Abstract
The CST20 gene of Candida albicans was cloned by functional complementation of a deletion of the STE20 gene in Saccharomyces cerevisiae. CST20 encodes a homolog of the Ste20p/p65PAK family of protein kinases. Colonies of C. albicans cells deleted for CST20 revealed defects in the lateral formation of mycelia on synthetic solid “Spider” media. However, hyphal development was not impaired in some other media. A similar phenotype was caused by deletion of HST7, encoding a functional homolog of the S. cerevisiae Ste7p protein kinase. Overexpression of HST7 partially complemented the deletion of CST20. Cells deleted for CST20 were less virulent in a mouse model for systemic candidiasis. Our results suggest that more than one signaling pathway can trigger hyphal development in C. albicans, one of which has a protein kinase cascade that is analogous to the mating response pathway in S. cerevisiae and might have become adapted to the control of mycelial formation in asexual C. albicans.
Candida albicans is the major fungal pathogen in humans, causing various forms of candidiasis. This fungus is diploid with no sexual cycle and is capable of a morphological transition from a unicellular budding yeast to a filamentous form. Extensive filamentous growth leads to the formation of a mycelium displaying hyphae with branches and lateral buds. In view of the observation that hyphae seem to adhere to and invade host tissues more readily than does the yeast form, the switch from the yeast to the filamentous form probably contributes to the virulence of this organism (for a review, see ref. 1).
Like C. albicans, bakers yeast Saccharomyces cerevisiae is also a dimorphic organism capable of switching under certain nutritional conditions from a budding yeast to a filamentous form. Under the control of nutritional signals, diploid cells switch to pseudohyphal growth (2), and haploid cells to invasive growth (3).
The similarities between the dimorphic switching of S. cerevisiae and C. albicans suggest that these morphological pathways may be regulated by similar mechanisms in both organisms. In S. cerevisiae, morphological transitions are controlled by signaling components that are also involved in the mating response of haploid cells (3, 4). The switch to pseudohyphal growth requires a transcription factor encoded by the STE12 gene, and a mitogen-activated protein (MAP) kinase cascade including Ste7p (a homolog of MAP kinase kinase or MEK), Ste11p (a MEK kinase homolog), and Ste20p (a MEK kinase kinase) (3, 4). The MAP kinases involved in this response are as yet unknown (3, 4).
We have recently shown that C. albicans contains a functional homolog of Ste7p (Hst7p; ref. 5). Cph1p, a homolog of the Ste12p transcription factor, is required for hyphal growth of C. albicans under certain in vitro conditions (6). Here we show a similar requirement for Hst7p and Cst20p, a C. albicans homolog of the Ste20p protein kinase. We also show in a mouse model for systemic candidiasis that Cst20p plays a role in virulence, as judged from significantly prolonged survival of mice infected with cst20-deleted cells. Our results suggest that Cst20p, Hst7p, and Cph1p act in a common regulatory pathway which is involved in hyphal growth of C. albicans.
MATERIALS AND METHODS
Yeast Manipulations.
The yeast form of C. albicans was cultured at 30°C in yeast extract/peptone/dextrose (YPD) medium (7). Hyphal growth was induced at 37°C on solid “Spider” media (6) containing 1% (wt/vol) nutrient broth, 0.2% (wt/vol) K2HPO4, 2% (wt/vol) agar, and 1% (wt/vol) of the indicated sugars (pH 7.2 after autoclaving). Cells were grown in liquid Spider media at 30°C to stationary phase, and then incubated for 5 days at 37°C on solid Spider media at a density of about 200 cells per 80-mm plate. All media were supplemented with uridine (25 μg/ml) for the growth of Ura− strains. Germ tube formation was induced at 37°C in either 10% fetal bovine serum (GIBCO/BRL), liquid Spider media containing the indicated sugars, or Lee’s medium (8) at an inoculation density of 107 cells per ml.
S. cerevisiae cells were transformed by the lithium acetate method, and C. albicans cells were transformed by the spheroplast protocol (7). Plasmid DNA was isolated from yeast cells as described (7). Quantitative mating of S. cerevisiae cells was performed with the MATα tester strain A281-30A as described (9).
Isolation of CST20.
The CST20 gene was isolated from a genomic C. albicans library constructed in plasmid YEp352 from genomic DNA of the clinical isolate WO1 (10). A plasmid carrying an amino terminally truncated version of CST20 missing the first 918 nt of coding sequence was isolated in a previously described screen (5) that sought suppressors of defects in basal FUS1::HIS3 expression and mating in S. cerevisiae strain YEL64 which was disrupted in STE20 (9). A fragment from nucleotides 958 to 1252 of CST20 was amplified by PCR and used as a probe to isolate a full-length clone by colony hybridization to the C. albicans genomic library transformed into Escherichia coli strain MC1061. Both DNA strands were sequenced by the dideoxy chain termination method (11). The full-length clone was subcloned between the SacI and HindIII sites of the S. cerevisiae centromere plasmid pRS316 (12) to yield plasmid pRL53.
Construction of C. albicans Strains and Plasmids.
To construct a cst20 null mutant, an EcoRI–SacI fragment from nucleotide positions 989 to 4134 of CST20 was subcloned into the pBluescript KS(+) vector (Stratagene) to yield plasmid pDH119. A plasmid that contained CST20-flanking sequences from nucleotides 989 to 1674 and 3423 to 4134 joined with BamHI sites, was then created by PCR using the divergent oligodeoxynucleotide primers ODH68 (5′-CGGGATCCAGACCAACCACTCGAACTACT-3′) and ODH69 (5′-CGGGATCCGAAGGTGAACCACCATATTTG-3′; newly introduced BamHI sites are underlined) and plasmid pDH119 as a template. The amplified DNA was cleaved with BamHI and ligated with a 4-kb BamHI–BglII fragment of a hisG-URA3-hisG cassette derived from plasmid pCUB-6 (13) to yield plasmid pDH183. This plasmid was linearized with XhoI and SacI and transformed into the Ura− C. albicans strain CAI4 (13) to partially replace the coding region of one of the chromosomal CST20 alleles with the hisG-URA3-hisG cassette by homologous recombination. Ura+ transformants were selected on Ura− medium, and integration of the cassette into the CST20 locus was verified by Southern blot analysis. Spontanous Ura− derivatives of two of the heterozygous disruptants were selected on medium containing 5-fluoroorotic acid. These clones were screened by Southern blot hybridization to identify those which had lost the URA3 gene by intrachromosomal recombination mediated by the hisG repeats. This procedure was then repeated to delete the remaining functional allele of CST20.
A similar procedure was employed to delete the HST7 gene. To construct the disruption plasmid pPF-7, a 7.1-kb SalI–BamHI fragment carrying the HST7 gene (5) was subcloned into the pBluescript KS(+) vector. A fragment from a HindIII site located 1.8-kb upstream of the HST7 coding sequence to the BglII site at nucleotide position 1518 of HST7 (5) was then replaced with a BglII–HindIII fragment of the hisG-URA3-hisG cassette from plasmid pMB7 (13) to yield plasmid pPF-7. This plasmid was linearized with ApaI and NotI for transformation into C. albicans strain CAI4. The deletion of both HST7 alleles was confirmed by Southern blot hybridization.
To reintegrate CST20 into the genome of mutant strains, the C. albicans integration plasmid pDH190 was constructed by subcloning a KpnI–PstI fragment of CST20 into pBS-cURA3 [pBluescript KS(+) into which the C. albicans URA3 gene was cloned between the NotI and XbaI sites of the polylinker; J. Douglas, I.D.B., and A.J.P.B., unpublished work]. The integration plasmid was then linearized with NsiI and transformed into C. albicans to target integration into the NsiI site of the cst20Δ::hisG fusion gene. Integrations were selected on Ura− medium and confirmed by Southern blot analysis.
The C. albicans CST20 expression plasmid pDH188 was constructed by subcloning a SacI–PstI fragment of CST20 into plasmid pVEC carrying a C. albicans autonomously replicating sequence and URA3 as selectable marker [kindly provided by C. Nombela (University of Madrid) and B. Magee (University of Minnesota)]. Similarly, the C. albicans HST7 expression plasmid pDH186 was constructed by subcloning a KpnI–SalI fragment of HST7 into pVEC. To construct plasmid pYPB1-ADHpt-HST7 carrying HST7 under control of the ADH1 promoter, the coding region of HST7 flanked by BamHI sites was amplified by PCR by using the oligodeoxynucleotide primers 5′-GCTCATTCATCATGGATCCAACAATGACAAG-3′ and 5′-GTATATGTATATGGGATCCGTTTACACTTTGC-3′ (the newly introduced BamHI sites are underlined), and cloned into the BglII site of the C. albicans expression vector pYPB1-ADHpt containing the C. albicans ADH1 promoter and terminator regions (14), an autonomously replicating sequence, C. albicans URA3 as selectable marker, and S. cerevisiae 2 μm sequences (G. Bertram, I.D.B., P. J. F. Feldmann, and A.J.P.B., unpublished).
Northern Blot Analyses.
Northern blots of total and poly(A)+ RNA from C. albicans cells were performed as described (5, 15) using an XbaI–BamHI fragment from nucleotide positions 2143 to 3100 of the CST20 DNA and the coding region of HST7 (5) as hybridization probes.
Animal Experiments.
Eight-week-old, male CFW-1 mice (Halan–Winkelmann, Paderborn, Germany) were inoculated with 1 × 105 or 1 × 106 cells by intravenous injection (16). Survival curves were calculated according to the Kaplan–Meier method using the prism program (GraphPad Software, San Diego) and compared using the log-rank test. A P value ≤0.05 was considered significant.
To quantify colony-forming C. albicans units in kidneys, mice were sacrificed by cervical dislocation 48 h after injection and kidneys were homogenized in 5 ml PBS, serially diluted, and plated on YNG medium (0.67% yeast nitrogen base/1% glucose, pH 7.0). Histological examination of kidney sections stained with periodic acid Schiff’s stain was as described (17).
RESULTS
Isolation and Characterization of CST20.
A C. albicans homolog of the S. cerevisiae STE20 gene was cloned by functional complementation of the pheromone signaling defect of S. cerevisiae cells that were deleted for the STE20 gene. We made use of strain YEL64 in which expression of the HIS3 gene fused to the pheromone-inducible promoter of FUS1 was dependent on the function of STE20 (9). A C. albicans genomic library was screened for plasmids that conferred both histidine prototrophy and ability to mate. By screening 2500 transformants, we isolated three plasmids containing amino terminally truncated versions of a STE20 homolog, designated CST20 (C. albicans STE20). Analogous screens identified HST7 and CPH1 encoding homologs of the S. cerevisiae Ste7p (5) and Ste12p (6) proteins, respectively. Full-length CST20 was subsequently isolated from the same library by colony hybridization (see Materials and Methods).
The mating defect of the ste20-deleted S. cerevisiae strain YEL206 (18) was fully complemented by introduction of the centromeric plasmid pRL53 carrying full-length CST20 (mating efficiency was 81 ± 9% in cells expressing CST20, compared with 85 ± 8% in cells expressing STE20; n = 3). Similarly, defects in growth arrest and morphological changes in response to pheromone were completely cured by transformation with the CST20 plasmid (data not shown). Moreover, nitrogen deficiency-induced pseudohyphae formation, which is blocked by disruption of STE20 in diploid cells (4), was restored by introduction of the CST20 plasmid (Fig. 1).
Figure 1.
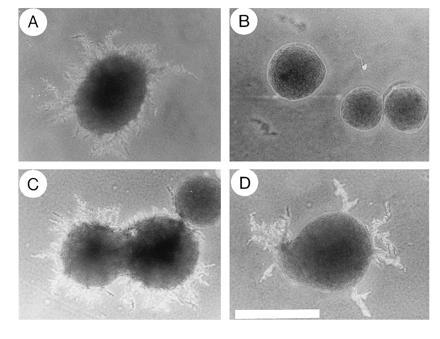
The C. albicans CST20 gene complements defects in pseudohyphal growth of ste20/ste20 S. cerevisiae diploid cells. Colonies of the diploid STE20 wild-type strain L5266 (4) (A) and the isogenic ste20/ste20 strain HLY492 (4) transformed with either the control plasmid pRS316 (B), the CST20 plasmid pRL53 (C), or the STE20 plasmid pSTE20-5 (15) (D) were grown on nitrogen starvation medium (2) for 5 days at 30°C. (×4; bar = 1 mm.)
Further characterization of the CST20 plasmid in S. cerevisiae cells showed that the cytokinesis defect caused by deletion of CLA4, encoding an S. cerevisiae isoform of Ste20p (19), was not complemented by CST20 (Fig. 2). However, the lethality caused by deletion of both STE20 and CLA4 (19) could be rescued by CST20 (Fig. 2). Thus, Cst20p acted as a fully functional homolog of Ste20p and did not overlap with the function of Cla4p.
Figure 2.
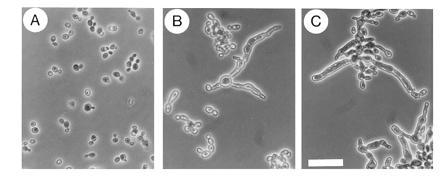
Morphology of S. cerevisiae MATa cells (strain YEL306-1A) deleted for STE20 and CLA4, and transformed with plasmids expressing CLA4 (A), STE20 (B) and C. albicans CST20 (C). The diploid strain YEL306 heterozygous for ste20Δ::TRP1/STE20 cla4Δ::LEU2/CLA4 (E.L., unpublished) was transformed with plasmid pRS316 carrying either no insert, CLA4 (pRL21), CST20 (pRL53), or STE20 (pSTE20-5), and then sporulated and dissected. No viable haploid ste20Δ cla4Δ spores were obtained from transformants with the plasmid without insert, but were obtained from transformants with plasmids carrying CLA4, STE20, or CST20. Cells were grown to mid-exponential phase in YPD medium at 30°C. No viable ste20Δ cla4Δ segregants were obtained in medium containing 5-fluoroorotic acid suggesting that the plasmids were essential for viability. Neither STE20 nor CST20 were able to suppress the morphological defect of cla4Δ cells. (×40; bar = 30 μm.)
The open reading frame of CST20 is capable of encoding
a protein of 1229 amino acids with a predicted molecular weight of 133
kDa and a domain structure characteristic of the
Ste20p/p65PAK family of protein kinases (Fig.
3
 A). The catalytic domain present in
the carboxyl terminal half of the protein has sequence identities of 76
and 56%, respectively, with S. cerevisiae Ste20p (15) and
Cla4p (19), 56% with Schizosaccharomyces pombe
Shk1p/Pak1p (21, 22), and 60% with rat p65PAK (23) (Fig.
3B). The amino-terminal, noncatalytic region contains a
sequence from amino acid residues 473 to 531 with 68% identity to the
p21 binding domain of Ste20p that has been shown to bind the small
GTPase Cdc42p (24) (Fig. 3C). This region contains the
sequence motif ISxPxxxxHxxH thought to be important for the interaction
of the p21 binding domain with the GTP-bound forms of Cdc42Hs and Rac1
(19, 23). The remaining noncatalytic sequences are less conserved.
Unique sequences not present in Ste20p and the other members of the
family are found at the amino terminus and between the p21 binding and
catalytic domains.
A). The catalytic domain present in
the carboxyl terminal half of the protein has sequence identities of 76
and 56%, respectively, with S. cerevisiae Ste20p (15) and
Cla4p (19), 56% with Schizosaccharomyces pombe
Shk1p/Pak1p (21, 22), and 60% with rat p65PAK (23) (Fig.
3B). The amino-terminal, noncatalytic region contains a
sequence from amino acid residues 473 to 531 with 68% identity to the
p21 binding domain of Ste20p that has been shown to bind the small
GTPase Cdc42p (24) (Fig. 3C). This region contains the
sequence motif ISxPxxxxHxxH thought to be important for the interaction
of the p21 binding domain with the GTP-bound forms of Cdc42Hs and Rac1
(19, 23). The remaining noncatalytic sequences are less conserved.
Unique sequences not present in Ste20p and the other members of the
family are found at the amino terminus and between the p21 binding and
catalytic domains.
Figure 3.
(A) Nucleotide and predicted amino acid sequences of CST20. Numbers on the left indicate nucleotide and amino acid positions. Nucleotide 1 corresponds to the first nucleotide of the initiation codon, and amino acid 1 to the first residue of the deduced protein. The putative p21 binding domain has been shadowed, and the kinase domain has been boxed. According to the presumed codon usage in C. albicans, the CTG codon has been translated into serine (20). Multiple amino acid alignments of the conserved kinase domains (B) and p21 binding domains (C) of Cst20p, S. cerevisiae Ste20p (15), S. cerevisiae Cla4p (19), Schizosaccharomyces pombe Shk1p/Pak1p (21, 22) and rat p65PAK (23). Sequence similarities with Cst20p are shaded. The following groups of amino acids were classified as similar: A, S, T; D, E; N, Q; R, K; I, L, M, V; and F, Y, W. Consensus residues conserved among at least three of the five kinases are shown below the protein sequences. Residues identical in all five kinases are underlined. The numbers at the right margin specify the amino acid positions within each protein.
A CST20 transcript of 4.9 kb in size was detected in Northern blots. This transcript was present at similar levels in yeast cells grown in YPD at room temperature and germ tubes induced by a temperature shift to 37°C (data not shown).
Chromosomal Deletions of CST20 and HST7.
Homologous recombination was used in a multistep procedure to partially delete CST20 and HST7 in a ura3/ura3 C. albicans strain (Fig. 4 A and C). The deletions were confirmed by Southern blot analyses (Fig. 4 B and D). Northern blots showed that the CST20 and HST7 transcripts were absent in the corresponding homozygous deletion strains (data not shown).
Figure 4.
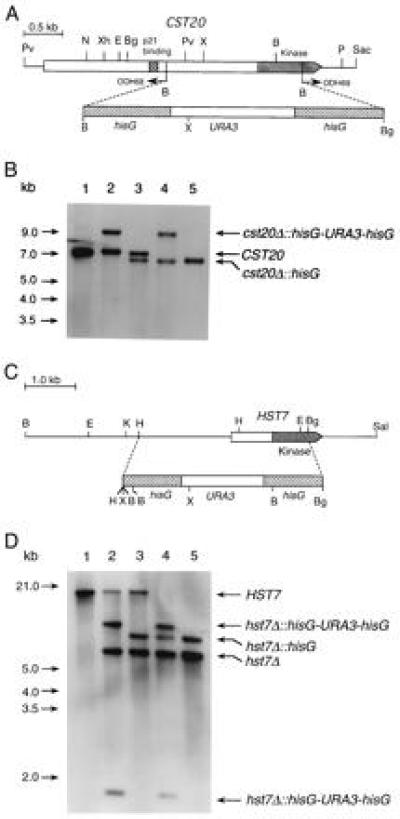
(A) Deletion of CST20 in C. albicans. PCR with the divergent oligodeoxynucleotides ODH68 and ODH69 was used to partially delete the coding sequence of CST20. A hisG-URA3-hisG cassette was then inserted. (B) Southern blot analysis with a CST20 fragment from EcoRI–XbaI as a probe. The genomic DNA samples digested with XhoI were from following strains: Lanes: 1, CAI4 (ura3/ura3 CST20/CST20); 2, CDH15 (ura3/ura3 CST20/cst20Δ::hisG-URA3-hisG); 3, CDH18 (ura3/ura3 CST20/cst20Δ::hisG); 4, CDH22 (ura3/ura3 cst20Δ::hisG-URA3hisG/cst20Δ::hisG); and 5, CDH25 (ura3/ura3 cst20Δ::hisG/cst20Δ::hisG). (C) Deletion of HST7 in C. albicans. A HindIII–BglII fragment was replaced by the hisG-URA3-hisG cassette to create plasmid pPF-7. (D) Southern blot analysis was performed with the two large BamHI fragments of pPF-7 as probes recognizing the HST7 sequence upstream of the HindIII site and the hisG-URA3-hisG cassette. The genomic DNA samples digested with XbaI were from following strains. Lanes: 1, CAI4 (ura3/ura3 HST7/HST7); 2, CDH5 (ura3/ura3 HST7/hst7Δ::hisG-URA3-hisG); 3, CDH8 (ura3/ura3 HST7/hst7Δ::hisG); 4, CDH10 (ura3/ura3 hst7Δ::hisG-URA3-hisG/hst7Δ::hisG); and 5, CDH12 (ura3/ura3 hst7Δ::hisG/hst7Δ::hisG). The endonuclease restriction sites are as follows: B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; K, KpnI; N, NsiI; P, PstI; Pv, PvuII; Sac, SacI; Sal, SalI; X, XbaI; Xh, XhoI.
The lateral outgrowth of hyphae from colonies grown on solid Spider media containing mannitol or sorbitol was completely blocked by deletion of CST20 (Fig. 5B). Mycelial formation was drastically reduced when the media contained galactose, mannose or raffinose (data not shown). The mutant strains regained the ability to form hyphae when wild-type CST20 was reintroduced by transformation with the CST20 expression plasmid pDH188 (data not shown) or reintegrated into the genome by targeted homologous recombination (Fig. 5C). The CST20 transcript was detected in these strains by Northern blot analysis (data not shown).
Figure 5.

Colonies of C. albicans cells grown for 5 days at 37°C on solid Spider medium containing mannitol. Wild-type strain SC5314 (13) (A), ura3/ura3 cst20Δ/cst20Δ::URA3 strain CDH22 (B), ura3/ura3 cst20Δ/cst20Δ::CST20::URA3 strain CDH36 (obtained by reintegration of CST20 into strain CDH25 by homologous recombination using linearized plasmid pDH190) (C), ura3/ura3 cst20Δ/cst20Δ strain CDH25 transformed with plasmids pYPB1-ADHpt (D) and pYPB1-ADHpt-HST7 (E), ura3/ura3 hst7Δ/hst7Δ strain CDH12 transformed with plasmids pVEC (F), pVEC-HST7 (G), pYPB1-ADHpt (H), and pYPB1-ADHpt-HST7 (I), and ura3/ura3 cph1/cph1 strain CDH72 [ura3/ura3 derivative of strain JK19 (6)] transformed with pYPB1-ADHpt-HST7 (J). (×2; bar = 2 mm.)
An identical phenotype was caused by deletion of HST7 (Fig. 5F). This phenotype could be reversed by introducing expression plasmids carrying HST7 either under control of its own promoter (Fig. 5G) or the C. albicans ADH1 promoter (Fig. 5I). Northern blot analysis indicated that expression of HST7 from the ADH1 promoter was approximately 180-fold higher than in HST7 wild-type cells. This overexpression of HST7 was able to partially rescue the deletion of CST20 (Fig. 5E) but failed to complement the deletion of CPH1 (Fig. 5J), which causes comparable defects in hyphal growth (6).
Mutant strains formed hyphae when colonies were grown on Spider media containing either glucose or N-acetyl glucosamine (data not shown). Normal hyphae formation was also observed on rice agar and on agar containing Lee’s medium (8) or 10% serum (data not shown). The frequency of germ-tube formation in either liquid Lee’s medium, 10% serum, or liquid Spider media containing any of the sugars tested above, were also normal (data not shown). These results indicate that Cst20p and Hst7p are not required for hypha formation under all conditions but are involved in the lateral formation of mycelia on some solid surfaces.
Virulence Studies.
To determine the role of Cst20p and Hst7p for virulence, mice were injected intravenously with wild-type and mutant strains and monitored for survival and for fungal invasion into kidneys. We found, in agreement with a previous study (25), that the Ura− strain CAI4 was not pathogenic (Fig. 6 A and B). However, infection with Ura+ wild-type cells resulted in rapid mortality with a rate that was dependent on the dose of injected cells (1 × 106 cells in Fig. 6A, and 1 × 105 cells in Fig. 6B). No difference in morbidity was found between mice infected with Ura+ cells that were either wild type for HST7 or deleted for both alleles of HST7 (hst7Δ/hst7Δ::URA3; Fig. 6 A and B). Also no difference was observed in Ura+ cells deleted for only one allele of HST7 (data not shown). Survival was significantly prolonged, however, in mice infected with Ura+ cells deleted for both alleles of CST20 (cst20Δ/cst20Δ::URA3). This effect, which was reproducible and statistically significant, was observed at high (Fig. 6A) or low (Fig. 6B) doses of infection (with P values of 0.027 and 0.001, respectively) and correlated with colony-forming units per kidney (1.5 × 106 for wild-type cells, 1.8 × 106 for hst7Δ/hst7Δ::URA3 mutant cells, and 7 × 105 for cst20Δ/cst20Δ::URA3 mutant cells) after 48 h of infection with 1 × 106 cells. These effects on virulence could be reversed by reintroducing CST20 into the strain deleted for both CST20 alleles, and were not observed in Ura+ cells deleted for only one CST20 allele (data not shown). A histological examination revealed that cells deleted for both alleles of HST7 or CST20, respectively, were able to form hyphae in infected kidneys (Fig. 6C).
Figure 6.

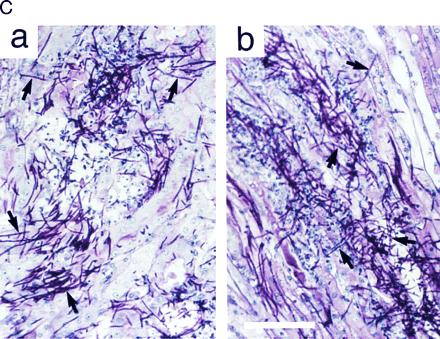
Virulence assays. Survival curves of mice (n = 10 for each C. albicans strain at each inoculation dose) infected with 1 × 106 (A) and 1 × 105 (B) cells of C. albicans strains SC5314 (wild type), CAI4 (ura3/ura3), CDH22 (ura3/ura3 cst20Δ/cst20Δ::URA3), and CDH10 (ura3/ura3 hst7Δ/hst7Δ::URA3). (C) Staining of mouse kidney sections with periodic acid Schiff’s stain 48 h after infection with cst20Δ/cst20Δ::URA3 mutant strain CDH22 (a) and hst7Δ/hst7Δ::URA3 mutant strain CDH10 (b). Some hyphal cells are indicated with arrows. (Bar = 0.1 mm.)
DISCUSSION
We have cloned and sequenced the C. albicans CST20 gene encoding a structural and functional homolog of the Ste20p protein kinase from S. cerevisiae. C. albicans cells deleted for the CST20 gene were defective in hyphal growth on solid media. The same phenotype was observed after deletion of the HST7 gene encoding a homolog of the Ste7p protein kinase. Cells deleted for CST20 were less virulent when injected into mice, as judged by prolonged survival of infected mice.
In S. cerevisiae, Ste20p fulfills multiple functions during mating (15), pseudohyphae formation (4), invasive growth (3), and cytokinesis (19). CST20 expression in S. cerevisiae fully complements these functions. Thus, Cst20p has the potential to fulfill similar functions in C. albicans.
The yeast-to-hyphal transition of C. albicans is a morphological change that can be triggered by a wide variety of factors (for a review, see ref. 26). Carbohydrates, amino acids, salts, and serum have been described as inducers of germ tube formation, as have pH changes, temperature increases, and starvation, but no single environmental factor could be defined as uniquely significant in stimulating the morphological switch (26). Hence C. albicans appears capable of responding to many divergent environmental signals. Disruption of both CPH1 alleles, which encode a homolog of the S. cerevisiae Ste12p transcription factor (6), suppressed the lateral formation of mycelia from colonies grown on solid Spider medium, but did not block hyphal development in other media. We have shown that C. albicans mutant cells deleted for CST20 or HST7 display a similar phenotype, and that the effect of these mutations on hyphal development is dependent on the carbon source in which the cells were grown.
We found that elevated expression of HST7 partially complemented the deletion of CST20 but not the deletion of CPH1. These results, and the identical phenotype caused by deletion of any of these genes, indicate an epistatic relationship between CST20, HST7, and CPH1. This is consistent with the hypothesis that the products of these genes participate in the same regulatory pathway. This pathway might be analogous to the mating response pathway of S. cerevisiae where Ste12p is the ultimate target of a MAP kinase cascade in which Ste20p and Ste7p are involved. In asexual C. albicans, this pathway might have become adapted to the control of morphological responses during the yeast-to-hyphal transition.
Taken together, these observations are consistent with the idea that several signaling pathways can trigger morphogenesis in C. albicans (for a review, see ref. 26). Furthermore, the behavior of C. albicans mutant strains deleted for either CPH1, CST20, or HST7 indicates that these pathways might operate independently to activate hyphal development under differing environmental conditions. C. albicans encounters a variety of different microenvironments during the development of superficial and sytemic infections. Hence, the existence of parallel morphogenetic signaling pathways might provide a distinct advantage to this pathogen.
Our results indicate that the pathway controlled by Cst20p and Hst7p is not essential for virulence in a mouse model of systemic infections. It is not inconceivable that this pathway plays a role in other forms of infections, for example in the development of superficial infections of the mucosal epithelia (thrush). An as yet undefined role of Cst20p in pathogenicity outside of the Cst20/Hst7 signaling pathway is suggested, however, by prolonged survival of mice infected with cst20-deleted cells. It is unlikely that this effect is caused by defects in hyphal formation since a histological examination of infected kidneys revealed that the cst20-deleted cells are not restricted in their capacity to form hyphae. Also this effect is not mediated through Hst7p, and hence is not dependent on the downstream MAP kinase pathway, since hst7 deleted cells were not less virulent. In this regard, it is important to note that Ste20p from S. cerevisiae plays a role in polarized morphogenesis during bud emergence which is not dependent on the functions of the pheromone response and pseudohyphal MAP kinase pathways (19), and the Ste20p homolog Shk1/Pak1 from Schizosaccharomyces pombe is essential for maintenance of polarity during vegetative growth independently of the mating response pathway (21, 22).
Our identification of one pathway that is involved in the regulation of hyphal growth under certain conditions provides an initial step for identifying components required for the induction of morphological switching of C. albicans and should therefore be helpful in establishing the relationship between morphological differentiation and the virulence of this pathogenic fungus.
Acknowledgments
We thank S. Badock, F.-U. Geschke, Robert Larocque, and M. Osterkamp for technical assistance; C. Boone for providing the genomic C. albicans library; and P. J. F. Feldmann, G. R. Fink, W. A. Fonzi, J. Köhler, B. Magee, C. Nombela, and S. Scherer for providing plasmids and C. albicans strains. We are grateful to M. Raymond and C. Csank for helpful discussions. I.D.B. was supported by an Aberdeen University Studenship, and A.J.R.B. was supported by a Research Fellowship from Aberdeen University. N.A.R.G. was awarded a Royal Society of Edinburgh/Caledonian Research Foundation Fellowship. This is National Research Council of Canada publication number 39926.
Footnotes
References
- 1.Fidel P L, Sobel J D. Trends Microbiol. 1994;2:202–205. doi: 10.1016/0966-842x(94)90112-i. [DOI] [PubMed] [Google Scholar]
- 2.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 3.Roberts R L, Fink G R. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Styles C, Fink G R. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 5.Clark K L, Feldmann P J F, Dignard D, Larocque R, Brown A J P, Lee M G, Thomas D Y, Whiteway M. Mol Gen Genet. 1995;249:609–621. doi: 10.1007/BF00418030. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Köhler J, Fink G R. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 7.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 8.Lee K L, Buckley H R, Campbell C C. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 9.Leberer E, Dignard D, Harcus D, Hougan L, Whiteway M, Thomas D Y. Mol Gen Genet. 1993;241:241–254. doi: 10.1007/BF00284675. [DOI] [PubMed] [Google Scholar]
- 10.Boone C, Sdicu A-M, Laroche M, Bussey H. J Bacteriol. 1991;173:6859–6864. doi: 10.1128/jb.173.21.6859-6864.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonzi W A, Irwin M Y. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram G, Swoboda R K, Gow N A R, Gooday G W, Brown A J P. Yeast. 1996;12:115–127. doi: 10.1002/(sici)1097-0061(199602)12:2<115::aid-yea889>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Leberer E, Dignard D, Harcus D, Thomas D Y, Whiteway M. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plempel M. J Antimicrob Chemother. 1984;13:447–463. doi: 10.1093/jac/13.5.447. [DOI] [PubMed] [Google Scholar]
- 17.Bancroft J D, Stevens A. Theory and Practice of Histological Techniques. Edinburgh: Churchill Livingstone; 1990. [Google Scholar]
- 18.Wu C, Whiteway M, Thomas D Y, Leberer E. J Biol Chem. 1995;270:15984–15992. doi: 10.1074/jbc.270.27.15984. [DOI] [PubMed] [Google Scholar]
- 19.Cvrckova F, De Virgilio C, Manser E, Pringle J R, Nasmyth K. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 20.Santos M A S, Tuite M F. Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus S, Polverino A, Chang E, Robbins D, Cobb M H, Wigler M H. Proc Natl Acad Sci USA. 1995;92:6180–6184. doi: 10.1073/pnas.92.13.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottilie S, Miller P J, Johnson D I, Creasy C L, Sells M A, Bagrodia S, Forsburg S L, Chernoff J. EMBO J. 1995;14:5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. Nature (London) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z-S, Leung T, Manser E, Lim L. Mol Cell Biol. 1995;15:5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole M F, Bowen W H, Zhao X, Cihlar R L. FEMS Microbiol Lett. 1995;126:177–180. doi: 10.1111/j.1574-6968.1995.tb07413.x. [DOI] [PubMed] [Google Scholar]
- 26.Odds F C. CRC Crit Rev Microbiol. 1985;12:45–93. doi: 10.3109/10408418509104425. [DOI] [PubMed] [Google Scholar]


