Abstract
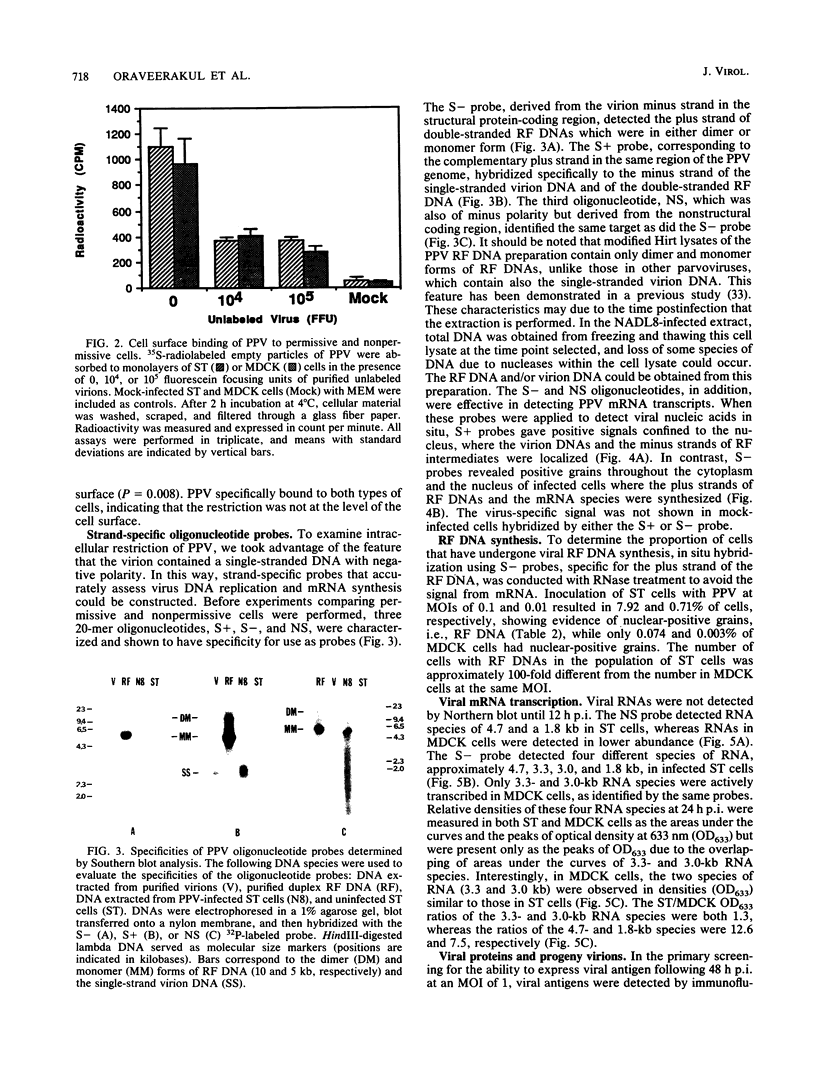
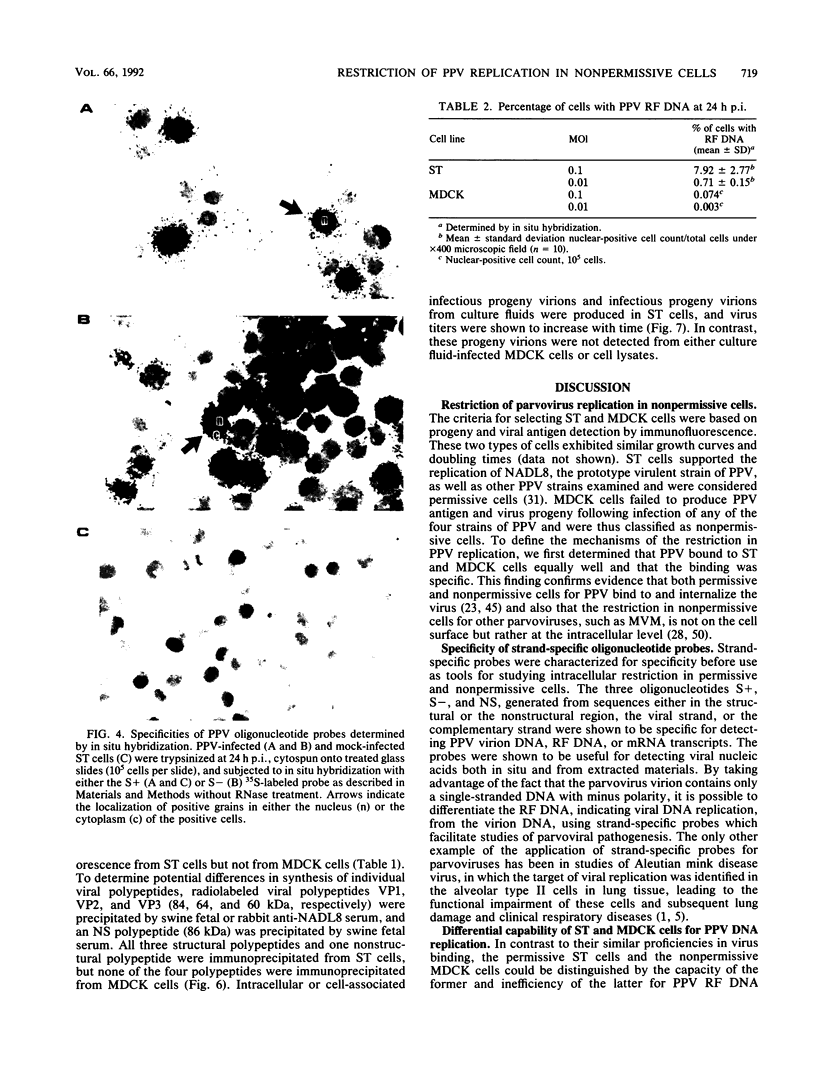
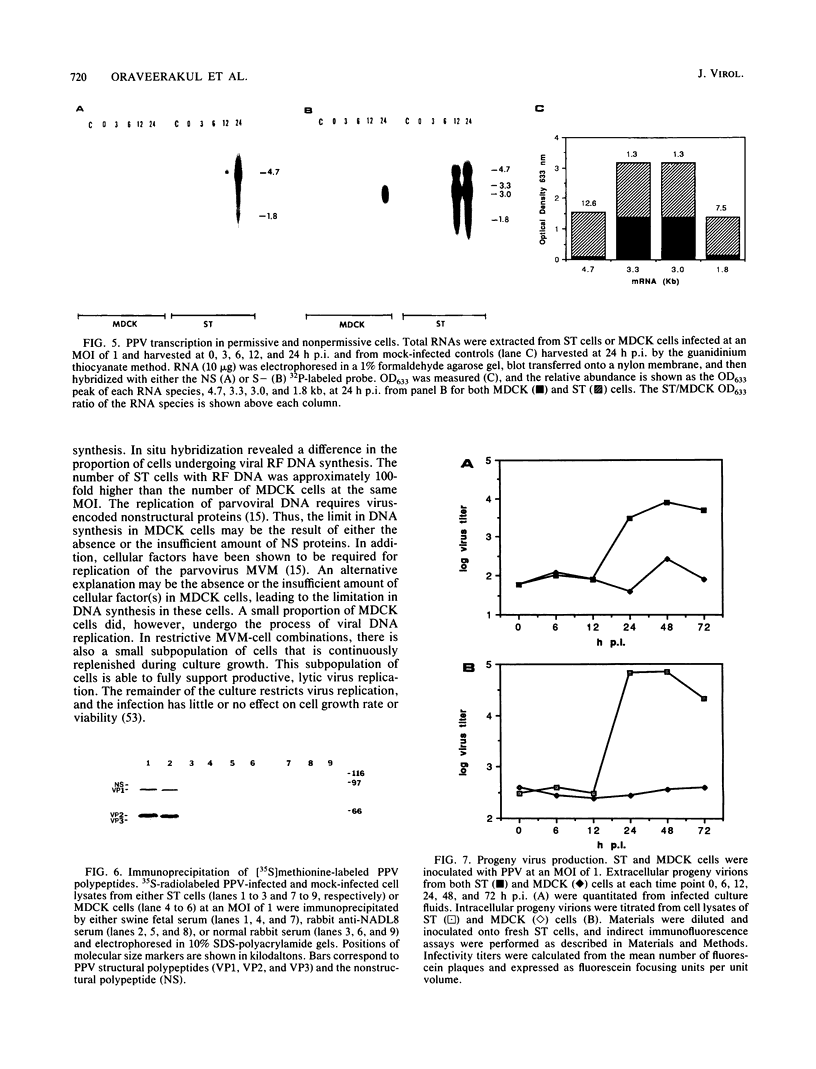
Swine testicle (ST) cells and Madin-Darby canine kidney (MDCK) cells differ in their ability to support replication of porcine parvovirus (PPV). Viral replication events in ST cells, a permissive cell type, and MDCK cells, a nonpermissive cell type, were compared in an attempt to elucidate putative mechanisms of restrictive virus replication. Radiolabeled PPV bound to the cell surface of both cell types equally well and the binding was shown to be PPV specific, indicating that the restriction was not at the cell surface level. In contrast, profound differences in intracellular events in PPV replication were observed between these two cell types. Synthesis of viral DNA was limited in MDCK cells in that the percentage of cells with replicative-form DNA as determined by strand-specific probe in situ hybridization was approximately 100-fold lower in MDCK cells than in ST cells at the same multiplicity of infection. Northern (RNA) blot analysis, using oligonucleotide probes derived from both structural and nonstructural protein-coding regions of the PPV genome, revealed four PPV mRNA transcripts from infected ST cells. Comparatively, RNA species from the structural protein coding region were actively transcribed in MDCK cells, but synthesis of RNA species from the nonstructural protein coding region was negligible. Immunoprecipitation of viral polypeptides revealed the three characteristic structural polypeptides, VP1, VP2, and VP3, along with the nonstructural polypeptide, NS-1 from ST cells. In contrast, neither viral structural or nonstructural polypeptides nor progeny virions were produced from MDCK cells. The data suggest that mechanisms controlling permissiveness of cells to PPV infection are associated with the level of viral DNA replication, RNA transcription, and viral antigen expression but not absorption to the cell surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonietti J. P., Sahli R., Beard P., Hirt B. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J Virol. 1988 Feb;62(2):552–557. doi: 10.1128/jvi.62.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel M. J., Scott F. W., Carmichael L. E. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Vet Rec. 1979 Aug 25;105(8):156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Legendre D., Avalosse B., Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990 Feb;174(2):576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chen Y. Q., Tuynder M. C., Cornelis J. J., Boukamp P., Fusenig N. E., Rommelaere J. Sensitization of human keratinocytes to killing by parvovirus H-1 takes place during their malignant transformation but does not require them to be tumorigenic. Carcinogenesis. 1989 Jan;10(1):163–167. doi: 10.1093/carcin/10.1.163. [DOI] [PubMed] [Google Scholar]
- Chen Y. Q., de Foresta F., Hertoghs J., Avalosse B. L., Cornelis J. J., Rommelaere J. Selective killing of simian virus 40-transformed human fibroblasts by parvovirus H-1. Cancer Res. 1986 Jul;46(7):3574–3579. [PubMed] [Google Scholar]
- Choi C. S., Molitor T. W., Joo H. S., Gunther R. Pathogenicity of a skin isolate of porcine parvovirus in swine fetuses. Vet Microbiol. 1987 Oct;15(1-2):19–29. doi: 10.1016/0378-1135(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Choi C. S., Molitor T. W., Joo H. S. Inhibition of porcine parvovirus replication by empty virus particles. Arch Virol. 1987;96(1-2):75–87. doi: 10.1007/BF01310991. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Chen Y. Q., Spruyt N., Duponchel N., Cotmore S. F., Tattersall P., Rommelaere J. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J Virol. 1990 Jun;64(6):2537–2544. doi: 10.1128/jvi.64.6.2537-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Dea S., Elazhary M. A., Martineau G. P., Vaillancourt J. Parvovirus-like particles associated with diarrhea in unweaned piglets. Can J Comp Med. 1985 Jul;49(3):343–345. [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Beard P., Antonietti J. P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988 Oct;69(Pt 10):2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- Duhamel G. E., Bargar T. W., Schmitt B. J., Molitor T. W., Lu W. Identification of parvovirus-like virus particles in intestinal crypt epithelial cells of pigs with diarrhea. J Vet Diagn Invest. 1991 Jan;3(1):96–98. doi: 10.1177/104063879100300126. [DOI] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Mapping of the fibrotropic and lymphotropic host range determinants of the parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2605–2613. doi: 10.1128/jvi.62.8.2605-2613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Harding M. J., Molitor T. W. Porcine parvovirus: replication in and inhibition of selected cellular functions of swine alveolar macrophages and peripheral blood lymphocytes. Arch Virol. 1988;101(1-2):105–117. doi: 10.1007/BF01314655. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Donaldson-Wood C. R., Johnson R. H. Observations on the pathogenesis of porcine parvovirus infection. Arch Virol. 1976;51(1-2):123–129. doi: 10.1007/BF01317841. [DOI] [PubMed] [Google Scholar]
- Kresse J. I., Taylor W. D., Stewart W. W., Eernisse K. A. Parvovirus infection in pigs with necrotic and vesicle-like lesions. Vet Microbiol. 1985 Dec;10(6):525–531. doi: 10.1016/0378-1135(85)90061-6. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeling W. L., Cutlip R. C. Pathogenesis of in utero infection: experimental infection of five-week-old porcine fetuses with porcine parvovirus. Am J Vet Res. 1975 Aug;36(08):1173–1177. [PubMed] [Google Scholar]
- Miller R. A., Ward D. C., Ruddle F. H. Embryonal carcinoma cells (and their somatic cell hybrids) are resistant to infection by the murine parvovirus MVM, which does infect other teratocarcinoma-derived cell lines. J Cell Physiol. 1977 Jun;91(3):393–401. doi: 10.1002/jcp.1040910309. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Identification and characterization of a porcine parvovirus nonstructural polypeptide. J Virol. 1985 Sep;55(3):554–559. doi: 10.1128/jvi.55.3.554-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. KBSH parvovirus: comparison with porcine parvovirus. J Virol. 1985 Aug;55(2):257–263. doi: 10.1128/jvi.55.2.257-263.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus DNA: characterization of the genomic and replicative form DNA of two virus isolates. Virology. 1984 Sep;137(2):241–254. doi: 10.1016/0042-6822(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus: virus purification and structural and antigenic properties of virion polypeptides. J Virol. 1983 Feb;45(2):842–854. doi: 10.1128/jvi.45.2.842-854.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Oraveerakul K., Zhang Q. Q., Choi C. S., Ludemann L. R. Polymerase chain reaction (PCR) amplification for the detection of porcine parvovirus. J Virol Methods. 1991 May;32(2-3):201–211. doi: 10.1016/0166-0934(91)90051-z. [DOI] [PubMed] [Google Scholar]
- Oraveerakul K., Choi C. S., Molitor T. W. Detection of porcine parvovirus using nonradioactive nucleic acid hybridization. J Vet Diagn Invest. 1990 Apr;2(2):85–91. doi: 10.1177/104063879000200201. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz A. I., Manclús J. J., Díaz-Aroca E., Casal J. I. Porcine parvovirus: DNA sequence and genome organization. J Gen Virol. 1989 Oct;70(Pt 10):2541–2553. doi: 10.1099/0022-1317-70-10-2541. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridpath J. F., Mengeling W. L. Uptake of porcine parvovirus into host and nonhost cells suggests host specificity is determined by intracellular factors. Virus Res. 1988 Apr;10(1):17–27. doi: 10.1016/0168-1702(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Yasuhara H., Matsui O., Hirahara T., Ohgitani T., Tanaka M., Kodama K., Nakai M., Sasaki N. Characterization of a parvovirus isolated from the diarrheic feces of a pig. Nihon Juigaku Zasshi. 1989 Apr;51(2):337–344. doi: 10.1292/jvms1939.51.337. [DOI] [PubMed] [Google Scholar]