Abstract
Membrane cofactor protein (MCP; CD46) is a widely expressed transmembrane complement regulator. Like factor H it inhibits complement activation by regulating C3b deposition on targets. Factor H mutations occur in 10–20% of patients with hemolytic uremic syndrome (HUS). We hypothesized that MCP mutations could predispose to HUS, and we sequenced MCP coding exons in affected individuals from 30 families. MCP mutations were detected in affected individuals of three families: a deletion of two amino acids (D237/S238) in family 1 (heterozygous) and a substitution, S206P, in families 2 (heterozygous) and 3 (homozygous). We evaluated protein expression and function in peripheral blood mononuclear cells from these individuals. An individual with the D237/S238 deletion had reduced MCP levels and ≈50% C3b binding compared with normal controls. Individuals with the S206P change expressed normal quantities of protein, but demonstrated ≈50% reduction in C3b binding in heterozygotes and complete lack of C3b binding in homozygotes. MCP expression and function was evaluated in transfectants reproducing these mutations. The deletion mutant was retained intracellularly. S206P protein was expressed on the cell surface but had a reduced ability to prevent complement activation, consistent with its reduced C3b binding and cofactor activity. This study presents further evidence that complement dysregulation predisposes to development of thrombotic microangiopathy and that screening patients for such defects could provide informed treatment strategies.
Hemolytic uremic syndrome (HUS) is characterized by the triad of thrombocytopenia, Coomb's test negative microangiopathic hemolytic anemia, and acute renal failure (1, 2). HUS is classified as either D+ when it is associated with a preceding diarrhoeal illness, which in most people is caused by infection with Escherichia coli O157, or less commonly nondiarrhoeal associated (D-) (also called “atypical”). D- HUS may be sporadic or familial. Mutations have been reported in the complement regulatory protein factor H in both sporadic (n = 9) and familial (n = 8 families) HUS (3–7) with mutations identified in 10–20% of cases studied. The majority of mutations are missense changes in the exon encoding complement control module 20 of factor H, an area important for both binding to anionic molecules and C3b (8). Such mutations result in impaired protection of host surfaces against complement activation (9–11).
Host cells are protected from complement activation by regulatory proteins that inactivate C3b deposited on their surface (12, 13). Membrane cofactor protein (MCP) is widely expressed on almost every human cell except erythrocytes (refs. 14–18 and reviewed in ref. 19). Together with factor I, it degrades C3b and C4b bound to the cell surface (20–25). The extracellular domain is composed of four complement control modules that house the sites for complement regulation. This is followed by a region of O-glycosylation and the cytoplasmic tail that mediates signaling events (19).
We first described factor H mutations (4) after a linkage study in three families that mapped HUS to a 26-centiMorgan region of chromosome 1 (1q32) containing a cluster of complement related genes, including factor H. However, we identified a factor H mutation in only one of the three families used in this linkage study.
The gene encoding MCP is in the cluster of complement-related genes on chromosome 1q32. Its function and genomic location made MCP a candidate gene. Consequently, we proceeded to sequence MCP in 30 HUS families and have identified functionally significant mutations in three, including one of our original families.
Families and Clinical Details
The study was approved by the Joint Ethics Committee of the University of Newcastle upon Tyne and Newcastle and North Tyneside Health Authority. All subjects gave informed consent. Table 1 gives details of the three families.
Table 1. Family details of affected individuals.
| Family | Inheritance | Patient ID | Sex | Age | Platelets × 103/mm3 | Serum C3 | Outcome of renal function | Transplant |
|---|---|---|---|---|---|---|---|---|
| 1 | Dominant (heterozygous) | A | Male | 27 | 35 | Normal | ESRF | No recurrence |
| B | Male | 31 | 71 | Normal | ESRF | No recurrence | ||
| C | Male | 35 | 89 | Normal | ESRF | No recurrence | ||
| 2 | Dominant (heterozygous) | D | Male | 8 | 32 | ND | Recovered | |
| E | Male | 15 | 9 | Normal | Recovered | |||
| 3 | Recessive (homozygous) | F | Female | 9 | 25 | Decreased | Recovered | |
| G | Male | 15 | 10 | Normal | Recovered |
ESRF, end stage renal failure; ND, not determined. Age, platelet count and serum C3 concentration are at presentation.
Family 1. Family 1 from Belgium has been described (26) and was one of three families used in our initial linkage study (4). Three male siblings were affected at the ages of 27, 31, and 35 years. The clinical features of all three were similar. In particular, C3 levels at presentation were normal, and there was no recovery of renal function. Subsequently, all three received a cadaver renal transplant with no recurrence of the disease. Since the original description of this family one of the brothers has died from hepatic failure with portal hypertension of unknown aetiology, one has developed Waldenstroms macroglobulinaemia, and the other remains well with a functioning transplant. The father of the affected individuals died from pancreatic carcinoma at the age of 65, and the mother is alive and well at the age of 80. In our original study (4), all of the affected members of family 1 shared a haplotype inherited from their father limited by the markers D1S212 and D1S245. This ≈32-megabase region contains the gene for MCP.
Family 2. Two male siblings of this German family have been affected. The elder presented at the age of 8 years with a short history of vomiting. On admission, platelet count was 32 × 103/mm3, and the blood smear demonstrated a microangiopathic hemolytic anemia. Urinalysis showed hematuria and 1.7 g/liter of proteinuria. Plasma creatinine was elevated. Dialysis was not necessary, and renal function recovered spontaneously. The younger sibling presented at the age of 15 years with a 2-day history of vomiting. On admission platelet count was 9 × 103/mm3, and the haematocrit was reduced secondary to a microangiopathic hemolytic anemia. There was an elevated serum lactate dehydrogenase (3,861 units/liter, normal range 50 to 150 units/liter). Plasma creatinine was 2.9 mg/dl (256 μmol/ liter) and rose to a zenith of 4.2 mg/dl (372 μmol/liter). Complement C3 levels were within the normal range at 98 mg/dl on presentation. Haemodialysis was initiated, and the child was also treated with plasma infusions and plasma exchange. After 14 days, renal function returned, and the child made a complete recovery. Neither parent reported a history of a similar syndrome.
Family 3. Two siblings, one male and one female, have been affected. Their Turkish parents are first-degree relatives. The female presented at the age of 9 years with oliguria. Platelet count on admission was 25 × 103/mm3, and there was a microangiopathic haemolytic anemia (Coombs negative). Complement C3 was decreased. Plasma creatinine and blood urea nitrogen levels were elevated. A renal biopsy showed features compatible with HUS. Treatment with peritoneal dialysis and plasma exchange was initiated. The child made a complete recovery although 2 years later she had a further episode that resolved spontaneously. Her brother presented at the age of 17 years with epistaxis, gingival bleeding, periorbital edema, and hematuria. Platelet count on admission was 10 × 103/mm3, and the blood smear indicated a microangiopathic hemolytic anemia that was Coomb's negative. Plasma creatinine and blood urea nitrogen were elevated. Complement C3 was normal. He was treated with plasma infusions and recovered completely. There was no history of an increase in childhood diseases in the two affected members of this family.
Methods
DNA Analysis. DNA was prepared from peripheral blood according to standard procedures. MCP exons were amplified by PCR. The sequences of primers and conditions for PCR and DNA sequencing are shown in Table 4, which is published as supporting information on the PNAS web site, www.pnas.org. The amplified products were directly sequenced by using a dye-terminator sequencing kit (BigDye, Applied Biosystems). To clarify the mutation in family 1 the PCR product spanning exon 6 was cloned by using the pGemTeasy Cloning kit (Promega) and sequenced as above.
Protein Analysis: Peripheral Blood Mononuclear Cell (PBMC) Lysates. PBMCs were isolated from whole heparinized blood by centrifugation through Ficoll–Paque (Pharmacia). PBMCs were lysed for 15 min at 4°C with 1% Triton X-100 in PBS, pH 7.4, containing 0.05% SDS and 2 mM PMSF. After centrifugation at 12,000 × g for 10 min, supernatants were collected and stored in aliquots at -70°C. After quantification of MCP in lysates, activity was evaluated as indicated below.
Flow Cytometry. Heparinized peripheral blood was incubated in the dark at room temperature for 15 min with Peridinin chlorophyll protein-conjugated anti-CD45 (a PBMC marker) Ab (Becton Dickinson) and either phycoerythrin-conjugated anti-MCP Ab (anti-CD46, clone 122-2, Serotec), or phycoerythrin-conjugated isotype control Ab (Serotec). The mAb binds complement control module 1 of MCP and recognized both mutants of MCP described in this article (data not shown). Bloods from nine normal healthy volunteers were used as controls.
Mutagenesis and Expression. Substitutions or deletions were produced by using the QuikChange site-directed mutagenesis kit (Stratagene). The template was MCP isoform BC1 (GenBank accession no. X59405) (19) cloned into the EcoRI site of plasmid pSG5 (Stratagene) for transient transfection or pHBApr1.neo for stable transfections (27). All cDNA clones were sequenced in their entirety to assure fidelity. Transfections were performed with Fugene-6 (Roche Molecular Biochemicals) into Chinese hamster ovary (CHO) K1 cells. Stable transfectants were selected in 0.5 mg/ml (active concentration) Geneticin (Invitrogen).
After cell lysis, MCP was quantified in an ELISA as described (28). Briefly, MCP mAb TRA-2–10 [that binds to complement control module 1 and recognizes the mutant proteins (verified by flow cytometry)] was coated at 5 μg/ml in microtiter wells followed by blocking in 4% BSA with 0.05% Tween 20 in PBS. Dilutions of cell lysates or standards were incubated in wells followed by reaction with rabbit anti-MCP antiserum (gift of Millennium Pharmaceuticals, Cambridge, MA), horseradishperoxidase-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch), and ImmunoPure TMB Substrate Kit (Pierce). MCP from cell lysates was characterized by Western blotting as described (28, 29). The blot was probed with a rabbit polyclonal Ab to MCP (30). Equal quantities of MCP were loaded. The negative control matched the highest amount of cell equivalents loaded.
Functional Assessment. Assays to evaluate complement activation on cell membranes have been described (20). Briefly, CHO transfectants were grown to 70–80% confluency and collected by brief trypsinization into 1% FCS-PBS. The cells were sensitized with anti-CHO antibodies (Cygnus Technologies, South-port, NC) at 0.25 mg/ml for 30 min at 4°C followed by complement activation for 1 hr at 37°C using C7-deficient serum (donated by P. Densen, University of Iowa, Iowa City) in gelatin veronal buffer. After complement activation, a murine mAb to human complement C3d (as an indicator for C3 fragment deposition, Quidel, San Diego) was added (10 μg/ml) for 30 min at 4°C followed by a similar incubation with FITC-goat anti-mouse IgG (Sigma–Aldrich). Cells were fixed in 0.5% paraformaldehyde and analyzed with a Becton Dickinson FACSCalibur system.
Ligand binding and cofactor assays have been described (28, 29). Briefly, an ELISA format was used for ligand binding in which C3b or C4b (Advanced Research Technologies, San Diego) was coated onto wells of a microtiter plate. Binding was detected by using a rabbit antiserum specific for MCP. For patient peripheral blood samples, use of the latter antiserum excluded measurement of any binding by complement receptor one. Binding assays were routinely performed in duplicate on two to three separate occasions by using diluted samples (from 1 × 109 to 1 × 1010 MCP molecules per ml as quantified in ELISA). The higher value was used to compare results. Binding of the cell lysates to the function-blocking MCP mAb, GB24, was assessed (described in ref. 29). Data were compiled from both stable and transient transfectants.
Cofactor assays have been described (28). These used biotinylated ligands in low-salt buffer (10 mM Tris/25 mM NaCl/1% Nonidet-P40), factor I (100 ng) and cell lysates (2.5 × 108 MCP molecules for C3b and 5.0 × 108 for C4b cofactor assays). Cleavage fragments were analyzed by using 10% reducing SDS/PAGE followed by transfer and Western blotting. Data were compiled from transient transfectants.
Results
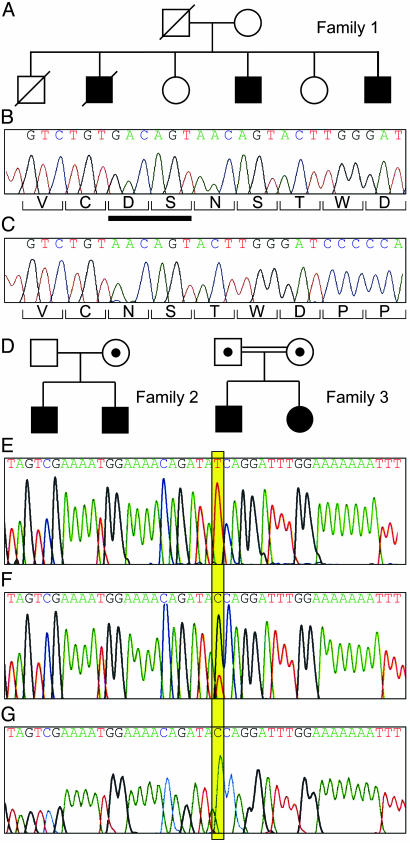
Family 1. The three affected family members in family 1 had a heterozygous 6-bp deletion (GACAGT) in MCP, resulting in the loss of amino acids D237 and S238 (Fig. 1 A–C). The mutation was not present in maternal DNA. No DNA was available from the deceased father. This change was not identified in 120 Northern European control subjects. We carried out fluorescence-activated cell sorting analysis of the PBMCs in the surviving affected male who did not have Waldenstroms macroglobulinaemia. The ratio of MCP fluorescence to isotype control was 5.0, and the mean ratio in eight normal controls also analyzed 24 h after sampling was 14.0 (SEM 0.9, range 11.7 to 18.4). Thus, the protein level in the affected individual studied was approximately half that of normal controls and suggests that the mutant protein is not expressed on the cell membrane.
Fig. 1.
(A) The pedigree of family 1. WT DNA sequence (B) and sequence (cloned PCR product) (C) from an affected individual from family 1. The nucleotide sequence is shown above and the corresponding amino acids below the sequence. There is a 6-bp deletion in the affected individual (GACAGT) resulting in deletion of an aspartic acid and serine (ΔD237/S238), denoted by the bar under B.(D) Shown are the pedigrees of families 2 and 3 (filled areas indicate an affected individual, • within □ or ○ indicates an unaffected heterozygote, and the double connecting line indicates consanguinity). (E) WT sequence. (F) A heterozygote T822C. (G) Homozygous T822C. This transition leads to an amino acid change, S206P. PCR products were directly sequenced.
C3b binding studies were performed on the cell lysates of the affected individual described above. There was a reduction of ≈50% in C3b binding as compared with WT MCP (Table 2). This finding is consistent with the inheritance of one normal and one mutant allele.
Table 2. C3b binding activity of MCP derived from lysates of PBMCs.
| Family | Patient ID | C3b binding, % WT MCP |
|---|---|---|
| 1 | C | Exp. 1: 56/57 |
| Exp. 2: 57/59 | ||
| 2 | D | 34 ± 4.5 |
| E | 40 ± 2.0 | |
| 3 | F | 1 ± 0.7 |
| G | 1 ± 0.4 |
These data were derived from two experiments for family 1 (each performed in duplicate) and three separate experiments for families 2 and 3 (each performed in triplicate with standard error shown). WT binding (assigned a value of 100%) was derived from a pool of PBMC lysates of normal individuals.
Families 2 and 3. A T822C transition (numbering from cDNA X59410), resulting in a serine to proline change, S206P, was identified in both family 2 and family 3 (Fig. 1 D–G). There is no known relationship between these two families. This change was not present in 112 control subjects including 62 from the region of Izmir in Turkey where family 3 lives. In family 2 this was a heterozygous mutation in the affected siblings, inherited from their unaffected mother. The ratios of MCP fluorescence to isotype control for the two affected siblings and their mother were 12, 9.8, and 10.1, respectively. In nine normal controls assayed at the same time interval after sampling (48 h) the mean ratio was 12.3 (SEM 1.1, range 8.2–18.9). In the two affected sibs in family 3, the change was homozygous. The ratios of MCP fluorescence to isotype control in the father, mother, and one affected sib from family 3 were 7.8, 10.8, and 10.7 respectively. The assays were performed 48 h after sampling. These results are consistent with normal expression of the mutant protein.
C3b binding studies of MCP in PBMC lysates demonstrated a reduction of ≈50% of the expected activity by affected members of family 2 (Table 2). There was no detectable binding in the affected individuals in family 3. These results are consistent with the respective heterozygous and homozygous nature of the mutational defect in these two families.
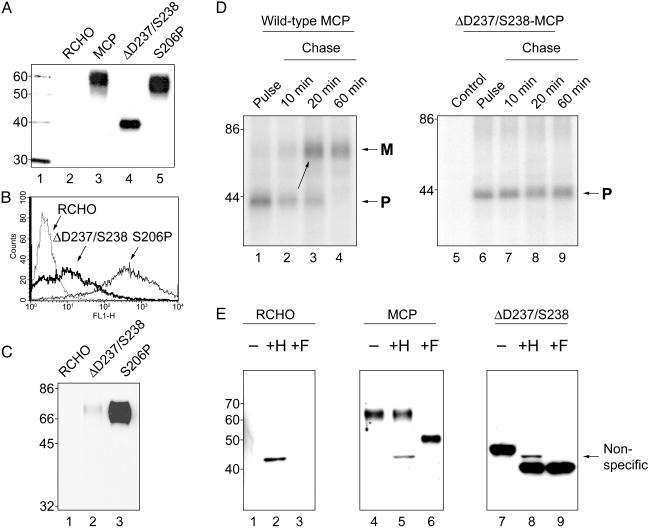
Functional and Structural Studies in Cell Lines Modeling MCP Mutations Observed in the Families. Mutant MCP constructs were evaluated after transfection into CHO cells. Whereas S206P migrated on SDS/PAGE with a mobility similar to WT MCP (Fig. 2A, lanes 3 and 5), the deletion mutant (ΔD237/S238) migrated as a low molecular mass species of ≈40 kDa (Fig. 2 A, lane 4). This molecular mass is similar to that of WT MCP precursor (see Fig. 2D and refs. 27 and 31), suggesting that the deletion mutant was retained intracellularly. Flow cytometry, cell surface labeling (biotinylation), and pulse–chase analysis supported this interpretation (see Fig. 2 B–D). The deletion mutant expressed <10% on the cell surface as compared with S206P (Fig. 2B) and a protein analogous to the ≈40-kDa sample was not observed after biotinylation of surface proteins (Fig. 2C, lane 2). Thus, this mutant protein is largely retained intracellularly.
Fig. 2.
(A) Western blot of CHO cell lysates probed with a rabbit polyclonal Ab to MCP. Lane 1 contains the size markers. Lane 2 is a CHO cell not expressing MCP. Lane 3 shows the phenotype of WT MCP as expressed by transfected CHO cells. Lanes 4 and 5 are the mutations in WT MCP identified in the HUS families. Lane 4 represents the cell line expressing the mutant observed in family 1. Its lower migration pattern is similar to a precursor form of MCP (see below). S206P is the single proline substitution for the native serine identified in families 2 and 3 (lane 5) that migrates similarly to WT. (B) Fluorescence-activated cell sorting analysis of expression of the MCP mutants ΔD237/S238 versus S206P shows low expression levels of the deletion mutant. (C) Biotinylation of cell surface proteins followed by immunoprecipitation with a mAb to MCP shows only a trace of deleted protein expressed on the cell surface. (D) Pulse–chase analysis of the deletion mutant (ΔD237/S238) versus WT MCP. The precursor of the deletion mutant does not chase into the mature protein. (E) Glycosidase digestion of deletion mutant (ΔD237/S238) versus WT MCP (see text for explanations).
In pulse–chase analyses, precursor proteins of both WT MCP and the deletion mutant aligned at ≈40 kDa (Fig. 2D, lanes 1 and 6), the same molecular mass as the mutant protein cell lysates immunoblotted (Fig. 2 A, lane 4). However, whereas the WT precursor chases into its higher molecular mass mature form largely by 20 min (Fig. 2D, lane 3) the deletion mutant protein remains at the same molecular mass and in the same quantity for at least 60 min (Fig. 2D, lane 9).
Next, glycosidase analyses were performed (Fig. 2E) with endoglycosidase H (which removes high mannose N-linked units characteristic of precursor proteins) and PNGase F [which removes both complex sugars (characteristic of mature proteins) and high mannose units]. In the case of WT MCP (Fig. 2E, lane 4), only PGNase F removed the sugars (compare lanes 5 and 6 of Fig. 2E), demonstrating this to be a complex type of N-linked sugar (i.e., the mature or cell membrane form). In contrast, for the mutant protein, both endoglycosidase H (Fig. 2E, lane 8) and PNGase F (Fig. 2E, lane 9) removed the N-linked sugars. This finding indicates that the attached sugars on the mutant protein are of the high mannose type consistent with it being the precursor or pro-form of MCP and thus intracellular (27, 31). Functional activity was evaluated on lysates containing the intracellular protein and demonstrated that it retained ligand binding activity but had minimal cofactor activity (data not shown).
As mentioned, substitution of a proline for a serine at position 206 (S206P) in families 2 and 3 resulted in expression levels similar to WT but reduced functional activity. Likewise, in CHO cells expressing this mutation regulatory activity was diminished (Table 3). This was evaluated in several ways. First, CHO transfectants were challenged by Ab and complement in a previously established model system to assess complement regulatory activity of membrane proteins (20, 28). Compared with WT MCP, the membranes of cells bearing the S206P mutation demonstrated less inhibition of C3b deposition. Thus, increased amounts of C3 fragments on the S206P cell surface indicate a reduced ability to protect against complement attack (Table 3). As expected, C3b ligand binding by the recombinantly expressed mutant protein also was diminished and no C3b cofactor activity was detected (Table 3). Further, lysates of S206P showed a nearly complete loss of reactivity to mAb GB24, which blocks C3b binding by MCP. From these data, we conclude that while this mutant demonstrated an electrophoretic mobility and expression levels similar to WT MCP (Fig. 2 A, lane 5), it has a reduced ability to interact with C3b.
Table 3. Functional consequences of the S206P MCP substitution expressed in CHO transfectants.
| Cell line | Description | Inhibition of C3b deposition on cell membranes,* % | C3b binding,† % | C3b cofactor‡ | C4b binding,† % | C4b cofactor‡ | GB24 binding,§ % |
|---|---|---|---|---|---|---|---|
| RCHO¶ | Control | 0 | 0.8 ± 0.1 | — | 0.6 ± 0.2 | — | 0.4 ± 0.1 |
| MCP | WT | 76 ± 3 | 100 | ++++ | 100 | ++++ | 100 |
| S206P | Families 2 & 3 model; MCP with substitution | 40 ± 6 | 15 ± 2 | — | 102 ± 3 | ++++ | 4 ± 1 |
These data represent the mean ± standard error for three to four experiments.
Inhibition calculated from mean fluorescent intensity derived from C3 fragment deposition assessed by flow cytometry in which RCHO (non-MCP expressing cells) was set at 0% inhibition and compared to WT MCP and the S206P-expressing CHO cells
WT activity was set at 100% by using 1 × 1010 MCP molecules per ml for each sample and evaluated in stable and transient transfectants
Cofactor activity was evaluated in transient transfectants and graded as follows: ++++, 90—120% of WT; +++, 70—89%; ++, 40—69%; +, 10—39%; —, <10%. See ref. 30
GB24 is a mAb to MCP that blocks C3b binding
RCHO is a CHO cell line with MCP cDNA cloned in the reverse orientation
Discussion
Our data provide strong evidence that mutations in MCP predispose to HUS. In family 1 we have identified a deletion removing two amino acids. The CHO cell model of this mutation establishes that the defective protein is improperly processed and, therefore, is retained intracellularly as a precursor. This result is consistent with the fluorescence-activated cell sorting analysis and functional activity of the patient PBMC samples indicating ≈50% of the expected expression in the heterozygous patient. It is likely that the mutation in family 1 was transmitted by the deceased father although he did not have HUS. Incomplete penetrance is also a feature in dominant HUS pedigrees with factor H mutations (5–7).
All three affected individuals in family 1 had successful cadaveric renal transplants without any recurrence of HUS in the allograft. In contrast, if patients with factor H mutations are transplanted, recurrence of HUS in the transplanted kidney is common (32). Factor H is a plasma protein synthesized by the liver, whereas MCP is synthesized locally by each cell. Our results suggest that the presence of WT MCP in the transplanted kidney may be sufficient to protect against recurrent HUS. Family 1 demonstrates why distinguishing between MCP and factor H deficiencies in specific patients could supply critical information for treatment decisions.
In contrast to family 1, in the second and third families MCP expression and quantity are normal but C3b binding is markedly decreased. The four affected individuals in families 2 and 3 have had full recovery of renal function. This would be unusual in patients with factor H mutations. The clinical picture in the affected individuals from families 2 and 3 is similar, despite the deficiency being heterozygous in family 2 and homozygous in family 3. The mother in family 2 and both parents in family 3 are heterozygous for the mutation but have not developed HUS. This raises the question as to why only homozygotes have manifested the HUS phenotype in family 3, whereas heterozgotes have shown the same phenotype in family 2. One possibility is that the affecteds in family 2 have a second functional change, either in one of the other complement regulatory proteins or another protein, that acts as a modifier. However, we have not detected factor H or decay-accelerating factor mutations in these three kindreds (unpublished work). We favor the hypothesis that the development of a thrombotic microangiopathy in association with MCP mutations (and factor H mutations) requires an endothelial injury that instead of resolving is maintained through excessive activation of the complement system (20, 21, 24). Disease onset thus would depend on an endothelial insult. In this scenario, mutations in factor H and MCP could be seen as predisposing to, rather than causing, HUS. Another consideration is that the S206P mutation leaves C4b binding and cofactor activities intact; therefore, only an alternative pathway triggering insult would predispose to HUS. A final possibility is that our findings represent an ascertainment artifact, but we think this is unlikely, as we have not found either of the function-altering mutations described here in a large number of normal controls.
These findings disclosing MCP mutations shed further light on the pathogenesis of HUS. The mutations in factor H have clustered largely in the exon coding for complement control protein domain 20, which is known to be important in substrate binding (33). Also, recent studies have documented impaired binding of mutant factor H to membrane-bound C3b (9–11). The S206P mutation in MCP lies within a hypervariable loop in complement control protein domain 4, a site previously found to be important for C3b regulation (29). Thus, these mutations in MCP, particularly S206P, suggest that a failure to inactivate or regulate C3b mediates disease development. In particular, both factor H and MCP bind C3b and possess cofactor activity for C3b (19). We hypothesize that factor H and MCP protect against C3 activation on vascular tissue. MCP provides protection because of its expression on endothelial cells. As the glomerular capillary bed is a fenestrated endothelium, the exposed basement membrane supplies a surface rich in polyanions for factor H binding. Once bound to such a substrate, factor H then functions like MCP to protect the tissue.
An immediate clinical consequence of our findings is that determination of the genetic defect underlying HUS could facilitate selection of therapy for patients with HUS because renal transplantation for a patient with an MCP mutation should not be associated with disease recurrence. Reduced complement levels in patients with HUS suggest a complement regulatory defect but the families reported here demonstrate again that normal serum C3 levels do not exclude such a defect. Based on the studies of factor H abnormalities in HUS and the MCP findings presented here we suggest that there is sufficient evidence to undertake a clinical trial in some HUS patients by using complement inhibitors that block the activation of C3 (reviewed in refs. 34 and 35).
Finally, this work has implications for how we think about syndromes involving essentially any type of tissue injury where the complement cascade is activated (autoantibodies, immune complexes, ischemia-reperfusion injury, sepsis, and many others). For a given degree of injury, individuals with defects in MCP, even if heterozygous, will likely be at increased risk for more severe tissue damage because they cannot appropriately regulate C3b activation and amplification.
Supplementary Material
Acknowledgments
A.R. was supported by a Medical Research Council Clinical Training Fellowship. The support of the National Kidney Research Fund is acknowledged. J.P.A. is supported by National Institutes of Health Grant R01 AI37618.
Abbreviations: HUS, hemolytic uremic syndrome; MCP, membrane cofactor protein; PBMC, peripheral blood mononuclear cell; CHO, Chinese hamster ovary.
References
- 1.Moake, J. L. (2002) N. Engl. J. Med. 347, 589-600. [DOI] [PubMed] [Google Scholar]
- 2.Richards, A., Goodship, J. A. & Goodship, T. H. J. (2002) Curr. Opin. Nephrol. Hypertens. 11, 431-435. [DOI] [PubMed] [Google Scholar]
- 3.Ying, L., Katz, Y., Schlesinger, M., Carmi, R., Shalev, H., Haider, N., Beck, G., Sheffield, V. C. & Landau, D. (1999) Am. J. Hum. Genet. 65, 1538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warwicker, P., Goodship, T. H. J., Donne, R. L., Pirson, Y., Nicholls, A., Ward, R. M. & Goodship, J. A. (1998) Kidney Int. 53, 836-844. [DOI] [PubMed] [Google Scholar]
- 5.Richards, A., Buddles, M. R., Donne, R. L., Kaplan, B. S., Kirk, E., Venning, M. C., Tielemans, C. L., Goodship, J. A. & Goodship, T. H. J. (2001) Am. J. Hum. Genet. 68, 485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprioli, J., Bettinaglio, P., Zipfel, P. F., Amadei, B., Daina, E., Gamba, S., Skerka, C., Marziliano, N., Remuzzi, G. & Noris, M. (2001) J. Am. Soc. Nephrol. 12, 297-307. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Caballero, D., González-Rubio, C., Gallardo, M. E., Vera, M., López-Trascasa, M. & Rodríguez de Córdoba, S. (2001) Am. J. Hum. Genet. 68, 478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins, S. J. & Goodship, T. H. J. (2002) J. Mol. Biol. 316, 217-224. [DOI] [PubMed] [Google Scholar]
- 9.Pangburn, M. K. (2002) J. Immunol. 169, 4702-4706. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Corral, P., Perez-Caballero, D., Huarte, O., Simckes, A. M., Goicoechea, E., Lopez-Trascasa, M. & Rodriguez De Cordoba, S. (2002) Am. J. Hum. Genet. 71, 1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manuelian, T., Hellwage, J., Meri, S., Caprioli, J., Noris, M., Heinen, S., Jozsi, M., Neumann, H. P., Remuzzi, G. & Zipfel, P. F. (2003) J. Clin. Invest. 111, 1181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker, C. J. (1992) Membrane Defenses Against Attack by Complement and Perforins (Springer, Berlin). [PubMed]
- 13.Morgan, B. P. & Harris, C. L. (1999) Complement Regulatory Proteins (Academic, London).
- 14.Endoh, M., Yamashina, M., Ohi, H., Funahashi, K., Ikuno, T., Yasugi, T., Atkinson, J. P. & Okada, H. (1993) Clin. Exp. Immunol. 94, 182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichida, S., Yuzawa, Y., Okada, H., Yoshioka, K. & Matsuo, S. (1994) Kidney Int. 46, 89-96. [DOI] [PubMed] [Google Scholar]
- 16.McNearney, T., Ballard, L., Seya, T. & Atkinson, J. P. (1989) J. Clin. Invest. 84, 538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi, I., Moutabarrik, A., Hara, T., Hatanaka, M., Hayashi, T., Syouji, T., Okada, N., Kitamura, E., Tsubakihara, Y., Matsumoto, M., et al. (1994) Eur. J. Immunol. 24, 1529-1535. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone, R. W., Loveland, B. E. & McKenzie, I. F. (1993) Immunology 79, 341-347. [PMC free article] [PubMed] [Google Scholar]
- 19.Liszewski, M. K., Farries, T. C., Lublin, D. M., Rooney, I. A. & Atkinson, J. P. (1996) Adv. Immunol. 61, 201-283. [DOI] [PubMed] [Google Scholar]
- 20.Barilla-LaBarca, M. L., Liszewski, M. K., Lambris, J. D., Hourcade, D. & Atkinson, J. P. (2002) J. Immunol. 168, 6298-6304. [DOI] [PubMed] [Google Scholar]
- 21.Devaux, P., Christiansen, D., Fontaine, M. & Gerlier, D. (1999) Eur. J. Immunol. 29, 815-822. [DOI] [PubMed] [Google Scholar]
- 22.Kojima, A., Iwata, K., Seya, T., Matsumoto, M., Ariga, H., Atkinson, J. P. & Nagasawa, S. (1993) J. Immunol. 151, 1519-1527. [PubMed] [Google Scholar]
- 23.Oglesby, T. J., Allen, C. J., Liszewski, M. K., White, D. J. & Atkinson, J. P. (1992) J. Exp. Med. 175, 1547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seya, T. & Atkinson, J. P. (1989) Biochem. J 264, 581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seya, T., Turner, J. R. & Atkinson, J. P. (1986) J. Exp. Med. 163, 837-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirson, Y., Lefebvre, C., Arnout, C. & Van Ypersele de Strihou, C. (1987) Clin. Nephrol. 28, 250-255. [PubMed] [Google Scholar]
- 27.Liszewski, M. K., Tedja, I. & Atkinson, J. P. (1994) J. Biol. Chem. 269, 10776-10779. [PubMed] [Google Scholar]
- 28.Liszewski, M. K., Leung, M. K. & Atkinson, J. P. (1998) J. Immunol. 161, 3711-3718. [PubMed] [Google Scholar]
- 29.Liszewski, M. K., Leung, M., Cui, W., Subramanian, V. B., Parkinson, J., Barlow, P. N., Manchester, M. & Atkinson, J. P. (2000) J. Biol. Chem. 275, 37692-37701. [DOI] [PubMed] [Google Scholar]
- 30.Wang, G., Liszewski, M. K., Chan, A. C. & Atkinson, J. P. (2000) J. Immunol. 164, 1839-1846. [DOI] [PubMed] [Google Scholar]
- 31.Ballard, L. L., Bora, N. S., Yu, G. H. & Atkinson, J. P. (1988) J. Immunol. 141, 3923-3929. [PubMed] [Google Scholar]
- 32.Ruggenenti, P. (2002) Kidney Int. 62, 1093-1104. [DOI] [PubMed] [Google Scholar]
- 33.Blackmore, T. K., Hellwage, J., Sadlon, T. A., Higgs, N., Zipfel, P. F., Ward, H. M. & Gordon, D. L. (1998) J. Immunol. 160, 3342-3348. [PubMed] [Google Scholar]
- 34.Kirschfink, M. (2001) Immunol. Rev. 180, 177-189. [DOI] [PubMed] [Google Scholar]
- 35.Lambris, J. D. & Holers, V. M. (2002) Therapeutic Interventions in the Complement System (Humana, Towata, NJ).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.