Abstract
Ionotropic glutamate and γ-aminobutyric acid type A (GABAA) receptors mediate critical excitatory and inhibitory actions in the brain. Cyclothiazide (CTZ) is well known for its effect of enhancing glutamatergic transmission and is widely used as a blocker for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor desensitization. Here, we report that in addition to its action on AMPA receptors, CTZ also exerts a powerful but opposite effect on GABAA receptors. We found that CTZ reversibly inhibited both evoked and spontaneous inhibitory postsynaptic currents, as well as GABA application-induced membrane currents, in a dose-dependent manner. Single-channel analyses revealed further that CTZ greatly reduced the open probability of GABAA receptor channels. These results demonstrate that CTZ interacts with both glutamate and GABAA receptors and shifts the excitation–inhibition balance in the brain by two independent mechanisms. Understanding the molecular mechanism of this double-faceted drug–receptor interaction may help in designing new therapies for neurological diseases.
Cyclothiazide (CTZ) was developed originally as a diuretic drug with clinical application in treating hypertension (1). In the early 1990s, CTZ was found to block the desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors and prolong glutamatergic synaptic currents (2, 3). Among a large family of thiazides, CTZ has been proven to be the most potent and therefore the most extensively studied and widely used positive AMPA receptor modulator (4–10). In addition to a postsynaptic allosteric modulation of AMPA receptors, CTZ has also been found to exert a presynaptic effect by increasing the frequency of spontaneous glutamate release (5, 7, 8).
Because AMPA receptors are important in mediating excitatory neurotransmission in the CNS, drugs positively modulating AMPA receptors have been explored for possible therapeutic applications (11). On the positive side, AMPA receptor modulators enhancing glutamate neurotransmission were found to facilitate long-term potentiation and improve learning and memory (11–14). On the negative side, administration of CTZ might cause seizures and cell death (15–17). So far, all of the effects induced by CTZ have been interpreted in the context of antagonistic action on AMPA receptor desensitization. Here we show that in addition to enhancing glutamatergic transmission, CTZ also strongly inhibits γ-aminobutyric acid (GABA) type A (GABAA) receptors and diminishes GABAergic transmission.
Methods
Cell Culture. Hippocampal microisland cultures were prepared from newborn Sprague–Dawley rats as described (18, 19). In brief, the hippocampal CA1–CA3 region was dissected and incubated for 30 min in 0.05% trypsin/EDTA solution (pH 7.2). After enzyme treatment, tissue blocks were triturated gently by using a fire-polished Pasteur pipette. Dissociated cells were plated onto microislands (0.4 mM poly-d-lysine/0.25 mM collagen) covered by a monolayer of astrocytes. The culture medium contained 500 ml of MEM (GIBCO), 5% FBS (HyClone), 10 ml of B-27 supplement (GIBCO), 100 mg of NaHCO3,20mM d-glucose, 0.5 mM l-glutamine, and 25 units/ml penicillin/streptomycin. Cells were maintained in a 5% CO2/95% air incubator for up to 3–4 weeks.
Electrophysiology. Whole-cell and outside-out patch recordings were performed by using Multiclamp 700A and Axopatch 200B patch-clamp amplifiers (Axon Instruments, Foster City, CA). Patch pipettes were pulled from borosilicate glass and fire-polished. The final resistance of pipettes was 2–4 MΩ for whole-cell and 6–10 MΩ for single-channel recordings. The recording chamber was perfused continuously with a bath solution that consisted of 128 mM NaCl, 30 mM d-glucose, 25 mM Hepes, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2 (pH 7.3, adjusted with NaOH). The pipette solution for whole-cell recordings contained 147 mM CsCl, 5 mM disodium phosphocreatine, 2 mM EGTA, 10 mM Hepes, 2 mM MgATP, and 0.3 mM Na2GTP (pH 7.3, adjusted with CsOH). For evoked inhibitory postsynaptic currents (IPSCs), 120 mM CsCl was replaced with equivocal CsMeSO3 to reduce the IPSC amplitude for better voltage control. For single-channel recordings, pipettes were coated with wax and filled with a solution that contained 120 mM CsCl, 20 mM tetraethylammonium-Cl, 2 mM MgCl2, 1 mM CaCl2, 11 mM EGTA, and 10 mM Hepes (pH 7.3, adjusted with CsOH). In whole-cell recordings, the series resistance was typically 10–20 MΩ and compensated by 50–70%. Data were acquired by using PCLAMP 8 software, sampled at 10 kHz and filtered at 1–2 kHz, and analyzed with CLAMPFIT 8 or CLAMPFIT 9 software (Axon Instruments). Miniature events were analyzed by using MINIANALYSIS software (Synaptosoft, Decatur, GA). Analyzed data were expressed as mean ± SE, and a paired Student t test was used for most comparisons between control and CTZ-treated groups, unless stated otherwise.
Drugs. CTZ was purchased from Sigma and Tocris Cookson (St. Louis) and dissolved in either DMSO or ethanol. The inhibitory effect of CTZ on GABAA receptors was similar irrespective of the source or solvent. The vehicle control for DMSO and ethanol had no significant effect on GABA-mediated responses at low concentrations (≤0.1%). When a high concentration of CTZ (500–1,000 μM) was used, DMSO was used to dissolve CTZ, and an equivalent concentration of DMSO was included in the control solution to diminish the solvent effect. Bicuculline (BIC), 6-nitro-7-cyanoquinoxaline-2,3-dione, tetrodotoxin, and GABA were obtained from Tocris Cookson. All of the drugs were freshly diluted in bath solution to final concentrations before experiments.
Results
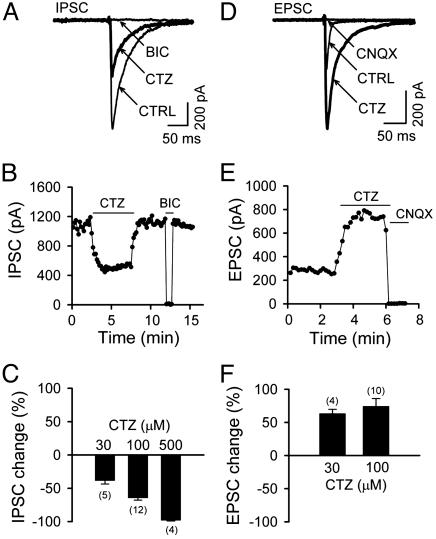
Opposite Effects of CTZ on Evoked IPSCs and Excitatory Postsynaptic Currents (EPSCs). Evoked synaptic currents were recorded from single autaptic neurons by a brief depolarization (-70 mV to 0 mV, 1.5 ms) in hippocampal microisland cultures (18, 19). CTZ is well known as an antagonist of AMPA receptor desensitization. During our studies of synaptic transmission, CTZ was applied when evoked IPSCs, instead of EPSCs, were recorded from autaptic neurons. Surprisingly, 100 μM CTZ, a concentration commonly used for the study of EPSCs (5, 8), potently reduced the amplitude of IPSCs (Fig. 1A). The IPSCs were blocked by BIC (40 μM), demonstrating their mediation by GABAA receptors. The CTZ effect on IPSCs was reversible (Fig. 1B) and dose-dependent (Fig. 1C). As summarized in Fig. 1C, IPSCs were almost abolished by a high concentration of CTZ (500 μM, 98.4 ± 0.8% reduction, n = 4) and significantly inhibited at low to medium concentrations (30 μM, 38.7 ± 4.9% reduction, n = 5; 100 μM, 64.6 ± 3.3% reduction, n = 12). As reported (5, 9), the same concentration of CTZ enhanced evoked autaptic EPSCs recorded from single glutamatergic neurons (Fig. 1 D and E). Potentiation of EPSCs by CTZ was seen at similar concentrations (30 μM, 163.7 ± 6.1% of control, n = 4; 100 μM, 174.5 ± 11.6% of control, n = 10) as the inhibition of IPSCs. These results demonstrate a dual effect of CTZ in modulating two different synaptic transmission systems: to enhance glutamatergic transmission and to inhibit GABAergic transmission, both of which will lead to an increase of neuronal activity.
Fig. 1.
CTZ potently inhibits IPSCs but enhances EPSCs. (A) A typical example showing evoked autaptic IPSCs (average five to eight traces) in control (CTRL), 100 μM CTZ, and specific GABAA receptor antagonist BIC (40 μM). Holding potential was -70 mV. (B) Time-effect plot illustrating the amplitude of IPSCs reversibly blocked by CTZ and BIC. (C) Pooled data showing that CTZ inhibited IPSCs in a dose-dependent manner. (D and E) CTZ (100 μM) enhanced the amplitude and prolonged the decay of evoked autaptic EPSCs in a representative neuron. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione. (F) Pooled data showing the percentage of EPSC amplitude increase by CTZ.
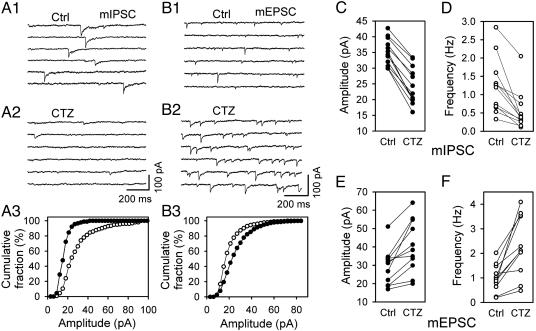
Effect of CTZ on Spontaneous Release Events. We next examined whether the CTZ-mediated inhibition of GABAergic transmission was due to changes in pre- or postsynaptic activity. To discriminate between pre- and postsynaptic action, we tested the effect of CTZ on miniature IPSCs (mIPSCs) in the presence of tetrodotoxin (1 μM) and 6-nitro-7-cyanoquinoxaline-2,3-dione (10 μM). In general, a significant change in the frequency of spontaneous releasing events would likely indicate a presynaptic effect, whereas a significant change in amplitude would indicate a postsynaptic effect. Fig. 2A1 shows representative traces of mIPSCs in control that were blocked by BIC and were, thus, GABAergic (BIC data not shown). In the presence of CTZ (100 μM), both the amplitude and frequency of mIPSCs were greatly reduced (Fig. 2 A2). The cumulative fraction plot in Fig. 2 A3 shows a left shift of the amplitude of mIPSCs toward smaller values in the presence of CTZ. Data obtained from 12 neurons are summarized in Fig. 2 C and D to show a significant decrease in both the amplitude (control, 35.2 ± 1.2; CTZ, 24.6 ± 1.7; P < 0.001) and frequency (control, 1.22 ± 0.21; CTZ, 0.54 ± 0.15; P < 0.001) of mIPSCs in the presence of CTZ. The marked reduction in the amplitude of BIC-sensitive mIPSCs suggests that CTZ may exert a postsynaptic effect on GABAA receptors. The CTZ inhibition of the mIPSC amplitude was probably an underestimate because many originally small mIPSCs might have been too small to be detected after the CTZ inhibition, which made the mIPSC amplitude during CTZ application larger than the true value. The frequency reduction of mIPSCs in the presence of CTZ may point to an additional presynaptic effect but also may be caused indirectly by the substantial reduction of the amplitude.
Fig. 2.
CTZ decreases spontaneous mIPSCs but increases mEPSCs. (A1) Consecutive traces showing control (Ctrl) mIPSCs. (A2) CTZ (100 μM) greatly inhibited mIPSCs. (A3) Cumulative fraction plot illustrating the decrease of mIPSC amplitude in the presence of CTZ (P < 0.001, Kolmogorov–Smirnov test; ○, Ctrl; •, CTZ). (B1) Typical example showing Ctrl mEPSCs. (B2) CTZ (100 μM) significantly enhanced mEPSCs. (B3) Cumulative fraction plot demonstrating the increase of mEPSC amplitude in the presence of CTZ (P < 0.001; ○, Ctrl; •, CTZ). (C) Pooled data showing consistent reduction of mIPSC amplitude by CTZ. (D) Pooled data showing marked reduction of mIPSC frequency by CTZ. (E) Pooled data illustrating significant enhancement of mEPSC amplitude by CTZ. (F) Pooled data illustrating remarkable increase of mEPSC frequency by CTZ. P < 0.001, paired t test for C–F.
In contrast to the effect on mIPSCs, CTZ significantly enhanced both the frequency and amplitude of mEPSCs, as reported (5, 9). Typical traces of control mEPSCs recorded in the presence of tetrodotoxin (1 μM) and BIC (40 μM) are shown in Fig. 2B1. Application of CTZ (100 μM) increased the frequency and right-shifted the amplitude of mEPSCs toward larger values (Fig. 2 B2 and B3). In Fig. 2 E and F, data pooled from 11 neurons showed a consistent increase in the amplitude (control, 29.1 ± 3.0; CTZ, 40.4 ± 4.3; P < 0.001) and frequency of mEPSCs in the presence of CTZ (control, 1.00 ± 0.17; CTZ, 2.34 ± 0.37; P < 0.001).
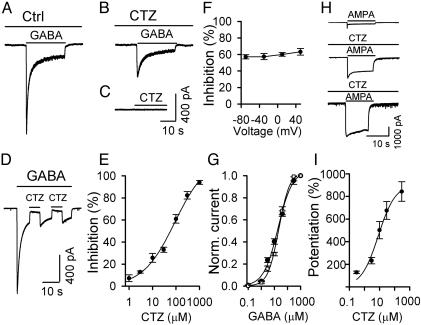
CTZ Inhibition of GABA-Application-Induced Membrane Currents. The marked reduction of mIPSC amplitude in the presence of CTZ prompted us to examine further its postsynaptic effect on GABAA receptors. Direct bath application of GABA (40 μM) under a whole-cell voltage clamp condition (holding potential -70 mV) induced a rapidly decaying inward current (Fig. 3A). In the presence of CTZ (100 μM for 60 s), the same concentration of GABA induced a much smaller current (Fig. 3B), suggesting that CTZ may act as an antagonist for GABAA receptors. Bath application of CTZ (100 μM) in the absence of GABA did not evoke any membrane currents (Fig. 3C). To test whether CTZ acts rapidly in blocking GABAA receptors, drug application was rapidly switched between GABA and GABA plus CTZ. As shown in Fig. 3D, the blocking effect of CTZ on GABAA receptors occurred instantaneously, implying a direct interaction rather than mediation through a cell-signaling pathway. The IC50 calculated from the dose–response curve of CTZ inhibition on the peak amplitude of GABA-evoked currents was 57.6 μM (Fig. 3E). This value appears to be similar to the IC50 for CTZ inhibition of evoked IPSCs (compare Figs. 3E and 1C), confirming a major postsynaptic effect of CTZ on GABAergic transmission. To examine whether the CTZ inhibition was voltage dependent, membrane potentials were changed from -70 mV to 50 mV when recording the GABA-evoked currents in the presence of CTZ (100 μM). No significant difference in the percentage inhibition was found across the 120-mV range (Fig. 3F; P > 0.36, one-way ANOVA). We further tested whether CTZ interferes with GABA binding by analyzing the effect of CTZ on the GABA dose–response curve. The EC50GABA value was 18.8 μM, similar to previous reports (20, 21). In the presence of CTZ (100 μM), the GABA dose–response curve was not apparently shifted (EC50GABA+CTZ = 22.2 μM), suggesting that CTZ may have only a slight effect, if any, on GABA binding (Fig. 3G).
Fig. 3.
CTZ blocks GABA-induced postsynaptic receptor responses. (A) Control (Ctrl) trace showing an inward current induced by bath application of GABA (40 μM). (B) CTZ (100 μM) inhibited the GABA-induced membrane current significantly. (C) Application of CTZ (100 μM) alone did not induce any membrane currents. (D) CTZ blocked the GABA-induced current instantaneously during rapid switch between GABA and GABA plus CTZ application. (E) Dose–response curve of CTZ inhibition on the peak amplitude of GABA-evoked inward currents. IC50 = 57.6 μM. (F) CTZ (100 μM) inhibition of GABA-evoked currents at different holding potentials. No significant difference was detected (P > 0.36, one-way ANOVA). (G) GABA dose–response curve in the absence (•) and presence (□) of CTZ (100 μM). EC50GABA = 18.8 μM. EC50GABA+CTZ = 22.2 μM. (H) Representative traces showing inward currents induced by bath application of AMPA (10 μM, Top), AMPA plus 10 μM CTZ (Middle), and AMPA plus 300 μM CTZ (Bottom). (I) Dose–response curve of CTZ potentiation of AMPA-evoked peak currents. EC50 = 10.4 μM. The number of experiments in E–G and I ranged from four to nine, with the majority being six or seven.
In contrast to the inhibitory effect of CTZ on GABA-evoked currents, bath application of CTZ greatly potentiated AMPA-evoked currents (10 μM; Fig. 3H). The potentiation of CTZ on AMPA receptor currents was also dose dependent, with an EC50 value of 10.4 μM (Fig. 3I), similar to previous studies (2, 22).
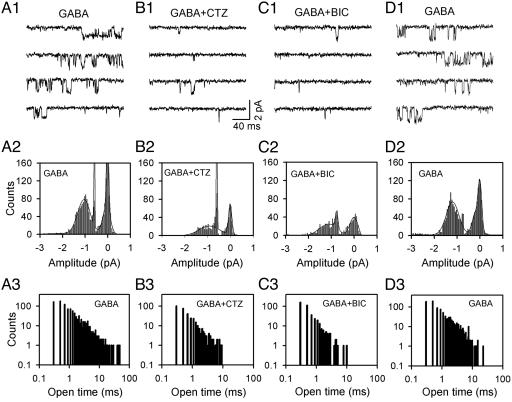
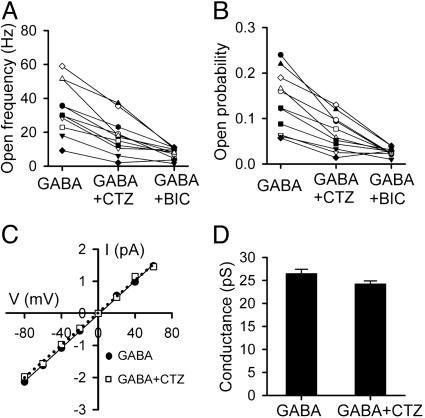
Single-Channel Analysis of CTZ Action on GABAA Receptors. The inhibitory effect of CTZ on bath application of GABA-induced membrane currents suggests a possible direct interaction between CTZ and GABAA receptors. To test this possibility, we performed single-channel recordings to examine whether CTZ can inhibit GABA-activated single-channel currents. Outside-out patches were excised from neuronal soma to monitor single-channel events in response to bath perfusion of GABA (2 μM), GABA plus CTZ (100 μM), GABA plus BIC (40 μM), and GABA after washing off the drugs (Fig. 4). Bath application of GABA activated many single-channel openings, with some of the duration lasting longer than 10 ms (Fig. 4 A1 and A3). As expected, the GABAA receptor channels displayed multiple conductance states (Fig. 4 A1 and A2). The main conductance of the single-channel currents was ≈27 pS (Fig. 5 C and D), consistent with previous reports (23, 24). When CTZ was applied with GABA, the number of single-channel events was greatly reduced (Fig. 4 B1 and B2). The channel open duration in the presence of CTZ was rarely longer than 10 ms (Fig. 4B3). To confirm that the single-channel events were indeed GABA activated, we applied BIC together with GABA on the same patch and found that the single-channel events were also greatly inhibited (Fig. 4C). At the single-channel level, the CTZ inhibition was also reversible, demonstrated by the recovery of GABA-activated single-channel events after washing off drugs (Fig. 4D). In a total of 11 outside-out patches, we found a consistent inhibition by CTZ and BIC on the single-channel open frequency and open probability (NPo; Fig. 5 A and B). Although CTZ greatly inhibited the number of channel events, the main conductance of GABAA receptor channels was only slightly reduced by CTZ (≈8% decrease; Fig. 5 C and D). These results at the single-channel level suggest that CTZ may bind directly to GABAA receptors and block channel openings without a great effect on the channel conductance.
Fig. 4.
CTZ inhibits GABAA receptor single-channel activity. (A1) Representative traces showing single-channel currents activated by bath perfusion of GABA (2 μM) from an outside-out patch. Channel openings are downward. (A2) The amplitude-distribution histogram of GABA-activated single-channel currents. (A3) The open time-distribution histogram of GABA-activated single-channel currents. (B1–B3) Representative traces (B1), amplitude histogram (B2), and open time-distribution histogram (B3) of single-channel currents in the presence of GABA plus CTZ (100 μM). Note a great reduction in the number of open events and a disappearance of long openings (>10 ms) in the presence of CTZ. (C1–C3) Representative traces (C1), amplitude histogram (C2), and open time-distribution histogram (C3) of single-channel currents in the presence of GABA plus BIC (40 μM). (D1–D3) Recovery of GABA-evoked single-channel events after washing off the drugs. All results in A–D were obtained from the same patch with the holding potential at -70 mV.
Fig. 5.
CTZ reduced the GABAA receptor channel NPo without marked effect on the channel conductance. (A) The channel open frequency was consistently reduced by CTZ (100 μM) and BIC (40 μM). (B) The channel NPo was also similarly reduced by CTZ and BIC (P < 0.001, for both CTZ and BIC in A and B). (C) Current–voltage (I–V) curve showing similar main GABAA receptor channel conductance (≈27 pS) with or without CTZ in the bath solution. (D) Bar graph illustrating only a slight reduction of the channel conductance by CTZ.
Discussion
The inhibitory effect of CTZ on GABAA receptors, in addition to its well known effect of enhancing glutamate responses, places CTZ at a unique position in modulating neuronal activities in the brain. Because glutamate and GABA are the most abundant excitatory and inhibitory neurotransmitters in the CNS, respectively, introduction of CTZ into a neural network may greatly shift the excitation–inhibition balance and dramatically accelerate the overall neuronal activity within a neural circuit. This possibility may raise considerable interest in making use of this special effect of CTZ in certain circumstances. For example, when an increase of neuronal activity is necessary, CTZ may be superior to other drugs that act only on either glutamate or GABA receptors alone. On the other hand, overdose of CTZ may cause potential excitotoxicity because of its one-way drive toward excitation.
The potent but opposite effects of CTZ on glutamate and GABA receptors may also have important implications in studying molecular mechanisms of drug–receptor interactions. The molecular structures of AMPA receptors and GABAA receptors are distinct, yet apparently they both possess a binding site for CTZ. More interestingly, the binding of CTZ leads to increased NPo of AMPA receptor channels but decreased NPo of GABAA receptor channels. This effect is reminiscent of the opposing effects of barbiturates. However, barbiturates enhance GABAA receptors and inhibit glutamate receptors (25, 26). CTZ does not resemble barbiturates in terms of chemical structure, but the possibility that they might act at the same site yet have opposite effects on both GABA and glutamate receptors cannot be ruled out. Unraveling the molecular mechanisms of the opposite interactions between CTZ and glutamate and GABAA receptors may yield critical insight for the development of new drugs to combat receptor dysfunction-related diseases.
In light of the newly discovered inhibitory effect of CTZ on GABAA receptors, some previous CTZ studies may have overstated the importance of glutamate receptor desensitization in determining the output of neural networks. Positive modulators of glutamate receptors have been suggested to facilitate the formation of long-term potentiation and possibly enhance memory storage (11, 14). Our results suggest that the inhibition of GABAergic neurotransmission by CTZ may have contributed to the overall increase of neuronal activity and might also play a role in long-term potentiation and memory enhancement. In fact, previous studies have shown a direct involvement of the GABAergic transmission system in modulating long-term synaptic plasticity, including learning and memory (27–30). Better understanding of the coordination between glutamatergic and GABAergic transmission systems, as well as the development of new drugs that interact with both glutamate and GABA receptors, may be a rationale in the design of new memory enhancement therapies.
Acknowledgments
We thank Drs. Bernhard Lüscher, Richard Ordway, and Cheryl Keller for critical reading of the manuscript. We especially thank Dr. Andy Ewing for lending us the Axopatch 200B amplifier for single-channel recordings. This work was partially supported by the Pennsylvania State University Life Sciences Consortium Innovative Biotechnology Research Fund.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BIC, bicuculline; CTZ, cyclothiazide; GABA, γ-aminobutyric acid; GABAA, GABA type A; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current; mEPSC/IPSC, miniature EPSC/IPSC; NPo, open probability.
References
- 1.Antlitz, A. M. & Valle, N. G. (1967) Curr. Ther. Res. Clin. Exp. 9 288-292. [PubMed] [Google Scholar]
- 2.Yamada, K. A. & Tang, C. M. (1993) J. Neurosci. 13 3904-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patneau, D. K., Vyklicky, L., Jr., & Mayer, M. L. (1993) J. Neurosci. 13 3496-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trussell, L. O., Zhang, S. & Raman, I. M. (1993) Neuron 10 1185-1196. [DOI] [PubMed] [Google Scholar]
- 5.Diamond, J. S. & Jahr, C. E. (1995) Neuron 15 1097-1107. [DOI] [PubMed] [Google Scholar]
- 6.Clements, J. D., Feltz, A., Sahara, Y. & Westbrook, G. L. (1998) J. Neurosci. 18 119-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellingham, M. C. & Walmsley, B. (1999) Neuron 23 159-170. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa, T. & Takahashi, T. (2001) J. Physiol. (London) 533 423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mennerick, S. & Zorumski, C. F. (1995) J. Neurosci. 15 3178-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes-Davies, M. & Forsythe, I. D. (1995) J. Physiol. (London) 488 387-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada, K. A. (1998) Neurobiol. Dis. 5 67-80. [DOI] [PubMed] [Google Scholar]
- 12.Stäubli, U., Perez, Y., Xu, F. B., Rogers, G., Ingvar, M., Stone-Elander, S. & Lynch, G. (1994) Proc. Natl. Acad. Sci. USA 91 11158-11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staubli, U., Rogers, G. & Lynch, G. (1994) Proc. Natl. Acad. Sci. USA 91 777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch, G. (2002) Nat. Neurosci. 5 Suppl., 1035-1038. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda, S., Ishida, N., Higashiyama, A., Morinobu, S. & Kato, N. (2000) Exp. Neurol. 164 396-406. [DOI] [PubMed] [Google Scholar]
- 16.May, P. C. & Robison, P. M. (1993) J. Neurochem. 60 1171-1174. [DOI] [PubMed] [Google Scholar]
- 17.Moudy, A. M., Yamada, K. A. & Rothman, S. M. (1994) Neuropharmacology 33 953-962. [DOI] [PubMed] [Google Scholar]
- 18.Bekkers, J. M. & Stevens, C. F. (1991) Proc. Natl. Acad. Sci. USA 88 7834-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, G. & van den Pol, A. N. (1996) J. Neurosci. 16 7711-7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, M. W., Reddy, P. A., Covey, D. F. & Rothman, S. M. (1998) J. Neurosci. 18 5103-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birnir, B., Eghbali, M., Cox, G. B. & Gage, P. W. (2001) J. Membr. Biol. 181 171-183. [DOI] [PubMed] [Google Scholar]
- 22.Yamada, K. A., Hill, M. W., Hu, Y. & Covey, D. F. (1998) Neurobiol. Dis. 5 196-205. [DOI] [PubMed] [Google Scholar]
- 23.Lorez, M., Benke, D., Lüscher, B., Mohler, H. & Benson, J. A. (2000) J. Physiol. (London) 527 11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, G., Trombley, P. Q. & van den Pol, A. N. (1996) J. Physiol. (London) 494 451-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamiya, Y., Andoh, T., Furuya, R., Hattori, S., Watanabe, I., Sasaki, T., Ito, H. & Okumura, F. (1999) Anesthesiology 90 1704-1713. [DOI] [PubMed] [Google Scholar]
- 26.Joo, D. T., Xiong, Z., MacDonald, J. F., Jia, Z., Roder, J., Sonner, J. & Orser, B. A. (1999) Anesthesiology 91 1329-1341. [DOI] [PubMed] [Google Scholar]
- 27.Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., Smith, A., Otu, F. M., Howell, O., Atack, J. R., et al. (2002) J. Neurosci. 22 5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trepel, C. & Racine, R. J. (2000) Synapse 35 120-128. [DOI] [PubMed] [Google Scholar]
- 29.Wang, J., Liu, S., Haditsch, U., Tu, W., Cochrane, K., Ahmadian, G., Tran, L., Paw, J., Wang, Y., Mansuy, I., et al. (2003) J. Neurosci. 23 826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crestani, F., Keist, R., Fritschy, J. M., Benke, D., Vogt, K., Prut, L., Bluthmann, H., Mohler, H. & Rudolph, U. (2002) Proc. Natl. Acad. Sci. USA 99 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]