Abstract
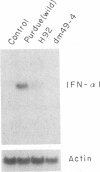
Transmissible gastroenteritis virus, an enteropathogenic coronavirus of swine, is a potent inducer of alpha interferon (IFN-alpha) both in vitro and in vivo. Previous studies have shown that virus-infected fixed cells or viral suspensions were able to induce an early and strong IFN-alpha synthesis by naive lymphocytes. Two monoclonal antibodies directed against the viral membrane glycoprotein M (29,000; formerly E1) were found to markedly inhibit virus-induced IFN production, thus assigning to M protein a potential effector role in this phenomenon (B. Charley and H. Laude, J. Virol. 62:8-11, 1988). The present report describes the selection and characterization of a collection of 125 mutant viruses which escaped complement-mediated neutralization by two IFN induction-blocking anti-M protein monoclonal antibodies. Two of these mutants, designated H92 and dm49-4, were found to exhibit a markedly reduced interferogenic activity. IFN synthesis by lymphocytes incubated with purified suspensions of these mutants was 30- to 300-fold lower than that of the parental virus. The transcription of IFN-alpha genes following induction by each mutant was decreased proportionally, as evidenced by Northern (RNA) blot analysis. The sequence of the M gene of 20 complement-mediated neutralization-resistant mutants, including the 2 defective mutants, was determined by direct sequencing of genome RNA. Thirteen distinct amino acid changes were predicted, all located at positions 6 to 22 from the N terminus of the mature M protein and within the putative ectodomain of the molecule. Two substitutions, Thr-17 to Ile and Ser-19 to Pro, were assumed to generate the defective phenotypes of mutants dm49-4 and H92, respectively. The alteration of an Asn-Ser-Thr sequence in dm49-4 virus led to the synthesis of an M protein devoid of a glycan side chain, which suggests a possible involvement of this structure in IFN induction. Overall, these data supported the view that an interferogenic determinant resides in the N-terminal, exposed part of the molecule and provided further evidence for the direct role of M protein in the induction of IFN-alpha by transmissible gastroenteritis virus. The acronym VIP (viral interferogenic protein) is proposed as a designation for this particular class of proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capobianchi M. R., Facchini J., Di Marco P., Antonelli G., Dianzani F. Induction of alpha interferon by membrane interaction between viral surface and peripheral blood mononuclear cells. Proc Soc Exp Biol Med. 1985 Apr;178(4):551–556. doi: 10.3181/00379727-178-42041. [DOI] [PubMed] [Google Scholar]
- Capobianchi M. R., Lorino G., Lun M. T., Mancini C., Di Marco P., Dianzani F. Membrane interactions involved in the induction of interferon-alpha by Mycoplasma pneumoniae. Antiviral Res. 1987 Oct;8(3):115–124. doi: 10.1016/0166-3542(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Cederblad B., Alm G. V. Infrequent but efficient interferon-alpha-producing human mononuclear leukocytes induced by herpes simplex virus in vitro studied by immuno-plaque and limiting dilution assays. J Interferon Res. 1990 Feb;10(1):65–73. doi: 10.1089/jir.1990.10.65. [DOI] [PubMed] [Google Scholar]
- Charley B., Laude H. Induction of alpha interferon by transmissible gastroenteritis coronavirus: role of transmembrane glycoprotein E1. J Virol. 1988 Jan;62(1):8–11. doi: 10.1128/jvi.62.1.8-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Lavenant L. Characterization of blood mononuclear cells producing IFN alpha following induction by coronavirus-infected cells (porcine transmissible gastroenteritis virus). Res Immunol. 1990 Feb;141(2):141–151. doi: 10.1016/0923-2494(90)90133-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Lavenant L., Delmas B. Glycosylation is required for coronavirus TGEV to induce an efficient production of IFN alpha by blood mononuclear cells. Scand J Immunol. 1991 Apr;33(4):435–440. doi: 10.1111/j.1365-3083.1991.tb01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Delmas B., Laude H. Carbohydrate-induced conformational changes strongly modulate the antigenicity of coronavirus TGEV glycoproteins S and M. Virus Res. 1991 Jul;20(2):107–120. doi: 10.1016/0168-1702(91)90103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Feldman M., Mendelsohn M., Curl S., Lopez C. Human mononuclear cells which produce interferon-alpha during NK(HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J Leukoc Biol. 1988 Apr;43(4):323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- Gobl A. E., Funa K., Alm G. V. Different induction patterns of mRNA for IFN-alpha and -beta in human mononuclear leukocytes after in vitro stimulation with herpes simplex virus-infected fibroblasts and Sendai virus. J Immunol. 1988 May 15;140(10):3605–3609. [PubMed] [Google Scholar]
- Ito Y., Nishiyama Y., Shimokata K., Nagata I., Takeyama H., Kunii A. The mechanism of interferon induction in mouse spleen cells stimulated with HVJ. Virology. 1978 Jul 1;88(1):128–137. doi: 10.1016/0042-6822(78)90116-2. [DOI] [PubMed] [Google Scholar]
- Kurane I., Ennis F. A. Induction of interferon alpha from human lymphocytes by autologous, dengue virus-infected monocytes. J Exp Med. 1987 Oct 1;166(4):999–1010. doi: 10.1084/jem.166.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Meager A., Ennis F. A. Induction of interferon alpha and gamma from human lymphocytes by dengue virus-infected cells. J Gen Virol. 1986 Aug;67(Pt 8):1653–1661. doi: 10.1099/0022-1317-67-8-1653. [DOI] [PubMed] [Google Scholar]
- La Bonnardiere C., Laude H. High interferon titer in newborn pig intestine during experimentally induced viral enteritis. Infect Immun. 1981 Apr;32(1):28–31. doi: 10.1128/iai.32.1.28-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Chapsal J. M., Gelfi J., Labiau S., Grosclaude J. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J Gen Virol. 1986 Jan;67(Pt 1):119–130. doi: 10.1099/0022-1317-67-1-119. [DOI] [PubMed] [Google Scholar]
- Laude H., Rasschaert D., Huet J. C. Sequence and N-terminal processing of the transmembrane protein E1 of the coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jun;68(Pt 6):1687–1693. doi: 10.1099/0022-1317-68-6-1687. [DOI] [PubMed] [Google Scholar]
- Lebon P., Bernard A., Boumsell L. Identification de populations lymphocytaires productrices d'interféron-alpha par des anticorps monoclonaux. C R Seances Acad Sci III. 1982 Sep 20;295(2):79–82. [PubMed] [Google Scholar]
- Lebon P., Commoy-Chevalier M. J., Robert-Galliot B., Chany C. Different mechanisms for alpha and beta interferon induction. Virology. 1982 Jun;119(2):504–507. doi: 10.1016/0042-6822(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Lebon P. Inhibition of herpes simplex virus type 1-induced interferon synthesis by monoclonal antibodies against viral glycoprotein D and by lysosomotropic drugs. J Gen Virol. 1985 Dec;66(Pt 12):2781–2786. doi: 10.1099/0022-1317-66-12-2781. [DOI] [PubMed] [Google Scholar]
- Lefevre F., La Bonnardiere C. Molecular cloning and sequencing of a gene encoding biologically active porcine alpha-interferon. J Interferon Res. 1986 Aug;6(4):349–360. doi: 10.1089/jir.1986.6.349. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Bandyopadhyay S., Miller D. S., Starr S. E. Cooperation between CD16(Leu-11b)+ NK cells and HLA-DR+ cells in natural killing of herpesvirus-infected fibroblasts. J Immunol. 1987 Oct 15;139(8):2799–2802. [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4(3):120–137. [PubMed] [Google Scholar]
- Peter H. H., Dallügge H., Zawatzky R., Euler S., Leibold W., Kirchner H. Human peripheral null lymphocytes. II. Producers of type-1 interferon upon stimulation with tumor cells, Herpes simplex virus and Corynebacterium parvum. Eur J Immunol. 1980 Jul;10(7):547–555. doi: 10.1002/eji.1830100712. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Liu L. L., Mowshowitz S. L. Interferon production by cultured murine splenocytes in response to influenza virus-infected cells. J Interferon Res. 1984;4(1):81–89. doi: 10.1089/jir.1984.4.81. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Rose J. K. Coronavirus E1 glycoprotein expressed from cloned cDNA localizes in the Golgi region. J Virol. 1987 Jun;61(6):2042–2045. doi: 10.1128/jvi.61.6.2042-2045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Welling G. W., Welling-Wester S., Niesters H. G., Lenstra J. A., Van der Zeijst B. A. Predicted membrane topology of the coronavirus protein E1. Biochemistry. 1986 Mar 25;25(6):1335–1339. doi: 10.1021/bi00354a022. [DOI] [PubMed] [Google Scholar]
- Rönnblom L., Cederblad B., Sandberg K., Alm G. V. Determination of herpes simplex virus-induced alpha interferon-secreting human blood leucocytes by a filter immuno-plaque assay. Scand J Immunol. 1988 Feb;27(2):165–170. doi: 10.1111/j.1365-3083.1988.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Gobl A. E., Funa K., Alm G. V. Characterization of the blood mononuclear leucocytes producing alpha interferon after stimulation with herpes simplex virus in vitro, by means of combined immunohistochemical staining and in situ RNA-RNA hybridization. Scand J Immunol. 1989 Jun;29(6):651–658. doi: 10.1111/j.1365-3083.1989.tb01169.x. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Matsson P., Alm G. V. A distinct population of nonphagocytic and low level CD4+ null lymphocytes produce IFN-alpha after stimulation by herpes simplex virus-infected cells. J Immunol. 1990 Aug 1;145(3):1015–1020. [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]