Abstract
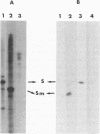
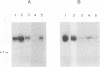
The transcriptase associated with Germiston virus was assayed in an in vitro reaction in which transcription was coupled to translation by adding reticulocyte lysate under the appropriate salt conditions. When analyzed in polyacrylamide gels, the major transcripts migrated like authentic S mRNAs and possessed 12- to 18-base-long nontemplated 5' extensions similar to the 5' end of viral mRNAs. These transcripts were functional for the synthesis of at least proteins N and NSS. When translation was inhibited by adding protein synthesis inhibitors such as puromycin, cycloheximide, and anisomycin, a drastic inhibitory effect was observed on the synthesis of the complete S mRNA transcripts. However, initiation and part of the elongation process were still active, since short and incomplete RNA molecules with RNA primers at their 5' ends were synthesized. On the other hand, we found that edeine, another inhibitor of protein synthesis, stimulated not only synthesis of S mRNAs but also that of the full-length S cRNAs. Taking into account the mode of action of this antibiotic, we discuss the results, which emphasize the crucial role of active ribosomes during bunyavirus transcription and confirm the observations reported on La Crosse virions. Moreover, we showed that the RNA transcripts synthesized in a transcription-translation reaction were capped and that most of them have acquired the 5' terminal sequences of the alpha- or beta-globin mRNA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle F. E. Structure-function relationship of 5' non-coding sequence of rabbit alpha- and beta-globin mRNA. Nature. 1977 May 19;267(5608):279–281. doi: 10.1038/267279a0. [DOI] [PubMed] [Google Scholar]
- Bellocq C., Kolakofsky D. Translational requirement for La Crosse virus S-mRNA synthesis: a possible mechanism. J Virol. 1987 Dec;61(12):3960–3967. doi: 10.1128/jvi.61.12.3960-3967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocq C., Raju R., Patterson J., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vitro studies. J Virol. 1987 Jan;61(1):87–95. doi: 10.1128/jvi.61.1.87-95.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M. Bunyaviridae: genome organization and replication strategies. Adv Virus Res. 1991;40:235–275. doi: 10.1016/s0065-3527(08)60281-x. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Studies on lumbo virus replication. I. RNA-dependent RNA polymerase associated with virions. Virology. 1976 Jan;69(1):258–264. doi: 10.1016/0042-6822(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Pardigon N., Vialat P., Gerbaud S., Girard M. Characterization of the 5' and 3' ends of viral messenger RNAs isolated from BHK21 cells infected with Germiston virus (Bunyavirus). Virology. 1990 Mar;175(1):50–58. doi: 10.1016/0042-6822(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Vialat P., Girard M., Pardigon N. A transcript from the S segment of the Germiston bunyavirus is uncapped and codes for the nucleoprotein and a nonstructural protein. J Virol. 1984 Mar;49(3):717–723. doi: 10.1128/jvi.49.3.717-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collett M. S. Messenger RNA of the M segment RNA of Rift Valley fever virus. Virology. 1986 May;151(1):151–156. doi: 10.1016/0042-6822(86)90114-5. [DOI] [PubMed] [Google Scholar]
- De B. P., Galinski M. S., Banerjee A. K. Characterization of an in vitro system for the synthesis of mRNA from human parainfluenza virus type 3. J Virol. 1990 Mar;64(3):1135–1142. doi: 10.1128/jvi.64.3.1135-1142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M. Molecular biology of the Bunyaviridae. J Gen Virol. 1990 Mar;71(Pt 3):501–522. doi: 10.1099/0022-1317-71-3-501. [DOI] [PubMed] [Google Scholar]
- Elliott R. M. Nucleotide sequence analysis of the large (L) genomic RNA segment of Bunyamwera virus, the prototype of the family Bunyaviridae. Virology. 1989 Dec;173(2):426–436. doi: 10.1016/0042-6822(89)90555-2. [DOI] [PubMed] [Google Scholar]
- Endres M. J., Jacoby D. R., Janssen R. S., Gonzalez-Scarano F., Nathanson N. The large viral RNA segment of California serogroup bunyaviruses encodes the large viral protein. J Gen Virol. 1989 Jan;70(Pt 1):223–228. doi: 10.1099/0022-1317-70-1-223. [DOI] [PubMed] [Google Scholar]
- Eshita Y., Ericson B., Romanowski V., Bishop D. H. Analyses of the mRNA transcription processes of snowshoe hare bunyavirus S and M RNA species. J Virol. 1985 Sep;55(3):681–689. doi: 10.1128/jvi.55.3.681-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J Virol. 1990 Dec;64(12):6196–6203. doi: 10.1128/jvi.64.12.6196-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud S., Vialat P., Pardigon N., Wychowski C., Girard M., Bouloy M. The S segment of the Germiston virus RNA genome can code for three proteins. Virus Res. 1987 Jul;8(1):1–13. doi: 10.1016/0168-1702(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Hacker D., Rochat S., Kolakofsky D. Anti-mRNAs in La Crosse bunyavirus-infected cells. J Virol. 1990 Oct;64(10):5051–5057. doi: 10.1128/jvi.64.10.5051-5057.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill V. M., Harmon S. A., Summers D. F. Stimulation of vesicular stomatitis virus in vitro RNA synthesis by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5410–5413. doi: 10.1073/pnas.83.15.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J Biol Chem. 1978 Sep 25;253(18):6568–6577. [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Pringle C. R., Elliott R. M. Nucleotide sequence of the Bunyamwera virus M RNA segment: conservation of structural features in the Bunyavirus glycoprotein gene product. Virology. 1986 Jan 15;148(1):1–14. doi: 10.1016/0042-6822(86)90398-3. [DOI] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardigon N., Vialat P., Gerbaud S., Girard M., Bouloy M. Nucleotide sequence of the M segment of Germiston virus: comparison of the M gene product of several bunyaviruses. Virus Res. 1988 Aug;11(1):73–85. doi: 10.1016/0168-1702(88)90068-8. [DOI] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Abraham G. Identification of four complementary RNA species in Akabane virus-infected cells. J Virol. 1983 Sep;47(3):452–462. doi: 10.1128/jvi.47.3.452-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Inhibitors of protein synthesis inhibit both La Crosse virus S-mRNA and S genome syntheses in vivo. Virus Res. 1986 Jul;5(1):1–9. doi: 10.1016/0168-1702(86)90061-4. [DOI] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1987 Jan;61(1):96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987 Mar;61(3):667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Kolakofsky D. The translational requirement for complete La Crosse virus mRNA synthesis is cell-type dependent. J Virol. 1989 Dec;63(12):5159–5165. doi: 10.1128/jvi.63.12.5159-5165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Pettersson R. F. Uukuniemi virus contains an RNA polymerase. J Virol. 1975 Dec;16(6):1420–1425. doi: 10.1128/jvi.16.6.1420-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Fujinami R. S. Characterization of in vitro transcription and transcriptional products of measles virus. J Virol. 1987 Nov;61(11):3381–3387. doi: 10.1128/jvi.61.11.3381-3387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C. S., Dalrymple J. M. Analysis of Hantaan virus RNA: evidence for a new genus of bunyaviridae. Virology. 1983 Dec;131(2):482–491. doi: 10.1016/0042-6822(83)90514-7. [DOI] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]