Abstract
Higher cognitive functions such as attention have been difficult to model in genetically tractable organisms. In humans, attention-distracting stimuli interfere with trace but not delay conditioning, two forms of associative learning. Attention has also been correlated with activation of anterior cingulate cortex (ACC), but its functional significance is unclear. Here we show that a visual distractor interferes selectively with trace but not delay auditory fear conditioning in mice. Trace conditioning is associated with increased neuronal activity in ACC, as assayed by relative levels of c-fos expression, and is selectively impaired by lesions of this structure. The effects of the ACC lesions are unlikely to be caused by indirect impairment of the hippocampus, which is required for mnemonic aspects of trace conditioning. These data suggest that trace conditioning may be useful for studying neural substrates of attention in mice, and implicate the ACC as one such substrate.
Selective attention is thought to contribute to conscious awareness, but its neural basis is poorly understood. The search for the neural substrates of attention has been concentrated in the primate visual system (1, 2). Although extremely useful for identifying neural correlates of attention, primates offer limited accessibility for functional perturbation experiments, prompting a search for alternative animal models more amenable to tests of causation. Although some attentional models have been established in rats (3), they depend on operant conditioning paradigms that have proven difficult to extend to mice, and require lengthy training periods.
Studies in humans have suggested that attention is required for certain forms of associative learning (4). Associative learning paradigms, such as fear conditioning, have been successfully extended from rats to mice (5). Two commonly used variants of this procedure are delay and trace conditioning. In delay fear conditioning, a conditioned stimulus (CS), such as a tone, is immediately followed by an unconditioned stimulus (US), such as a foot shock. In trace conditioning, a time gap is introduced between the end of the CS and the start of the US. In human eye blink conditioning, another associative learning paradigm, distracting stimuli interfere with trace but not delay conditioning, suggesting that attention is necessary for the former type of learning (4, 6–10). More recent studies have suggested a similar requirement for attention in trace but not delay fear conditioning in humans (11).
Potential neural substrates of attention have been identified by functional imaging in humans. For example, attention has been correlated with increased activity in the anterior cingulate cortex (ACC) (12–17). Furthermore, the ACC is preferentially activated during presentation of the conditional stimulus, compared with that of a meaningless stimulus, during aversive trace conditioning (18). The ACC has also been implicated in tasks requiring visual attention in rats (3, 19–23). Lesion studies have shown that the medial prefrontal cortex, including the ACC, is critical for trace but not for delay eye blink conditioning in rabbits (24, 25). However, a direct link between trace conditioning and attention has not been established in this species.
We investigated whether trace and delay fear conditioning can be used to study neural substrates of attention in mice, where genetic manipulations are possible. We show that a visual distractor selectively interferes with trace but not delay nor contextual conditioning, suggesting an attentional requirement for this type of learning in mice, as in humans. Furthermore, as in humans, the acquisition of trace conditioning is associated with increased activation of ACC, as determined by using the induction of c-fos mRNA as a surrogate marker of neuronal activity (26–28). To extend these correlational studies to a test of causation, we specifically lesioned the ACC by using excitotoxins. Such lesions produced selective deficits in trace but not delay or contextual conditioning. These studies establish a system for studying the neural basis of attention in a genetically tractable organism, and further implicate the ACC in this process.
Materials and Methods
Subjects. C57BL/6N male mice from Harlan Sprague–Dawley (San Diego), aged 6–10 weeks and weighing 24–32 g, were used. All subjects were maintained on a 12 h/12 h light/dark cycle and allowed free access to food and water. Mice were allowed at least 1 week of rest with their littermates after their arrival, before they were singly housed for 3 days before the experiments. The sample size for each group is shown in the figures. The experimental protocol was approved by the California Institute of Technology Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines.
Apparatus. The conditioning chamber was 18 × 18 × 30 cm in dimension with 16 metal grids spaced 1.1 cm center-to-center on the floor connected to a shock scrambler (Coulbourn Instruments, Allentown, PA). The speaker was mounted on the back wall. The onsets and durations of the sound and shock were controlled by a PC. Before each use, the box was washed thoroughly with 95% alcohol, and the floor and drop pan were washed with detergent and disinfectant. Testing of fear conditioning was performed in a room different from the training and housing rooms. Ordinary clean home cages without bedding, food, and water were used as testing boxes. The paper filter on the cage lid was removed for sound transmission. The speaker was mounted on the metal rack 10 cm above the cage lid. A video camera was positioned in front of the cages to record the behavior.
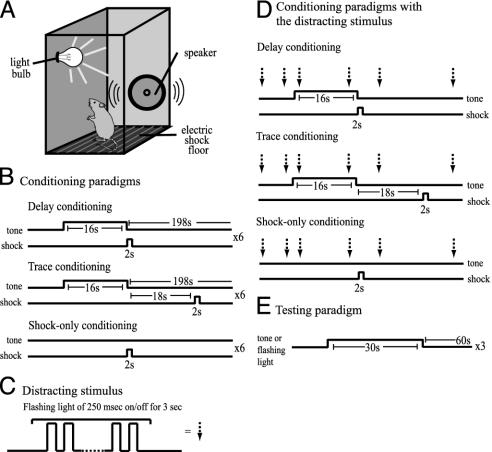
Conditioning Procedure for the Distraction Study. Both training and testing were carried out under dim red light illumination conditions. On day 1, mice were brought to the training room and placed individually in the conditioning boxes for 20 min, and then returned to their home cages. On day 2, mice received a 20-min baseline period in the conditioning boxes, and then six trials of delay, trace, or shock-only fear conditioning. In delay conditioning, a foot shock (2 sec at 0.5 mA) was delivered immediately after a tone (85 dB, 2 kHz, 16 sec). The time between the end of the tone and next tone was 198 sec. In trace conditioning, the shock was delivered 18 sec after the cessation of the tone. In shock-only conditioning, no tone was presented, but other parameters were the same as in delay conditioning (Fig. 1B). For animals in the distraction conditions, the presentation of a distractor (Fig. 1C) commenced 1 min before the first tone–shock pairing. The distractor consisted of a flashing white light (250 msec on/off for 3 sec, 8 lux), emitting from two dome-shaped lamps (Lamp Type 1864). The interstimulus interval sequence was randomly chosen from 5, 10, 15, or 20 sec by computer in the beginning of the experiment, and the same sequence was used for all animals (Fig. 1D). The distractor sequence was terminated 1 min after the final shock presentation. Three minutes after the last footshock, mice were taken out of the training chambers and put back into their home cages. On day 3, mice were brought to the testing room and placed in the testing boxes. Mice received tone and light tests. The time between the tone and light tests was 5 min, and the order of tests was counterbalanced for each animal. In the tone test, three trials of tone testing were presented after 3 min of baseline. Each trial consisted of a tone (85 dB, 2 kHz, 30 sec) followed by an interval (60 sec) (Fig. 1E). In the light test, the flashing light (250 msec on/off for 30 sec) was used instead of the tone (Fig. 1E). Because six tone–shock training trials were given in each session, it is possible that mice formed the light–shock association at the expense of the tone–shock association in the first few training trials in trace conditioning, but that this light–shock association was extinguished in later training trials. To investigate whether a light–shock association is formed at any time point, a different group of mice received one to six trials of tone–shock pairings. The behavior of mice was videotaped throughout the session and later analyzed. For contextual fear conditioning, mice were brought to the training boxes and one 2-sec-long footshock was delivered after a 3-min baseline on day 1. On day 2, mice were brought back to the training boxes and their behavior was recorded for 5 min.
Fig. 1.
The conditioning and testing procedures. See Materials and Methods for parameters. (A, B, and E) The training box, the conditioning paradigms, and the testing paradigm. (C) A light presentation served as the distractor. (D) For animals in the distraction conditions, the distractor is presented with a random interstimulus interval of 5, 10, 15, or 20 sec.
Conditioning Procedure for the Lesion Study. The training and testing procedure was similar to the procedure described above, except that no distractor was used and the experiments were conducted under normal lighting.
Surgery. Mice were anesthetized with isoflurane gas and mounted in a stereotaxic frame. A scalp incision was made after disinfecting the skin, and holes corresponding to the lesion sites were drilled on the skull. A guide cannula (28 gauge, Small Parts, Inc., Miami Lakes, FL) secured on the stereotaxic arm was inserted to the target brain area. The coordinates for the ACC were (anteriorposterior, mediolateral, and dorsoventral, respectively, in mm relative to bregma): 1.3, ±0.3, -2.2 and 0.3, ±0.3, -2.0, and those for the primary visual cortex (V1) were: -2.5, ±3.0, -1.0, and -3.5, ±3.0, -1.5. N-methyl-D-aspartate was infused through the guide cannula (0.2 μl of 136 mM) manually, and the cannula stayed in for 10 min. Sham surgery was identical to the ACC surgery except that no cannula was inserted. The scalp incisions were closed with stainless steel wound clips. Mice rested for at least 2 weeks before behavioral training.
Histology. After the behavioral experiments, mice were killed by cervical dislocation and then decapitated. Brains were removed from the skull and placed in a 10% paraformaldehyde solution for 3 days before sectioning. On the day of sectioning, brains were frozen in 0.7% alcohol solution. Coronal sections (50 μm) were cut by cryostat and stained with thionin (0.25%). Microscopic photographs of the brain sections were imported to computer graphic processing programs to superimpose them onto the digital atlas of corresponding levels (29). Individual brain images were digitally morphed to best fit the contours of the clearly visible anatomical landmarks such as the central fissure and the genu of the corpus callosum. The outlines of the lesions were drawn on the atlas plates by using the graphic programs. Mice were excluded from the analysis when the histology showed unilateral lesions, lesions extending ventrally into the hippocampus or septum, shallow dorsal lesions not including Cg2, or lesions restricted to the either frontal or caudal parts of the ACC. None of the animals that received V1 lesions were excluded.
Fear Conditioning for in Situ Hybridization Study. Forty-four mice were divided into four identical groups of 11. Within each group, the 11 mice were further divided into a delay training subgroup of four, a trace training subgroup of four, and a shock-only training subgroup of three. All mice in a given subgroup were trained and tested in parallel. On day 1, mice were housed overnight individually in one of the four training boxes to reduce the basal level of c-fos mRNA expression. On day 2, one of the three types of training was administered without any handling or other disturbance. The paradigms and parameters of the three types of conditioning were the same as in the distraction study described above. Thirty minutes after the last stimulus, one of the four mice in the delay or trace conditioning paradigm was killed by cervical dislocation, and the brains processed for c-fos in situ hybridization. A total of eight brains (four trace and four delay) were thus obtained. The other three mice were removed from the conditioning box and returned to their home cages. All of the mice in the shock-only conditioning paradigm were returned to their home cages. On day 3, mice were transported to the testing room and placed in the testing boxes to receive tone testing. After 3 min of baseline, three trials of tone testing were presented. Each trial consisted of a tone (85 dB, 2 kHz, 30 sec) followed by a 60-sec interval. We did not include a shock-only group in the c-fos study, because our hypothesis was simply that neuronal activity in the ACC is higher during trace than during delay conditioning. However, shock-only conditioning was included in the behavior testing as a control for the efficacy of trace and delay conditioning.
Behavioral Data Analysis. Freezing was defined as total lack of movement, except for breathing, while significant muscle tone is exhibited. It was scored every 2 sec for tone testing and every 8 sec for contextual testing by a human observer in a blind fashion. Percent time spent freezing was derived from dividing the sum of scores by the total number of observations, and multiplied by 100. Locomotor activities were assessed by the number of times the mouse crossed the midline of the testing box and the number of times the mouse showed rearing behavior during the 3-min baseline in the tone freezing test before the first tone was presented. ANOVA was used to detect differences between groups. When a significant difference was detected, the F value was reported and the post hoc Student–Newman–Keuls (SNK) test was conducted to detect all pair-wise differences.
c-fos mRNA in Situ Hybridization and Bias-Free Stereology. Immediately after death, the mouse brain was cut into 4-mm-thick slabs by using Rodent Brain Matrix (RBM-2000, ASI Instruments, Warren, MI) and fixed for 24 h in diethyl pyrocarbonate-treated paraformaldehyde at 4°C. The slabs were then cryoprotected in 30% sucrose and stored overnight at -20°C. The slabs were sectioned at 120 μm and processed for nonisotopic in situ hybridization with a c-fos antisense cRNA probe as described (30). Bias-free stereology using an Optical Dissector and the Stereoinvestigator program (MicroBrightField, Williston, VT) was used to measure the density of c-fos-positive cells. The counting brick was Δx = 50 μm, Δy = 50 μm, and Δz = 60 μm in size. Sampling error was ≤5% by using the Schaffer test. The contours of the ACC (divided into Cg1 and Cg2) and the primary motor cortex (M1) were fitted electronically on the section with reference to a mouse brain atlas (29) and calibrated in relation to relevant anatomical landmarks such as the corpus callosum. Stained cells were identified and scored by an experienced but blinded experimenter. Sixteen coronal sections spanning from 1.10 nm anterior to 0.82 nm posterior to bregma were hybridized. Alternating sections, i.e., eight sections, were analyzed. For more details, see ref. 30.
Results
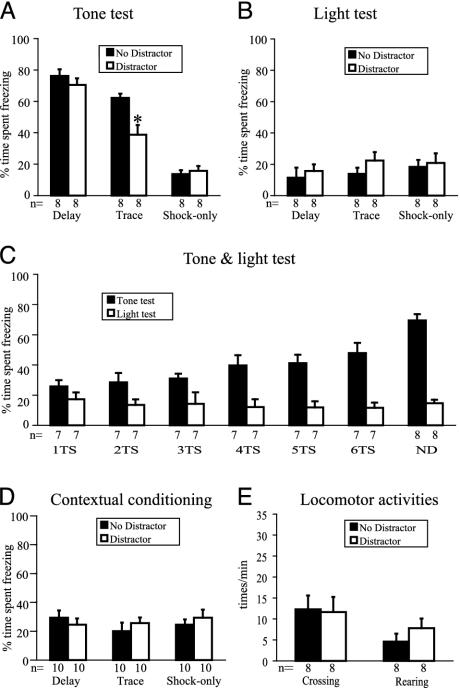
Distraction Disrupts Trace but Not Delay Conditioning. The flashing light disrupted freezing in the trace conditioning group, as indicated by a significant reduction in the percent time spent freezing to the tone, compared with the control mice that did not receive the distractor (P < 0.05) (Fig. 2A). No significant effect of the flashing light in either the delay or shock-only conditions was observed. In control animals, both delay and trace conditioning were observed, as shown by a significantly higher level of freezing elicited by the tone, compared with the shock-only conditioning (P < 0.05) (Fig. 2 A; no distractor).
Fig. 2.
Animals received either a distractor or no distractor during training. On the testing day, animals were presented with the tone and the light, or vice versa. (A) Percent time spent freezing during tone testing. The distractor during conditioning selectively disrupts trace learning, without affecting delay learning. An asterisk indicates significant reduction in time spent freezing for the distractor group compared with the nondistractor group of trace conditioning training (P < 0.05). Both delay and trace conditioning are significantly different from the shock-only conditioning for the nondistracted animals (black bars, P < 0.05). Error bars indicate SEM. (B) For light testing, there is no difference in percent time spent freezing between the animals that did and did not receive the distractor during training. (C) Six groups of mice received one to six tone–shock pairings and the flashing light as the distractor during trace conditioning. One group of mice received standard six tone–shock pairings and no distractor during trace conditioning. There is no difference in freezing to the flashing light for any of the six tone–shock pairings, compared with mice that received no flashing light. As a positive control, the ND group shows a high level of freezing in the tone test. TS, tone–shock pairing; ND, no distractor. (D) No difference was observed between the distractor and no distractor groups, indicating that the distractor did not affect contextual fear conditioning. (E) No difference was found in locomotor activity, as assessed by the number of crossing and rearing events between the distractor and no distractor groups.
We attribute the effects of the distractor to a disruption of the attentional processes necessary for trace conditioning. However, it is also known that multiple stimuli can compete with each other for the relative strength of association with the unconditional stimulus (31). It does not seem that this associative competition (between the flashing light and tone) can account for our results for the following reasons. First, if associative competition did occur, it would be expected that delay conditioning would also be affected by the presentation of the flashing light, which is not the case (Fig. 2 A). Second, animals that received the flashing light during training showed low freezing levels, comparable to those that did not receive the flashing light, when exposed to the light as a test stimulus (Fig. 2B). This observation suggests that the flashing light did not compete with the tone as a CS. The observed absence of freezing to the light distractor cannot be explained by the poor associability of the light to the shock. Previous studies have shown that light can serve as a CS in fear conditioning experiments (32). In a separate study involving 24 animals, we confirmed that the flashing light stimulus (in the absence of a tone) was able to acquire CS properties, in a delay fear conditioning procedure (percent time spent freezing to light ± SEM: Delay conditioning, 64 ± 8.76%; Shock-only, 4.89 ± 2.95%, P < 0.05).
We also examined the disrupting effect of the flashing light at different time points of the trace conditioning trials. No differences in freezing to the flashing light were observed across any of the six tone–shock pairings, compared with mice that received no flashing light (Fig. 2C). Therefore, it is unlikely that the flashing light became associated with the shock at the expense of the tone–shock association in the first few training trials in trace conditioning, but that this light–shock association was extinguished in later training trials. The presentation of the flashing light also did not increase contextual freezing (Fig. 2D), ruling out the possibility that the flashing light increases the saliency of the context, which in turn competes with learning to the tone.
Taken together, these results provide evidence that the distracting effect of the flashing light on trace conditioning is not caused by associative competition at any point of training. In addition, locomotor activity, as assessed by the number of crossing and rearing events (see Materials and Methods), was not significantly different between the light-exposed and nonexposed groups (Fig. 2E), arguing against the possibility that the decreased freezing level in trace conditioning, or its performance, is caused by hyperactivity. The most reasonable alternative explanation is that the flashing light interferes with trace conditioning by distracting the animals' attention during the acquisition phase.
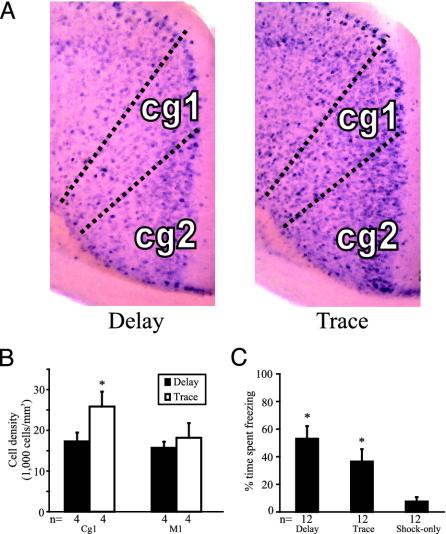
Higher Density of c-fos-Positive Cells in the ACC After Trace Conditioning Compared with Delay Conditioning. To determine whether trace fear conditioning in mice is associated with higher neuronal activity in the ACC compared with delay conditioning, we performed in situ hybridization to detect c-fos mRNA, a marker of neuronal activation. After training, a subset of the mice was killed for c-fos analysis, and the rest were saved for tone testing on the next day. The contours of the ACC (divided into subregions Cg1 and Cg2) and M1 were fitted electronically on the section with reference to a mouse brain atlas (29), and were calibrated in relation to relevant anatomical landmarks such as the corpus callosum. Mice that received trace conditioning showed, on average, ≈50% more c-fos-positive cells in the Cg1 subregion of the ACC than mice that received delay conditioning [t(6) = 3.24, P < 0.05] (Fig. 3 A and B). In each pair of brains (trace vs. delay) from four separate in situ hybridization experiments, the Cg1 of the mouse that received trace conditioning had more c-fos-positive cells than the Cg1 of the mouse that received delay conditioning. In the Cg2 subregion, there was a trend of more c-fos-positive cells in the trace conditioning group, but the difference was not significant. There was no difference between the trace conditioning and delay conditioning groups in the primary motor cortex (Fig. 3B, M1). Therefore, the increased number of the c-fos-positive cells in the Cg1 subregion in the trace conditioning group does not simply reflect a general increase in activity across all cortical areas. Among mice that were saved for tone testing, animals that received trace conditioning exhibited a significantly higher percent time freezing than those that received the shock-only training (Fig. 3C; P < 0.05), strongly suggesting that the mice killed for c-fos mRNA in situ hybridization were also successfully conditioned. These data suggest that the ACC in mice is more activated during the acquisition of trace fear conditioning compared with delay conditioning.
Fig. 3.
c-fos-positive cell counts after trace or delay conditioning. (A) Representative c-fos in situ hybridization expression in the ACC during delay and trace conditioning. Darker dots are c-fos-positive cells stained by BCIP/NBT. (B) c-fos-positive cell density in the Cg1 and M1. Mice that received trace conditioning have significantly more c-fos-positive cells in the ACC compared with mice that received delay conditioning (*P < 0.05). There is no such difference in M1. (C) Percent time spent freezing during the tone test of the mice trained simultaneously with the mice killed for c-fos mRNA in situ hybridization. Mice trained on delay and trace conditioning exhibit significantly more freezing than the shock-only group (*P < 0.05).
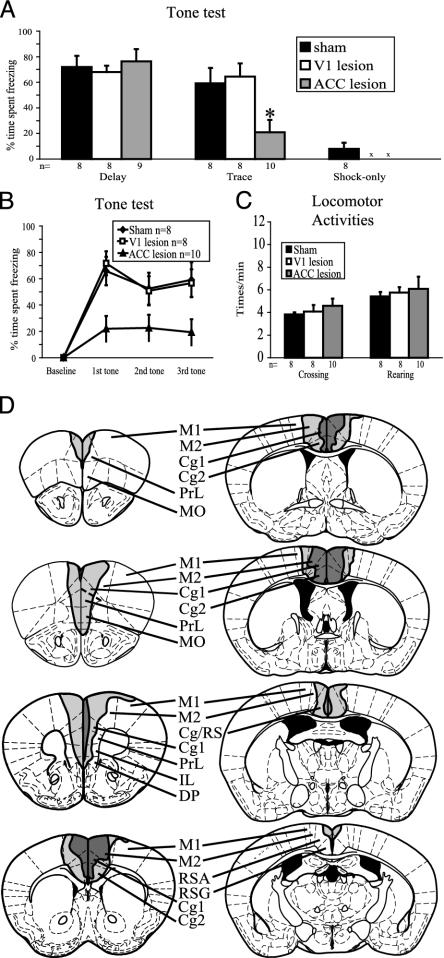
The ACC Is Required for Trace but Not Delay Conditioning. To assess the requirement of an intact ACC for trace fear conditioning in mice, we used an excitotoxin, N-methyl-D-aspartate, to lesion the ACC (Fig. 4D). We also included two control groups. One group received sham surgery to control for the effect of the general surgical procedure, and another group received lesions to the V1 to control for the effect of general cortical damage. The extent of the lesion was verified after the experiment for each mouse by histology. Animals received trace, delay, or shock-only training (see Materials and Methods). Mice that received sham operations exhibited both successful delay and trace conditioning in comparison to the control shock-only group (P < 0.05) (Fig. 4A). In contrast, animals that received ACC lesions showed a significant reduction in trace conditioning by comparison to the sham group and the V1 group (P < 0.05) (Fig. 4A). The level of freezing in the ACC-lesioned animals is not significantly different from the freezing level in animals that only received the shock. There was no difference in trace conditioning between the sham and V1 groups, suggesting that the impairment in the ACC group was not caused by general cortical damage. Importantly, all of the lesion groups exhibited successful delay conditioning in comparison to shock-only controls (Fig. 4A, P < 0.05). These data indicate that ACC lesions selectively disrupt trace but not delay fear conditioning in mice. To delineate at what point during testing the impairment of the ACC group first became evident, the time spent freezing was analyzed separately at each of the three consecutive tone testing trials for trace conditioning (Fig. 4B). The ACC lesion group displayed significantly less freezing as early as the first trial, compared with the sham group (P < 0.01) or the V1 lesion group (P < 0.01). No difference was found between the ACC lesion and control groups in locomotor activity by measures of crossing and rearing (Fig. 4C). For contextual conditioning, the percent time freezing for the ACC lesion group and sham group was 37 ± 9% and 36 ± 8% (mean ± SEM), respectively. No significant difference was found.
Fig. 4.
The effects of the pretraining ACC lesion on trace and delay conditioning. (A) Tone test. The ACC lesion group show impaired freezing performance in trace conditioning, but not in delay conditioning, compared with the sham control group. There is no difference between the V1 lesion group and the sham group in either delay or trace group. An asterisk identifies signifi-cantly less time spent freezing of ACC lesions compared with sham operation and V1 lesions in trace conditioning (P < 0.05). An “x” means the corresponding V1 and ACC groups did not exist. (B) Percent time spent freezing as a function of trial number for trace conditioning. The impairment of the ACC lesions can be seen as early as the first trial. (C) Locomotor activities. No difference is found between the ACC, V1, and sham lesion groups trained in trace, assessed by crossing and rearing activities. (D) Schematic representations of the ACC lesions. The dark gray area indicates the smallest and the light gray area indicates the greatest extent of the lesions. Cg, ACC; DP, dorsal peduncular cortex; IL, infralimbic cortex; M2, secondary motor cortex; MO, medial orbital cortex; RS, retrosplenial cortex (posterior cingulate cortex); RSA, retrosplenial cortex agranular; RSG, retrosplenial cortex granular; PrL, prelimbic cortex.
Discussion
Our results show that a distractor can selectively interfere with trace but not delay or contextual fear conditioning. Moreover, the density of c-fos-positive cells is ≈50% higher in portions of the ACC in those animals that received trace fear conditioning compared with animals that received delay fear conditioning. We also show that the pretraining lesions of the ACC impair trace but not delay or contextual fear conditioning. In contrast, V1 lesions did not affect either trace or delay conditioning, demonstrating the relative specificity of the ACC lesions. These data suggest that trace conditioning can be a useful model of attention in mice, and that the correlation between increased neuronal activity in the ACC and trace conditioning indeed reflects a functional requirement for the ACC in trace conditioning.
Although there are more c-fos-positive cells in the ACC during trace than during delay conditioning, there is substantial c-fos expression in the ACC during both delay and trace conditioning. One possible explanation is that the animals in both groups attend to the CS–US contingency, which is mediated by the ACC. This form of attention is required for trace but not delay conditioning, as shown by the distraction experiments. However, animals in the trace group need heightened attention to identify the less obvious CS–US contingency, reflected by more c-fos-positive cells. Alternatively, the increased activity in the ACC may reflect attention only in the case of trace conditioning, and in delay conditioning may reflect other activities, such as the response to shock.
Because of the decreasing size of the ACC toward its anterior tip and the relatively large domain of excitotoxic cell killing, it was difficult to lesion this region of the ACC without including parts of the prelimbic and the infralimbic cortex. Lesions that spared these latter two areas also spared the anterior tip of the ACC. Nevertheless, when the ACC lesion group was separated into two subgroups, one including and one excluding those subjects with lesions spanning the prelimbic/infralimbic/ anterior tip of the ACC, no significant performance difference between these two subgroups was detected, arguing that the effect of our lesions on trace conditioning indeed reflects a requirement for the ACC. Our c-fos analysis suggested enhanced activity in the Cg1 subdomain of ACC during trace conditioning, whereas Cg2 did not show a significant difference. Anatomically, Cg1 projects to the insular cortex, another brain area that was shown to be activated during aversive trace conditioning (18), but Cg2 does not (33). Firm conclusions about the subregions of the ACC necessary for trace conditioning will demand a more refined method of functional perturbation, such as lesion or inactivation driven by specific gene promoters, or other approaches available in mice.
Because ACC activity is implicated in attentionally demanding tasks in humans (12–17) and animals (3, 19–23) and attention to the tone-airpuff contingency is necessary for trace but not delay eye blink conditioning (6, 7), it is possible that the ACC lesions disrupt attention to the tone–shock contingency. The ACC lesion group displayed significantly less freezing as early as the first trial, compared with the sham group or the V1 lesion group (Fig. 4B), indicating that the impairment likely lies in early learning stages such as acquisition, when attending to the tone–shock contingency takes place, but not in late stages such as extinction. The ACC is known to have an important role in motor functions (12). In principle, ACC lesions might cause hyperactivity, which lowers the freezing level, and/or interfere with learning. Mice receiving the ACC lesions showed no difference in locomotor activity (Fig. 4C). These data, along with the finding that the ACC lesions do not impair delay conditioning or contextual conditioning, argue that the impairment in freezing seen in the ACC lesion group is unlikely to reflect simply motor hyperactivity. However, we cannot exclude the possibility that the impairment of trace conditioning by ACC lesions reflects an interference with nonattentional processes.
The hippocampus has been shown to be required for trace but not delay conditioning (6, 34–40). Contextual fear conditioning also requires short-term memory and an intact hippocampus (38, 39, 41–43). Because the hippocampal formation and the ACC have bidirectional connections (44), it is possible that ACC lesions impair trace conditioning by disrupting hippocampal functions. However, the ACC lesions did not impair contextual conditioning. Therefore, it is unlikely that the ACC lesions impair trace conditioning by affecting hippocampal functions. In addition, it is thought that the ACC is involved in emotion and pain (12, 20, 45). The lack of effects of the ACC lesions on delay and contextual conditioning argues against a general effect on fear. One possible explanation for the lack of effects of the ACC lesions on contextual conditioning is that contextual conditioning does not require attention to the CS–US contingency. If so, it would be consistent with the idea that this form of attention is mediated by the ACC.
Our data provide evidence that the pretraining lesions of the ACC impair trace but not delay fear conditioning, but it is unclear whether the acquisition, consolidation, and/or retrieval processes of trace conditioning is affected. To reveal at which stage the ACC is required, posttraining lesions will be necessary. If the posttraining lesions do not affect trace conditioning, the ACC is likely only required for the acquisition phase. If the posttraining lesions impair trace conditioning, some method of reversible inactivation will be required to determine whether the ACC is required during the acquisition phase.
We show that a visual distractor can selectively interfere with auditory trace but not delay fear conditioning. Such disruption does not reflect competition between the distractor and the tone for association with the shock, suggesting that trace fear conditioning in mice, as in humans (11), requires some form of attention. Our data therefore establish a correlation between a requirement for attention and for the ACC in trace fear conditioning in mice, but do not yet prove that the ACC is required for attention. Nevertheless, the ability to model an attentionally demanding task in mice opens up the problem to genetic manipulations. Finally, we wish to point out that awareness of the tone–air puff contingency relationship has been implicated as being necessary for trace but not delay eye blink conditioning in humans (6–9). Given the close relationship between attention and awareness (46), the murine system described here may be useful for better understanding the neuronal basis of this relationship.
Acknowledgments
We thank E. Chiang, W. Lerchner, R. M. Carter, H. Lester, M. A. MacIver, G. Mosconi, and M. R. Tinsley for support and assistance throughout the development of this work. This research was supported by California Institute of Technology, the Moore Discovery Award, The Gordon and Betty Moore Foundation, the W. M. Keck Foundation fund for Discovery, and the National Institute of Mental Health and the National Science Foundation under Award Nos. EEC-9402726 and IBN-0091487. D.J.A. is an Investigator of the Howard Hughes Medical Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACC, anterior cingulate cortex; CS, conditioned stimulus; US, unconditioned stimulus; V1, primary visual cortex; M1, primary motor cortex.
References
- 1.Parasuraman, R. (1998) The Attentive Brain (MIT Press, Cambridge, MA).
- 2.Braun, J., Koch, C. & Davis, J. L. (2001) Visual Attention and Cortical Circuits (MIT Press, Cambridge, MA).
- 3.Robbins, T. W. (2002) Psychopharmacology 163 362-380. [DOI] [PubMed] [Google Scholar]
- 4.Clark, R. E., Manns, J. R. & Squire, L. R. (2002) Trends Cognit. Sci. 6 524-531. [DOI] [PubMed] [Google Scholar]
- 5.Balogh, S. A., Radcliffe, R. A., Logue, S. F. & Wehner, J. M. (2002) Behav. Neurosci. 116 947-957. [DOI] [PubMed] [Google Scholar]
- 6.Clark, R. E. & Squire, L. R. (1998) Science 280 77-81. [DOI] [PubMed] [Google Scholar]
- 7.Clark, R. E. & Squire, L. R. (1999) Psychol. Sci. 10 14-18. [Google Scholar]
- 8.Manns, J. R., Clark, R. E. & Squire, L. R. (2000) Learn. Mem. 7 267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manns, J. R., Clark, R. E. & Squire, L. R. (2000) Hippocampus 10 181-186. [DOI] [PubMed] [Google Scholar]
- 10.Manns, J. R., Clark, R. E. & Squire, L. R. (2002) J. Exp. Psychol. Anim. Behav. Process. 28 32-37. [PubMed] [Google Scholar]
- 11.Carter, R. M., Hofstötter, C., Tsuchiya, N. & Koch, C. (2003) Proc. Natl. Acad. Sci. USA 100 1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devinsky, O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118 279-306. [DOI] [PubMed] [Google Scholar]
- 13.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cognit. Sci. 4 215-222. [DOI] [PubMed] [Google Scholar]
- 14.Bush, G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A. & Rosen, B. R. (2002) Proc. Natl. Acad. Sci. USA 99 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovicich, J., Peters, R. J., Koch, C., Braun, J., Chang, L. & Ernst, T. (2001) J. Cognit. Neurosci. 13 1048-1058. [DOI] [PubMed] [Google Scholar]
- 16.Davis, K. D., Hutchison, W. D., Lozano, A. M., Tasker, R. R. & Dostrovsky, J. O. (2000) J. Neurophysiol. 83 3575-3577. [DOI] [PubMed] [Google Scholar]
- 17.Bush, G., Whalen, P. J., Rosen, B. R., Jenike, M. A., McInerney, S. C. & Rauch, S. L. (1998) Hum. Brain Mapp. 6 270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Büchel, C., Dolan, R. J., Armony, J. L. & Friston, K. J. (1999) J. Neurosci. 19 10869-10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bussey, T. J., Muir, J. L., Everitt, B. J. & Robbins, T. W. (1996) Behav. Brain Res. 82 45-56. [DOI] [PubMed] [Google Scholar]
- 20.Bussey, T. J., Everitt, B. J. & Robbins, T. W. (1997) Behav. Neurosci. 111 908-919. [DOI] [PubMed] [Google Scholar]
- 21.Bussey, T. J., Muir, J. L., Everitt, B. J. & Robbins, T. W. (1997) Behav. Neurosci. 111 920-936. [DOI] [PubMed] [Google Scholar]
- 22.Muir, J. L., Everitt, B. J. & Robbins, T. W. (1996) Cereb. Cortex 6 470-481. [DOI] [PubMed] [Google Scholar]
- 23.Rogers, R. D., Baunez, C., Everitt, B. J. & Robbins, T. W. (2001) Behav. Neurosci. 115 799-811. [DOI] [PubMed] [Google Scholar]
- 24.Kronforst-Collins, M. A. & Disterhoft, J. F. (1998) Neurobiol. Learn. Mem. 69 147-162. [DOI] [PubMed] [Google Scholar]
- 25.Weible, A. P., McEchron, M. D. & Disterhoft, J. F. (2000) Behav. Neurosci. 114 1058-1067. [DOI] [PubMed] [Google Scholar]
- 26.Dragunow, M. & Faull, R. (1989) J. Neurosci. Methods 29 261-265. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri, A., Zangenehpour, S., Rahbar-Dehgan, F. & Ye, F. C. (2000) Acta Neurobiol. Exp. 60 403-410. [DOI] [PubMed] [Google Scholar]
- 28.Bahu, S. J., Kaltenbach, J. A., Zhang, J. S., Khariwala, S. S., Afman, C. E. & Hnatiuk, M. (2001) Neurosci. Res. Commun. 29 107-117. [Google Scholar]
- 29.Paxinos, G. & Franklin, K. B. J. (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 30.Mongeau, R., Miller, G. A., Chiang, E. & Anderson, D. J. (2003) J. Neurosci. 23 3855-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rescorla, R. A. & Wagner, A. R. (1972) in Classical Conditioning II: Current Research and Theory, eds. Black, A. H. & Prokasy, W. F. (Appleton-Century-Crofts, New York), pp. 64-99.
- 32.Kim, S. D., Rivers, S., Bevins, R. A. & Ayres, J. J. (1996) J. Exp. Psychol. Anim. Behav. Process. 22 87-104. [DOI] [PubMed] [Google Scholar]
- 33.Zilles, K. & Wree, A. (1995) in The Rat Nervous System, ed. Paxinos, G. (Academic, Sydney), p. 654.
- 34.Moyer, J. R., Deyo, R. A. & Disterhoft, J. F. (1990) Behav. Neurosci. 104 243-252. [DOI] [PubMed] [Google Scholar]
- 35.James, G. O., Hardiman, M. J. & Yeo, C. H. (1987) Behav. Brain Res. 23 109-116. [DOI] [PubMed] [Google Scholar]
- 36.Solomon, P. R., Vanderschaaf, E. R., Thompson, R. F. & Weisz, D. J. (1986) Behav. Neurosci. 100 729-744. [DOI] [PubMed] [Google Scholar]
- 37.Port, R. L., Romano, A. G., Steinmetz, J. E., Mikhail, A. A. & Patterson, M. M. (1986) Behav. Neurosci. 100 745-752. [DOI] [PubMed] [Google Scholar]
- 38.McEchron, M. D., Bouwmeester, H., Tseng, W., Weiss, C. & Disterhoft, J. F. (1998) Hippocampus 8 638-646. [DOI] [PubMed] [Google Scholar]
- 39.McEchron, M. D., Tseng, W. & Disterhoft, J. F. (2000) Hippocampus 10 739-751. [DOI] [PubMed] [Google Scholar]
- 40.Quinn, J. J., Oommen, S. S., Morrison, G. E. & Fanselow, M. S. (2002) Hippocampus 12 495-504. [DOI] [PubMed] [Google Scholar]
- 41.Bast, T., Zhang, W. N. & Feldon, J. (2001) Hippocampus 11 828-831. [DOI] [PubMed] [Google Scholar]
- 42.Anagnostaras, S. G., Gale, G. D. & Fanselow, M. S. (2001) Hippocampus 11 8-17. [DOI] [PubMed] [Google Scholar]
- 43.Kim, J. J. & Fanselow, M. S. (1992) Science 256 675-677. [DOI] [PubMed] [Google Scholar]
- 44.Kang, E. & Gabriel, M. (1998) Hippocampus 8 491-510. [DOI] [PubMed] [Google Scholar]
- 45.Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E. & Hof, P. (2001) Ann. N.Y. Acad. Sci. 935 107-117. [PubMed] [Google Scholar]
- 46.Crick, F. & Koch, C. (2003) Nat. Neurosci. 6 119-126. [DOI] [PubMed] [Google Scholar]