632-1639. [DOI] [PubMed] [Google Scholar]
- 46.Krowka, J. F., Sarin, S., Namikawa, R., McCune, J. M. & Kaneshima, H. (1991) J. Immunol. 146, 3751-3756. [PubMed] [Google Scholar]
- 47.McCune, J. M. (1992) Bone Marrow Transplant. 9, Suppl. 1, 74-76. [PubMed] [Google Scholar]
- 48.McCune, J. M. (1996) Semin. Immunol. 8, 187-196. [DOI] [PubMed] [Google Scholar]
- 49.Korin, Y. D. & Zack, J. A. (1998) J. Virol. 72, 3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jamieson, B. D. & Zack, J. A. (1998) J. Virol. 72, 6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aldrovandi, G. M. & Zack, J. A. (1996) J. Virol. 70, 1505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scripture-Adams, D. D., Brooks, D. G., Korin, Y. K. & Zack, J. A. (2002) J. Virol. 76, 13077-13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy, M., Pike-Nobile, L., Soo, V. W. & Epstein, L. B. (1994) Thymus 23, 177-194. [PubMed] [Google Scholar]
- 54.Chene, L., Nugeyre, M. T., Guillemard, E., Moulian, N., Barre-Sinoussi, F. & Israel, N. (1999) J. Virol. 73, 7533-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryan, T. C., Cruikshank, W. W., Kornfeld, H., Collins, T. L. & Center, D. M. (1995) J. Biol. Chem. 270, 17081-17086. [DOI] [PubMed] [Google Scholar]
- 56.Bureau, F., Vanderplasschen, A., Jaspar, F., Minner, F., Pastoret, P. P., Merville, M. P., Bours, V. & Lekeux, P. (2002) Blood 99, 3683-3691. [DOI] [PubMed] [Google Scholar]
- 57.Doppler, C., Schalasta, G., Amtmann, E. & Sauer, G. (1992) AIDS Res. Hum. Retroviruses 8, 245-252. [DOI] [PubMed] [Google Scholar]
- 58.Adams, M., Sharmeen, L., Kimpton, J., Romeo, J., Garcia, J., Peterlin, B., Groudine, M. & Emerman, M. (1994) Proc. Natl. Acad. Sci. USA 91, 3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Z., Schuler, T., Zupancic, M., Wietgrefe, S., Staskus, K. A., Reimann, K. A., Reinhart, T. A., Rogan, M., Cavert, W., Miller, C. J., et al. (1999) Science 286, 1353-1357. [DOI] [PubMed] [Google Scholar]
- 60.Brooks, D. G. & Zack, J. A. (2002) J. Virol. 76, 1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinter, A. L., Umscheid, C. A., Arthos, J., Cicala, C., Lin, Y., Jackson, R., Donoghue, E., Ehler, L., Adelsberger, J., Rabin, R. L. & Fauci, A. S. (2003) J. Immunol. 170, 2449-2455. [DOI] [PubMed] [Google Scholar]
- 62.Quill, H. & Schwartz, R. H. (1987) J. Immunol. 138, 3704-3712. [PubMed] [Google Scholar]
- 63.Chun, T. W., Finzi, D., Margolick, J., Chadwick, K., Schwartz, D. & Siliciano, R. F. (1995) Nat. Med. 1, 1284-1290. [DOI] [PubMed] [Google Scholar]
- 64.Chun, T. W., Engel, D., Mizell, S. B., Ehler, L. A. & Fauci, A. S. (1998) J. Exp. Med. 188, 83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crowe, S. M. & Sonza, S. (2000) J. Leukocyte Biol. 68, 345-350. [PubMed] [Google Scholar]
- 66.Lambotte, O., Taoufik, Y., de Goer, M. G., Wallon, C., Goujard, C. & Delfraissy, J. F. (2000) J. Acquired Immune Defic. Syndr. 23, 114-119. [DOI] [PubMed] [Google Scholar]
- 67.Zack, J. A., Arrigo, S. J., Weitsman, S. R., Go, A. S., Haislip, A. & Chen, I. S. (1990) Cell 61, 213-222. [DOI] [PubMed] [Google Scholar]
- 68.Bukrinsky, M. I., Stanwick, T. L., Dempsey, M. P. & Stevenson, M. (1991) Science 254, 423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cole, S. W., Korin, Y. D., Fahey, J. L. & Zack, J. A. (1998) J. Immunol. 161, 610-616. [PubMed] [Google Scholar]
- 70.Yang, X. & Gabuzda, D. (1999) J. Virol. 73, 3460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francois, F. & Klotman, M. E. (2003) J. Virol. 77, 2539-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.