Abstract
Activation of β-adrenergic receptors and consequent phosphorylation by cAMP-dependent protein kinase A (PKA) greatly increases the L-type Ca2+ current through CaV1.2 channels in isolated cardiac myocytes. A kinase-anchoring protein 15 (AKAP15) coimmunoprecipitates with CaV1.2 channels isolated from rat heart membrane extracts and transfected cells, and it colocalizes with CaV1.2 channels and PKA in the transverse tubules of isolated ventricular myocytes. Site-directed mutagenesis studies reveal that AKAP15 directly interacts with the distal C terminus of the cardiac CaV1.2 channel via a leucine zipper-like motif. Disruption of PKA anchoring to CaV1.2 channels via AKAP15 using competing peptides markedly inhibits the β-adrenergic regulation of CaV1.2 channels via the PKA pathway in ventricular myocytes. These results identify a conserved leucine zipper motif in the C terminus of the CaV1 family of Ca2+ channels that directly anchors an AKAP15-PKA signaling complex to ensure rapid and efficient regulation of L-type Ca2+ currents in response to β-adrenergic stimulation and local increases in cAMP.
Voltage-gated L-type calcium (Ca2+) channels play a pivotal role in the regulation of a wide range of cellular processes, including membrane excitability, Ca2+ homeostasis, protein phosphorylation, and gene regulation. In cardiac myocytes, Ca2+ influx through CaV1.2 channels contributes to the plateau phase of the cardiac action potential and is responsible for initiating excitation-contraction coupling (1-3). Voltage-gated L-type Ca2+ channels are multisubunit complexes composed of a poreforming α1 subunit and auxiliary β and α2δ subunits (4). In cardiac muscle, a distinct α1 subunit (α11.2a) (5, 6), an α2δ subunit (7), and several isoforms of β subunits [β1b and β2a-d (8-11)] have been identified and implicated to form the CaV1.2 channel. As for the skeletal muscle CaV1.1 channel (12, 13), two size forms of the α11.2a subunit of CaV1.2 channels, ≈240 and 210 kDa, are present in cardiac muscle and differ by truncation at the C terminus (14). Whereas the majority of Ca2+ channel α1 subunits isolated from cardiac muscle are truncated (11, 14, 15), the cleaved distal C terminus remains associated with the truncated α1 subunit of CaV1.2 after proteolytic processing, and peptides derived from it can regulate channel activity (16, 17).
CaV1.2 channels can be modulated by a variety of receptor-mediated processes, including stimulation through activation of the β-adrenergic receptor/cAMP signaling pathway (4, 18-21). Activation of β-adrenergic receptors increases cardiac L-type Ca2+ currents through cAMP-dependent protein kinase A (PKA)-mediated phosphorylation of the CaV1.2 channel protein and/or associated proteins (22, 23). As for skeletal muscle Cav1.1 channels (24, 25), both the α1 and β subunits of CaV1.2 channels are substrates for phosphorylation by PKA (14, 26-28). PKA phosphorylates only the full-length form of the α1 subunit, on a single site containing serine 1928 in the C-terminal domain (14, 26). In contrast, the C-terminal truncated α1 subunit is not a substrate for phosphorylation by PKA in vitro (14, 26). CaV1.2 channels consisting of only α1 subunits can be regulated by PKA, implicating phosphorylation of serine 1928 in channel regulation (29, 30). Mutation of serine 1928 to alanine prevents PKA-dependent phosphorylation and the low level of functional modulation of CaV1.2 channels that is observed in heterologous expression systems (31).
The importance of PKA anchoring through the association with A kinase-anchoring proteins (AKAPs) has recently been established in the regulation of L-type Ca2+ channels. AKAPs belong to a family of functionally related proteins that contain a targeting domain that directs the AKAP to a specific subcellular compartment or substrate and a kinase-anchoring domain containing an amphipathic α helix that binds the regulatory (R) subunit dimer of PKA (32-35). Intracellular dialysis of ”kinase-anchoring inhibitor peptides,” which competitively inhibit PKA-AKAP interactions, effectively inhibits PKA-dependent increase of Ca2+ channel activity in skeletal muscle cells (36, 37) and cardiac myocytes (31). A low molecular weight AKAP, AKAP15 (refs. 38 and 39; also known as AKAP18 and AKAP7; refs. 40 and 41; bioinfo.cnio.es/cgi-bin/db/genecards/carddisp? AKAP7) has been identified as the anchoring protein that targets PKA to CaV1.1 channels in skeletal muscle. In contrast, in the brain, PKA is anchored near CaV1.2 channels via the microtubule-associated protein MAP2B (42). Because MAP2B is not expressed in the heart, it is not a likely candidate for recruiting PKA to cardiac CaV1.2 channels.
Insight into the molecular mechanisms underlying the targeting of kinases and phosphatases to ion channels has recently emerged. Marx et al. (43) identified a novel role of atypical leucine zipper (LZ) motifs in targeting kinases and phosphatases to the ryanodine-sensitive Ca2+-release channel of the sarcoplasmic reticulum. Similarly, we found that AKAP15 targets PKA to the C terminus of skeletal muscle CaV1.1 channels through modified LZ interactions (44). Disruption of the LZ interaction between AKAP15 and the C-terminal domain of CaV1.1 channels with a competing peptide effectively inhibits PKA-dependent potentiation of L-type Ca2+ channel activity in skeletal muscle cells (44). Sequence alignment of the LZ-like region of CaV1.1 with other members of the CaV1 channel family reveals a striking conservation of LZ-like motifs, suggesting that AKAP15 may also target PKA to CaV1.2 channels. Here, we report that AKAP15 coimmunoprecipitates and colocalizes with CaV1.2 channels in rat ventricular myocytes. Site-directed mutagenesis studies reveal that AKAP15 directly interacts with the C terminus of the cardiac CaV1.2 channel via an LZ-like interaction, similar to that identified in skeletal muscle. Disruption of PKA anchoring to CaV1.2 channels via AKAP15, using competing peptides, markedly inhibits PKA regulation of CaV1.2 channels activated by β-adrenergic receptors in ventricular myocytes. Our results imply that PKA anchored directly to the CaV1.2 channel by AKAP15 via an LZ motif is required for regulation of Ca2+ channels in cardiac myocytes by the sympathetic nervous system.
Experimental Procedures
Antibodies and Peptides. Rabbit polyclonal anti-CNC1 and anti-CH1 antibodies were generated against peptides corresponding to residues 821-838 in the intracellular loop between domains II and III (CNC1) and residues 2155-2171 (CH1) in the distal C terminus of CaV1.2a, respectively, and characterized as described (14). A chicken polyclonal anti-CNC1 antibody was generated against peptides corresponding to residues 823-838 [TTKINMDDLQPSENEDKS(C)] in the intracellular loop between domains II and III of CaV1.2a (26). The cysteine residue was added to the C terminus of the peptide to facilitate crosslinking. The peptide was synthesized, purified, coupled to keyhole limpet hemocyanin, and used for immunization of chickens (Affinity BioReagents, Neshanic Station, NJ). The resulting antibody was affinity-purified on peptide columns as described (45), and specificity of the antibody was confirmed by immunoblotting. Anti-AKAP15 antibodies and RII-biotin protein were prepared as described (39). Anti-RIIα antibody against the type IIα regulatory subunit of PKA was kindly provided by G. Stanley McKnight (University of Washington). Monoclonal anti-Myc antibody was purchased from Invitrogen. AKAP15LZ(38-54) (acetyl-ENAVLKAVQQYLEETQN-amide) and AKAP15LZM(38-54) peptides (acetyl-ENAVAKAVQQYAEETQN-amide) were synthesized and purified by Genemed Biotechnologies (South San Francisco, CA). PKI(5-24)-amide was obtained from Peninsula Laboratories, and HT31 peptide was synthesized and purified as described (38).
Molecular Biology. The N-terminal acylated (NAc) CaV1.2 AKAP15-binding domain (ABD, residues 2057-2115) of the α1 subunit of the rabbit cardiac muscle Ca2+ channel (5) was constructed by incorporating the myristoylation and palmitoylation sequence (MGQLCC) of AKAP15 onto the N terminus of the CaV1.2 ABD with specific primers by PCR and cloned in frame into pcDNA3mycHisA. CaV1.2 ABD(LZm) incorporating mutations I2073A, F2080A, and I2087A was constructed by using PCR overlap extension and cloned into pcDNA3mycHisA. AKAP15LZM was generated as described (44). The orientation and reading frame of all constructs were confirmed by DNA sequencing.
Expression of CaV1.2 Channels and AKAP15 in tsA-201 Cells. TsA-201 cells were cultured in DMEM/Ham's F-12 supplemented with 10% FBS, 100 units/ml penicillin, and streptomycin plated on 15-cm dishes. Cells were transfected with 30-50 μg of an equimolar ratio of expression plasmid cDNA by using the calcium phosphate method. For coexpression of AKAP15 with the full-length CaV1.2 channel, cDNA encoding the Ca2+ channel subunits α11.2a, β1b, and α2δ were cotransfected with AKAP15. After 48 h, cells were washed in PBS, solubilized in ice-cold bRIA (50 mM Tris·HCl, pH 7.4/150 mM NaCl/1 mg/ml BSA/5 mM NaF/1 mM EGTA/5 mM EDTA/1% Triton X-100/plus protease inhibitors), and rotated at 4°C for 30 min. Unsolubilized material was removed by centrifugation, and the solubilized extracts containing AKAP15 and CaV1.2 were frozen for further analysis.
Preparation of Rat Heart Extracts. Hearts from adult male Wistar rats were pulverized under liquid N2 and homogenized in buffer containing 20 mM Tris, 150 mM NaCl, 10 mM EGTA, 10 mM EDTA (pH 7.4), plus protease inhibitors. Homogenates were centrifuged at 8,900 × g at 4°C to remove debris, and membranes were collected by ultracentrifugation at 100,000 × g (4°C) for 45 min. Membranes were solubilized in homogenization buffer containing 1% digitonin for 30 min at 4°C, and insoluble material was removed by centrifugation.
Coimmunoprecipitation Experiments. Rat heart and transfected tsA-201 extracts were precleared with either protein A- or G-Sepharose. Precleared extracts were incubated with either 10 μg of affinity-purified CaV1.2 channel antibody (anti-CNC1), 10 μg of rabbit IgG, 2 μg of anti-Myc antibody, or 2 μg of mouse IgG for 3 h at 4°C, followed by the addition of protein A- or G-Sepharose (5 mg) for an additional 2 h. Immune complexes bound to the Sepharose beads were washed extensively, and proteins were separated by SDS/PAGE, transferred to nitrocellulose, and analyzed by immunoblotting. CaV1.2 channel protein was detected with the anti-CNC1 antibody. Myc-tagged NAc-CaV1.2 ABD was detected by using monoclonal anti-Myc antibody (Invitrogen). For AKAP15 detection, immunoblots were probed by using an anti-AKAP15 antibody or the RII-biotin overlay assay (38). Each immunoprecipitation experiment was repeated at least three times.
Cell Isolation. Ventricular myocytes were isolated from male Wistar rats (201-225 g) as described (46) and maintained at 37°C until use.
Immunocytochemistry. For double-labeling studies, ventricular myocytes were plated on laminin-coated glass coverslips and incubated (5% CO2, 37°C) for ≈2 h. Myocytes were fixed with 4% paraformaldehyde for 30 min, rinsed with 0.1 M Tris-buffered saline (TBS) and blocked sequentially with 2% avidin and 2% biotin. For double-labeling, myocytes were incubated overnight at 4°C with the following primary antibodies (diluted in 10% goat serum and 0.2% Triton X-100 in TBS): chicken anti-CNC1 and anti-CH1, chicken anti-CNC1 and anti-AKAP15, and chicken anti-CNC1 and anti-RIIα. Myocytes were then rinsed in TBS and incubated with biotinylated chicken antibody against chicken IgY (Vector Laboratories) for 2 h at room temperature to label the chicken anti-CNC1, rinsed, and then incubated with avidin D fluorescein (Vector Laboratories). After extensive washing (12 × 5 min TBS), myocytes were next incubated with biotinylated antibody against rabbit IgG (to label AKAP15 and CH1) or goat IgG (to label RIIα) overnight at 4°C, rinsed, and then incubated with avidin D Texas red (Vector Laboratories) for 2 h at room temperature. After final washes, coverslips were mounted on slides by using Vectashield (Vector Laboratories). Cells were viewed by using a Bio-Rad MRC 600 confocal microscope in the W. M. Keck Imaging Facility. Control experiments were routinely performed to exclude the possibility of nonspecifc labeling, crossreactivity of secondary antibodies with primary antibodies, or bleed-through from one channel to the other.
Electrophysiology. Whole-cell Ca2+ currents (ICa) were recorded at room temperature from rod-shaped, striated, Ca2+-tolerant myocytes (46) within 1-10 h of isolation. The extracellular bath solution contained: 140 mM TEA-Cl, 2 mM MgCl2, 1.8 mM CaCl2, 10 mM Hepes, and 10 mM glucose (pH 7.4). Patch pipettes (1-1.5 MΩ) were filled with an intracellular solution containing 100 mM CsCl, 20 mM TEA-Cl, 1 mM MgCl2, 5 mM MgATP, 10 mM Hepes, and 10 mM EGTA (pH 7.3). Series resistance ranged between 3 and 6 MΩ (60-80% compensated), and recordings were discarded if the series resistance was >6 MΩ before compensation. ICa was elicited by either a repetitive train of 300-ms depolarizing pulses to 0 mV or a series of 10-mV depolarizing pulses to different test potentials (-40 to +60 mV) from a holding potential of -40 mV. ICa was measured as the difference between the peak inward current and the current at the end of the test pulse. Membrane capacitance was measured by integrating the capacitance current recorded during a 10-mV hyperpolarizing pulse from a holding potential of -40 mV. Currents were recorded with an Axopatch 200B amplifier (Axon Instruments, Union City, CA) and sampled at 5 kHz after anti-alias filtering at 2 kHz. Data acquisition and command potentials were controlled by PCLAMP software (v8.0, Axon Instruments), and data were stored for later off-line analysis. HT31, AKAP15LZ(38-54), and AKAP15LZM(38-54) peptides were prepared and used as described (44). The effect of isoproterenol (1 μM) on ICa in the absence and presence of peptides was examined 10-15 min after establishing the whole-cell configuration. All data are expressed as the mean ± SE of n cells. Statistical significance was tested by using Student's t test. Values of P < 0.01 were considered significant.
Results and Discussion
Coimmunoprecipitation of AKAP15 with CaV1.2 Channels. AKAP15 targets PKA to the C terminus of skeletal muscle CaV1.1 channels through modified LZ interactions (44). Sequence alignment of the LZ-like region of CaV1.1 with other members of the CaV1 family revealed a striking conservation of LZ-like motifs (Fig. 1A), including the cardiac CaV1.2 channel, raising the possibility that AKAP15 may also target PKA to CaV1.2 channels. To examine the possibility that AKAP15 associates with cardiac CaV1.2 channels, immunoprecipitation experiments were performed from rat heart extracts. CaV1.2 channels were immunoprecipitated with anti-CNC1 and assayed for coimmunoprecipitation of AKAP15 by immunoblotting. Fig. 1B shows that AKAP15 coimmunoprecipitated with CaV1.2 channels (Fig. 1B, lane 2). The interaction between AKAP15 and the Ca2+ channel was specific because AKAP15 was excluded from precipitates obtained with control IgG (Fig. 1B, lane 3). Furthermore, whereas other AKAPs were detected in rat heart extracts by using the RII overlay assay (e.g., mAKAP and yotiao), these AKAPs failed to coimmunoprecipitate with CaV1.2 channels (data not shown).
Fig. 1.
AKAP15 coimmunoprecipitates with CaV1.2 channels isolated from rat heart. (A) Amino acid sequence alignment of the LZ-like region identified in the distal C-terminal domain of CaV1.1 that interacts with AKAP15 with other members of the CaV1 family (44). Conserved leucine residues or hydrophobic residues in the ”a” and ”d” positions of the heptad repeats are indicated and asterisked. (B) Coimmunoprecipitation of AKAP15 with CaV1.2 channels isolated from rat heart extracts. Proteins were immunoprecipitated from heart extracts with an anti-CaV1.2 antibody (CNC1; lane 2) or control IgG (lane 3). Immunoblots were probed with anti-CNC1 (Upper) or anti-AKAP15 (Lower). Positive control for immunoblotting was 20 μl of heart extract (lane 1).
To verify the interaction between AKAP15 and CaV1.2 channels, immunoprecipitation experiments were performed in transfected cells. TsA-201 cells were cotransfected with equimolar ratios of Ca2+ channel α11.2a, β1b, and α2δ subunits and AKAP15. Cell lysates were immunoprecipitated with anti-CNC1 or control IgG, and the immunoprecipitated AKAP15 protein was detected with an anti-AKAP15 antibody. Fig. 2A shows that AKAP15 also coimmunoprecipitated with CaV1.2 channels when heterologously expressed in tsA-201 cells. Thus, AKAP15 may bind directly to the CaV1.2 channel complex.
Fig. 2.
AKAP15 associates with the distal C terminus of CaV1.2 via LZ-like motifs in tsA-201 cells. (A) Lysates of tsA-201 cells transfected with CaV1.2 and AKAP15 were immunoprecipitated with anti-CNC1 (lane 2) or control IgG (lane 3). Immunoblots were probed with anti-CNC1 (Upper) or anti-AKAP15 (Lower). Positive control for immunoblotting was 20 μl of lysate (lane 1). (B) TsA-201 cells were cotransfected with wtCaV1.2 ABD and wtAKAP15 (lanes 1), wtCaV1.2 ABD and AKAP15LZm (lanes 2), CaV1.2 ABDLZm and wtAKAP15 (lanes 3), or CaV1.2 ABDLZm and AKAP15LZm (lanes 4). Cell lysates were immunoprecipitated with an anti-myc antibody or nonimmune IgG, and the immunoprecipitated AKAP15 protein was detected using the RII-biotin assay (Upper). Immunoblotting of cell lysates with RII-biotin (Lower Left) or an anti-myc antibody (Lower Right) shows equivalent expression of the AKAP15 and CaV1.2 ABD protein, respectively. (C) Immunoblot of tsA-201 cell lysate expressing CaV1.2 was probed with chicken anti-CNC1 to verify that this antibody specifically recognizes CaV1.2 channel protein.
Interaction of AKAP15 with the Distal C Terminus of CaV1.2 via an LZ. Having established that AKAP15 associates with the cardiac CaV1.2 channel, we next tested the hypothesis that AKAP15 directly interacts with the C terminus of CaV1.2 via an LZ interaction similar to that identified in the skeletal muscle CaV1.1 channel (Fig. 1 A and ref. 44). A myc-epitope-tagged C-terminal construct of CaV1.2 containing the putative AKAP15-binding domain (CaV1.2 ABD, residues 2057-2115) was generated, and its ability to interact with AKAP15 was examined by coimmunoprecipitation experiments in transfected tsA-201 cells, as described (44). Lysates of tsA-201 cells coexpressing AKAP15 and the CaV1.2 ABD were immunoprecipitated with an anti-Myc antibody or control IgG, and the immunoprecipitated AKAP15 was detected by using the anti-AKAP15 antibody (Fig. 2). Immunoblotting of cell lysates with anti-AKAP15 or anti-myc antibody demonstrated robust expression of AKAP15 and the CaV1.2 ABD, respectively (Fig. 2B). Fig. 2B shows that AKAP15 coimmunoprecipitated with the CaV1.2 ABD. To test the hypothesis that AKAP15 binds to the CaV1.2 ABD via an LZ interaction, isoleucine and phenylalanine residues at positions 2073, 2087, and 2080 were mutated to alanine to generate CaV1.2 ABDLZm, and the leucine residues at positions 42 and 49 in AKAP15 were mutated to alanine to generate AKAP15LZm as described (44). Lysates of tsA-201 cells coexpressing wtAKAP15 or AKAP15(LZm) with either the CaV1.2 ABD or CaV1.2 ABDLZm were immunoprecipitated with an anti-myc antibody or control IgG, and the immunoprecipitated proteins were detected by using the RII-biotin overlay assay, which gives robust labeling of both wild-type and mutant AKAP15. We found that binding of either AKAP15LZm to wtCaV1.2 ABD, or wtAKAP15 to CaV1.2 ABDLZm was substantially reduced (Fig. 2B). Binding of AKAP15LZm to CaV1.2 ABDLZm was not detectable (Fig. 2B). Thus, the LZ motif in the distal C terminus of CaV1.2 is required for AKAP15 targeting of PKA to the Ca2+ channel.
Colocalization of CaV1.2 Channels, AKAP15, and the Regulatory Subunit of PKA in the Transverse Tubules of Rat Ventricular Myocytes. We next determined the subcellular localization of AKAP15 in rat ventricular myocytes and examined whether AKAP15, PKA, and CaV1.2 channels colocalize in vivo by using double-labeling immunofluorescence techniques and chicken anti-CNC1, which specifically recognizes CaV1.2 (Fig. 2C). CaV1.2 channels (Fig. 3A, green) and AKAP15 (Fig. 3B, red) are concentrated in the transverse tubules, as revealed by the regular, punctate pattern of the two proteins along the z-line and throughout the depth of the cell. A clear colocalization of these proteins is revealed when images of their respective staining patterns are merged (Fig. 3C, yellow). These results confirm our biochemical data suggesting that AKAP15 and CaV1.2 channels are associated and support a role for AKAP15 in targeting PKA to Ca2+ channels in the heart. To examine whether PKA colocalizes with AKAP15 and CaV1.2 channels, myocytes were double-labeled with antibodies specific for the CaV1.2 channel and the Type IIα regulatory subunit of PKA (anti-RIIα, Fig. 3 D-F). Whereas a clear overlap of CaV1.2 channel and RIIα staining was detected along the z-line in the transverse tubules (Fig. 3F), a significant portion of RIIα staining did not colocalize with CaV1.2 channels, as revealed by red staining in Fig. 3F. These findings suggest that a pool of PKA containing RIIα subunits is associated with AKAP15 and CaV1.2 channels, but PKA containing RIIα subunits is also targeted to other compartments of the cells, as expected from the many physiological effects of PKA in cardiac cells. Whereas PKA containing Type I regulatory subunits (RI) is also highly expressed in the rodent heart, it is differently distributed than RIIα-containing PKA (47).
Fig. 3.
CaV1.2 channels, AKAP15, and PKA colocalize at the transverse tubules in rat ventricular myocytes. Ventricular myocytes were double-labeled with antibodies specific for CaV1.2 and AKAP15 (A-C), CaV1.2 and the Type IIα regulatory subunit of PKA (RIIα; D-F), and CaV1.2 and CaV1.2 (distal; G-I). A punctate transverse tubule pattern of CaV1.2 channels, AKAP15, and PKA:RIIα was detected along the z-line of rat ventricular myocytes. The plane of focus was within the depth of the cell for these images. Double-labeled structures appear yellow. (Scale bar = 10 μM.)
Two size forms of the α1 subunit of CaV1.2 channels, ≈240 and 210 kDa, are present in cardiac muscle and differ by truncation at the C terminus (14). Interestingly, the AKAP15 interaction site in CaV1.2 channels is located in the C-terminal domain that is cleaved in the truncated form of CaV1.2. Therefore, CaV1.2 channels may have intact, truncated and bound, or truncated and dissociated C-terminal domains in vivo. To examine the subcellular localization pattern of CaV1.2 channels and the distal C terminus, myocytes were double-labeled with anti-CNC1, which recognizes both full-length and truncated CaV1.2 channels, and anti-CH1, which recognizes only the distal C terminus. Fig. 3 G and H shows that the staining pattern for anti-CNC1 (Fig. 3G, green) was indistinguishable from that of anti-CH1 [Fig. 3 H (red) and I (merged)]. Evidently, the distal C-terminal domain containing the CaV1.2 ABD is present in intact ventricular myocytes and colocalized with the remainder of the CaV1.2 channel protein in the transverse tubules at the junctions with the sarcoplasmic reticulum. Taken together, these data show that CaV1.2 channels, their distal C-terminal domain, AKAP15, and PKA colocalize in the t-tubules of ventricular myocytes.
Disruption of PKA/AKAP15 Anchoring to Cav1.2 Channels with Competing Peptides. It is well established that activation of β-adrenergic receptors by isoproterenol increases CaV1.2 channel activity through activation of a cAMP- and PKA-dependent pathway (19, 20, 23). The results presented above demonstrate that AKAP15 directly associates with the C terminus of the cardiac CaV1.2 channel through modified LZ motifs and colocalizes with CaV1.2 channels in ventricular myocytes. To examine the functional significance of AKAP15 binding to the C terminus of CaV1.2, we used a competing peptide approach to examine the functional importance of disrupting anchoring of PKA and AKAP15 to CaV1.2 channels in PKA regulation of Ca2+ currents in rat ventricular myocytes.
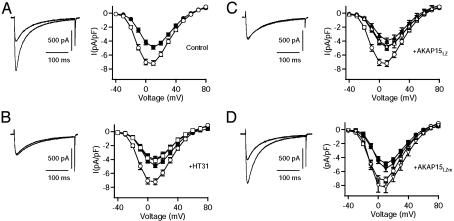
Fig. 4A shows superimposed records of ICa from a typical myocyte recorded at 0 mV from a holding potential of -40 mV before (filled circles) and after (open circles) 5-min exposure to isoproterenol (1 μM). Isoproterenol markedly increased the amplitude of ICa and caused a 9.1 ± 0.8-mV hyperpolarizing shift in the current-voltage (I-V) relationship (Fig. 4A). To test whether anchoring of PKA near Ca2+ channels is required for β-adrenergic receptor regulation of ICa in rat ventricular myocytes, we examined the effects of HT31, a specific peptide inhibitor of PKA-AKAP interactions (48), on the response of ICa to isoproterenol. Inclusion of HT31 (100 μM) in the patch pipette substantially reduced the isoproterenol-induced increase in ICa (Fig. 4B) and blocked the hyperpolarizing shift in the voltage-dependence of activation. The mean increase measured at 10 mV was 1.68 ± 0.11-fold in the absence of peptide compared with 1.01 ± 0.10-fold in the presence of HT31. Interestingly, the presence of HT31 also produced a small, but consistent, decrease in basal ICa. Thus, it seems that PKA anchoring close to Ca2+ channels via an AKAP is required for PKA regulation of cardiac CaV1.2 channels under basal conditions and during stimulation of the β-adrenergic receptor-signaling pathway by isoproterenol.
Fig. 4.
Disruption of the AKAP15-LZ interaction inhibits β-adrenergic receptor regulation of ICa in rat ventricular myocytes. (A-D Left) Representative currents elicited by 300-ms test pulses to 0 mV before (filled symbols) and after (open symbols) 5-min exposure to isoproterenol (1 μM). (A-D Right) Mean (± SEM) current-voltage relationships before (filled symbols) and after (open symbols) 5-min exposure to isoproterenol. (A) The effect of isoproterenol on ICa in the absence of peptide dialysis. Shown are the effects of HT31 (100 μM, n = 7, B), AKAP15LZ(38-54) (100 μM, n = 14, C), and AKAP15LZm(38-54) (100 μM, n = 6, D) on the response of ICa to isoproterenol as compared with control (circles).
We next examined the functional importance of AKAP15 binding to the C-terminal domain of CaV1.2. The synthetic peptide corresponding to the LZ motif of AKAP15 [AKAP15 LZ(38-54)] was introduced into the intracellular pipette solution. This peptide, which prevents AKAP15 association with the C terminus of the CaV1.1 channel, has been used successfully as a competing peptide to prevent PKA- and voltage-dependent potentiation of Ca2+ channel activity in skeletal muscle (44). In cells dialyzed with AKAP15LZ(38-54) (100 μM), the isoproterenol-induced increase in ICa was greatly reduced (1.21 ± 0.17-fold), and the hyperpolarizing shift in the voltage dependence of activation was blocked (Fig. 4C). In contrast, dialysis of a mutant form of the same peptide, in which alanine residues were substituted for the two critical leucine residues [AKAP15LZm(38-54)], had no significant effect on the increase in ICa induced by isoproterenol 1.55 ± 0.21-fold (Fig. 4D). Thus, our data show that targeting of PKA to Ca2+ channels via an LZ-like interaction between AKAP15 and the C-terminal domain of CaV1.2 channels plays an essential role in β-adrenergic receptor regulation of Ca2+ currents in rat ventricular myocytes. Remarkably, the block of PKA regulation is essentially complete, indicating that PKA not bound to AKAP15 is ineffective in regulation of CaV1.2 channels in ventricular myocytes in response to activation of the β-adrenergic receptor pathway.
Regulation of the CaV1 Family of Ca2+ Channels by PKA and AKAP15. In the present study, we have identified AKAP15 as the anchoring protein important for targeting of PKA to CaV1.2 channels and regulating L-type Ca2+ currents in the heart in response to β-adrenergic stimulation. AKAP15 directly associates with the C terminus of the cardiac CaV1.2 channel through modified LZ motifs and colocalizes with CaV1.2 channels in isolated ventricular myocytes. This association is specific for AKAP15 because neither mAKAP nor yotiao, which interact with the RyR (43, 49) and KNCQ1-KNCE1 potassium channel (50), respectively, was detected in CaV1.2 channel complexes. Disruption of PKA anchoring to CaV1.2 channels via AKAP15 using competing peptides markedly inhibits PKA regulation of CaV1.2 currents in ventricular myocytes. Our results identify a conserved LZ motif in the C terminus of the CaV1 family of Ca2+ channels that directly anchors an AKAP15-PKA signaling complex to CaV1.2 channels and is necessary for efficient β-adrenergic regulation of Ca2+ currents in ventricular myocytes.
The importance of PKA anchoring through the association with AKAPs for Ca2+ channel regulation was first established for skeletal muscle Ca2+ channels (36, 37). In skeletal muscle, AKAP15 has been identified as the anchoring protein that targets PKA to CaV1.1 channels (38), and PKA-dependent potentiation of Ca2+ channel activity in skeletal muscle cells is effectively blocked by peptides that disrupt the CaV1.1-AKAP15 interaction (39, 44). In contrast to cardiac and skeletal muscle, PKA is associated with CaV1.2 channels via the microtubule-associated protein MAP2B in the brain (42). In addition, AKAP79/150 is also expressed in the brain and has been implicated in PKA-independent trafficking of CaV1.2 channels (51). Because AKAP15 and CaV1.2 channels are both present in the dendrites and cell bodies of brain neurons (52, 53), it is possible that AKAP15 may be involved in PKA regulation of CaV1.2 channels in neurons as well. Moreover, because CaV1.3 and CaV1.4 channels also contain the conserved AKAP15-binding domain, regulation of these channels in neurons, endocrine cells, cardiac pacemaker cells, retinal rods and cones, and auditory hair cells may also involve the direct targeting of PKA by AKAP15. Anchoring of an AKAP15-PKA-signaling complex to the C terminus of the CaV1 family of Ca2+ channels via LZ motifs may define a general mechanism for the precise targeting of PKA for regulation of L-type Ca2+ currents.
Molecular Mechanism of PKA Regulation of CaV1.2 Channels. Despite much effort, it has not been possible to fully reconstitute β-adrenergic regulation or PKA regulation of holomeric CaV1.2 channels in nonmuscle cells (e.g., refs. 54-56). Small increments in CaV1.2 channel activity have been observed, but typically <10% of the increases in ICa that were observed in cardiac myocytes. Our present results reveal new complexity in PKA regulation of CaV1.2 channels. PKA anchoring by AKAP15 is required for regulation, and its interaction site is in the distal C terminus that is proteolytically cleaved in vivo. Effective regulation apparently requires continued association of the distal C-terminal domain, AKAP15, and PKA with the CaV1.2 channel. Other essential regulatory proteins may also be associated with this domain. Effective reconstitution of PKA regulation of CaV1.2 channels may therefore require reestablishment of these protein-protein interactions in nonmuscle cells.
Acknowledgments
This research was supported by National Institutes of Health Research Grant P01 HL44948 (to W.A.C.) and American Heart Association Grant 0330303N (to J.T.H.).
Abbreviations: PKA, protein kinase A; AKAP, A kinase-anchoring protein; LZ, leucine zipper; ABD, AKAP15-binding domain.
References
- 1.Reuter, H. (1979) Annu. Rev. Physiol. 41 413-424. [DOI] [PubMed] [Google Scholar]
- 2.Bers, D. M. (2002) Nature 415 198-205. [DOI] [PubMed] [Google Scholar]
- 3.Reuter, H. (1967) J. Physiol. (London) 192 479-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Biol. 16 521-555. [DOI] [PubMed] [Google Scholar]
- 5.Mikami, A., Imoto, K., Tanabe, T., Niidome, T., Mori, Y., Takeshima, H., Narumiya, S. & Numa, S. (1989) Nature 340 230-233. [DOI] [PubMed] [Google Scholar]
- 6.Ertel, E. A., Campbell, K. P., Harpold, M. M., Hofmann, F., Mori, Y., Perez-Reyes, E., Schwartz, A., Snutch, T. P., Tanabe, T., Birnbaumer, L., et al. (2000) Neuron 25 533-535. [DOI] [PubMed] [Google Scholar]
- 7.Ellis, S. B., Williams, M. E., Ways, N. R., Brenner, R., Sharp, A. H., Leung, A. T., Campbell, K. P., McKenna, E., Koch, W. J., Hui, A., et al. (1988) Science 241 1661-1664. [DOI] [PubMed] [Google Scholar]
- 8.Ruth, P., Röhrkasten, A., Biel, M., Bosse, E., Regulla, S., Meyer, H. E., Flockerzi, V. & Hofmann, F. (1989) Science 245 1115-1118. [DOI] [PubMed] [Google Scholar]
- 9.Colecraft, H. M., Alseikhan, B., Takahashi, S. X., Chaudhuri, D., Mittman, S., Yegnasubramanian, V., Alvania, R. S., Johns, D. C., Marban, E. & Yue, D. T. (2002) J. Physiol. (London) 541 435-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Reyes, E., Castellano, A., Kim, H. S. B. P., Baggstrom, E., Lacerda, A. E., Wei, X. & Birnbaumer, L. (1992) J. Biol. Chem. 267 1792-1797. [PubMed] [Google Scholar]
- 11.Gao, T. Y., Puri, T. S., Gerhardstein, B. L., Chien, A. J., Green, R. D. & Hosey, M. M. (1997) J. Biol. Chem. 272 19401-19407. [DOI] [PubMed] [Google Scholar]
- 12.De Jongh, K. S., Merrick, D. K. & Catterall, W. A. (1989) Proc. Natl. Acad. Sci. USA 86 8585-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jongh, K. S., Warner, C., Colvin, A. A. & Catterall, W. A. (1991) Proc. Natl. Acad. Sci. USA 88 10778-10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jongh, K. S., Murphy, B. J., Colvin, A. A., Hell, J. W., Takahashi, M. & Catterall, W. A. (1996) Biochemistry 35 10392-10402. [DOI] [PubMed] [Google Scholar]
- 15.Schneider, T. & Hofmann, F. (1988) Eur. J. Biochem. 174 369-375. [DOI] [PubMed] [Google Scholar]
- 16.Gerhardstein, B. L., Gao, T., Bunemann, M., Puri, T. S., Adair, A., Ma, H. & Hosey, M. M. (2000) J. Biol. Chem. 275 8556-8563. [DOI] [PubMed] [Google Scholar]
- 17.Gao, T., Cuadra, A. E., Ma, H., Bunemann, M., Gerhardstein, B. L., Cheng, T., Eick, R. T. & Hosey, M. M. (2001) J. Biol. Chem. 276 21089-21097. [DOI] [PubMed] [Google Scholar]
- 18.Reuter, H. (1974) J. Physiol. (London) 242 429-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter, H. (1983) Nature 301 569-574. [DOI] [PubMed] [Google Scholar]
- 20.Tsien, R. W., Bean, B. P., Hess, P., Lansman, J. B., Nilius, B. & Nowycky, M. C. (1986) J. Mol. Cell. Cardiol. 18 691-710. [DOI] [PubMed] [Google Scholar]
- 21.Tsien, R. W. (1973) Nat. New Biol. 245 120-122. [DOI] [PubMed] [Google Scholar]
- 22.Osterrieder, W., Brum, G., Hescheler, J., Trautwein, W., Flockerzi, V. & Hofmann, F. (1982) Nature 298 576-578. [DOI] [PubMed] [Google Scholar]
- 23.McDonald, T. F., Pelzer, S., Trautwein, W. & Pelzer, D. J. (1994) Physiol. Rev. 74 365-507. [DOI] [PubMed] [Google Scholar]
- 24.Curtis, B. M. & Catterall, W. A. (1985) Proc. Natl. Acad. Sci. USA 82 2528-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flockerzi, V., Oeken, H.-J., Hofmann, F., Pelzer, D., Cavalie, A. & Trautwein, W. (1986) Nature 323 66-68. [DOI] [PubMed] [Google Scholar]
- 26.Hell, J. W., Yokoyama, C. T., Wong, S. T., Warner, C., Snutch, T. P. & Catterall, W. A. (1993) J. Biol. Chem. 268 19451-19457. [PubMed] [Google Scholar]
- 27.Haase, H., Bartel, S., Karczewski, P., Morano, I. & Krause, E. G. (1996) Mol. Cell. Biochem. 163-164, 99-106. [DOI] [PubMed] [Google Scholar]
- 28.Puri, T. S., Gerhardstein, B. L., Zhao, X. L., Ladner, M. B. & Hosey, M. M. (1997) Biochemistry 36 9605-9615. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida, A., Takahashi, M., Nishimura, S., Takeshima, H. & Kokubun, S. (1992) FEBS Lett. 309 343-349. [DOI] [PubMed] [Google Scholar]
- 30.Sculptoreanu, A., Rotman, E., Takahashi, M., Scheuer, T. & Catterall, W. A. (1993) Proc. Natl. Acad. Sci. USA 90 10135-10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao, T., Yatani, A., Dell'Acqua, M. L., Sako, H., Green, S. A., Dascal, N., Scott, J. D. & Hosey, M. M. (1997) Neuron 19 185-196. [DOI] [PubMed] [Google Scholar]
- 32.Rubin, C. S. (1994) Biochim. Biophys. Acta 1224 467-479. [PubMed] [Google Scholar]
- 33.Scott, J. D. & McCartney, S. (1994) Mol. Endocrinol. 8 5-11. [DOI] [PubMed] [Google Scholar]
- 34.Dell'Acqua, M. L. & Scott, J. D. (1997) J. Biol. Chem. 272 12881-12884. [DOI] [PubMed] [Google Scholar]
- 35.Gray, P. C., Scott, J. D. & Catterall, W. A. (1998) Curr. Opin. Neurobiol. 8 330-334. [DOI] [PubMed] [Google Scholar]
- 36.Johnson, B. D., Scheuer, T. & Catterall, W. A. (1994) Proc. Natl. Acad. Sci. USA 91 11492-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson, B. D., Brousal, J. P., Peterson, B. Z., Gallombardo, P. A., Hockerman, G. H., Lai, Y., Scheuer, T. & Catterall, W. A. (1997) J. Neurosci. 17 1243-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray, P. C., Tibbs, V. C., Catterall, W. A. & Murphy, B. J. (1997) J. Biol. Chem. 272 6297-6302. [DOI] [PubMed] [Google Scholar]
- 39.Gray, P. C., Johnson, B. D., Westenbroek, R. E., Hays, L. G., Yates, I. J., Scheuer, T., Catterall, W. A. & Murphy, B. J. (1998) Neuron 20 1017-1026. [DOI] [PubMed] [Google Scholar]
- 40.Fraser, I. D. C., Tavalin, S. J., Lester, L. B., Langeberg, L. K., Westphal, A. M., Dean, R. A., Marrion, N. V. & Scott, J. D. (1998) EMBO J. 17 2261-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trotter, K. W., Fraser, I. D., Scott, G. K. Stutts, M. J., Scott, J. D. & Milgram, S. L. (1999) J. Cell Biol. 147 1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davare, M. A., Dong, F., Rubin, C. S. & Hell, J. W. (1999) J. Biol. Chem. 274 30280-30287. [DOI] [PubMed] [Google Scholar]
- 43.Marx, S. O., Reiken, S., Hisamatsu, Y., Gaburjakova, M., Gaburjakova, J., Yang, Y. M., Rosemblit, N. & Marks, A. R. (2001) J. Cell Biol. 153 699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulme, J. T., Ahn, M., Hauschka, S. D., Scheuer, T. & Catterall, W. A. (2002) J. Biol. Chem. 277 4079-4087. [DOI] [PubMed] [Google Scholar]
- 45.Westenbroek, R. E., Hell, J. W., Warner, C., Dubel, S. J., Snutch, T. P. & Catterall, W. A. (1992) Neuron 9 1099-1115. [DOI] [PubMed] [Google Scholar]
- 46.Hulme, J. T. & Orchard, C. H. (2000) Am. J. Physiol. 278 H50-H59. [DOI] [PubMed] [Google Scholar]
- 47.Yang, J., Drazba, J. A., Ferguson, D. G. & Bond, M. (1998) J. Cell Biol. 142 511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carr, D. W., Hausken, Z. E., Fraser, I. C. D., Stofko-Hahn, R. E. & Scott, J. D. (1992) J. Biol. Chem. 267 13376-13382. [PubMed] [Google Scholar]
- 49.Dodge, K. L., Khouangsathiene, S., Kapiloff, M. S., Mouton, R., Hill, E. V., Houslay, M. D., Langeberg, L. K. & Scott, J. D. (2001) EMBO J. 20 1921-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marx, S. O., Kurokawa, J., Reiken, S., Motoike, H., D'Armiento, J., Marks, A. R. & Kass, R. S. (2002) Science 295 496-499. [DOI] [PubMed] [Google Scholar]
- 51.Altier, C., Dubel, S. J., Barrere, C., Jarvis, S. E., Stotz, S. C., Spaetgens, R. L., Scott, J. D., Cornet, V., De Waard, M., Zamponi, G. W., et al. (2002) J. Biol. Chem. 277 33598-33603. [DOI] [PubMed] [Google Scholar]
- 52.Hell, J. W., Westenbroek, R. E., Warner, C., Ahlijanian, M. K., Prystay, W., Gilbert, M. M., Snutch, T. P. & Catterall, W. A. (1993) J. Cell Biol. 123 949-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cantrell, A. R., Tibbs, V. C., Westenbroek, R. E., Scheuer, T. & Catterall, W. A. (1999) J. Neurosci. 19 RC21, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer-Lahat, D., Lotan, I., Biel, M., Flockerzi, V., Hofman, F. & Dascal, N. (1994) Recept. Channels 2 215-226. [PubMed] [Google Scholar]
- 55.Zong, X., Schreieck, J., Mehrke, G., Welling, A., Schuster, A., Bosse, E., Flockerzi, V. & Hofmann, F. (1995) Pflügers Arch. 430 340-347. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Reyes, E., Yuan, W., Wei, X. & Bers, D. M. (1994) FEBS Lett. 342 119-123. [DOI] [PubMed] [Google Scholar]