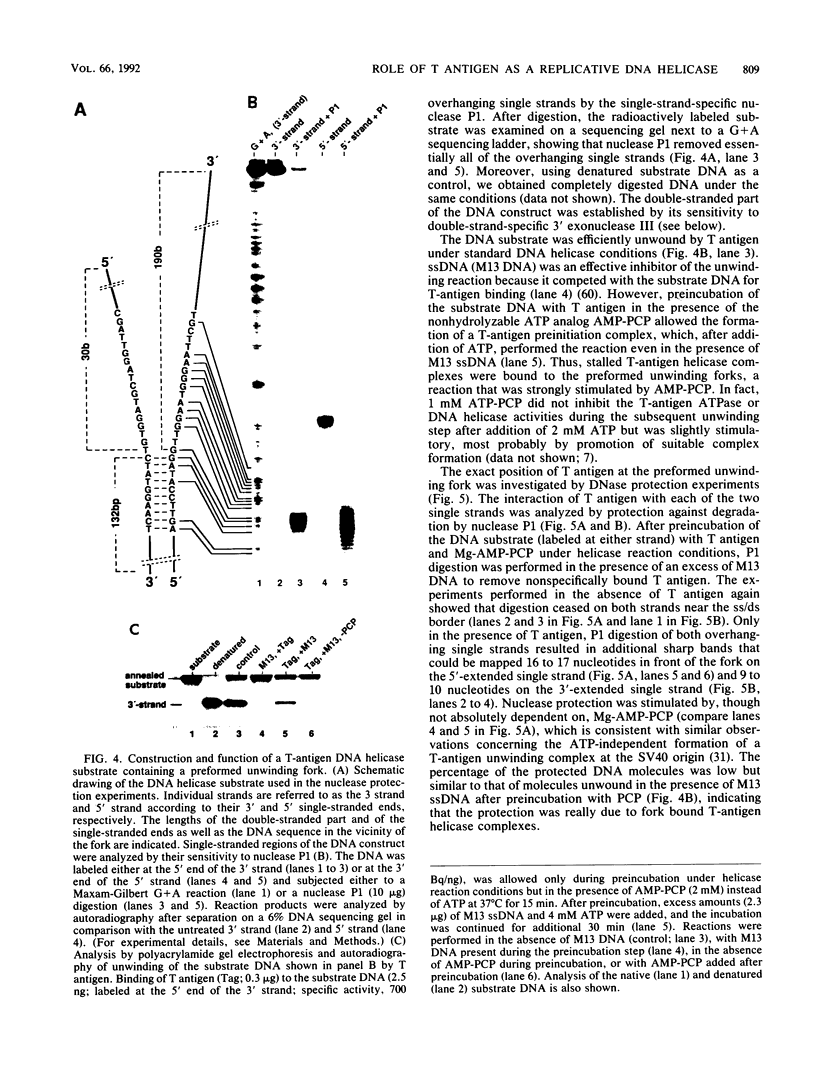
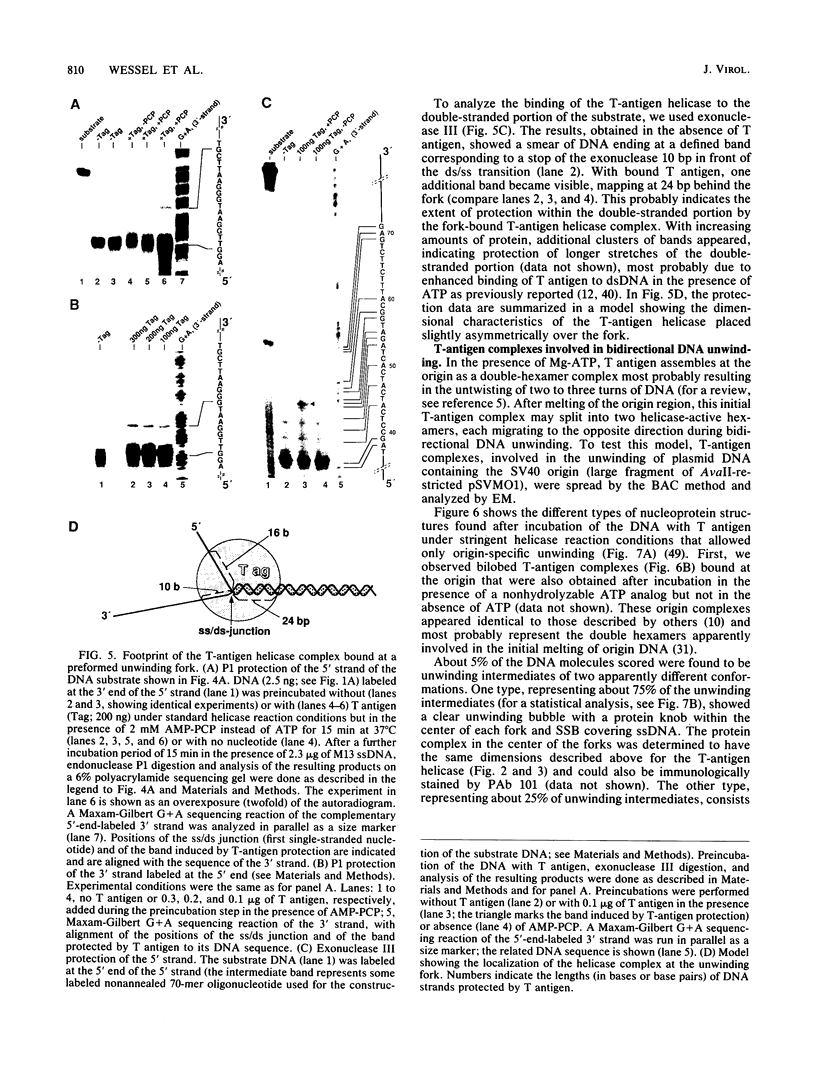
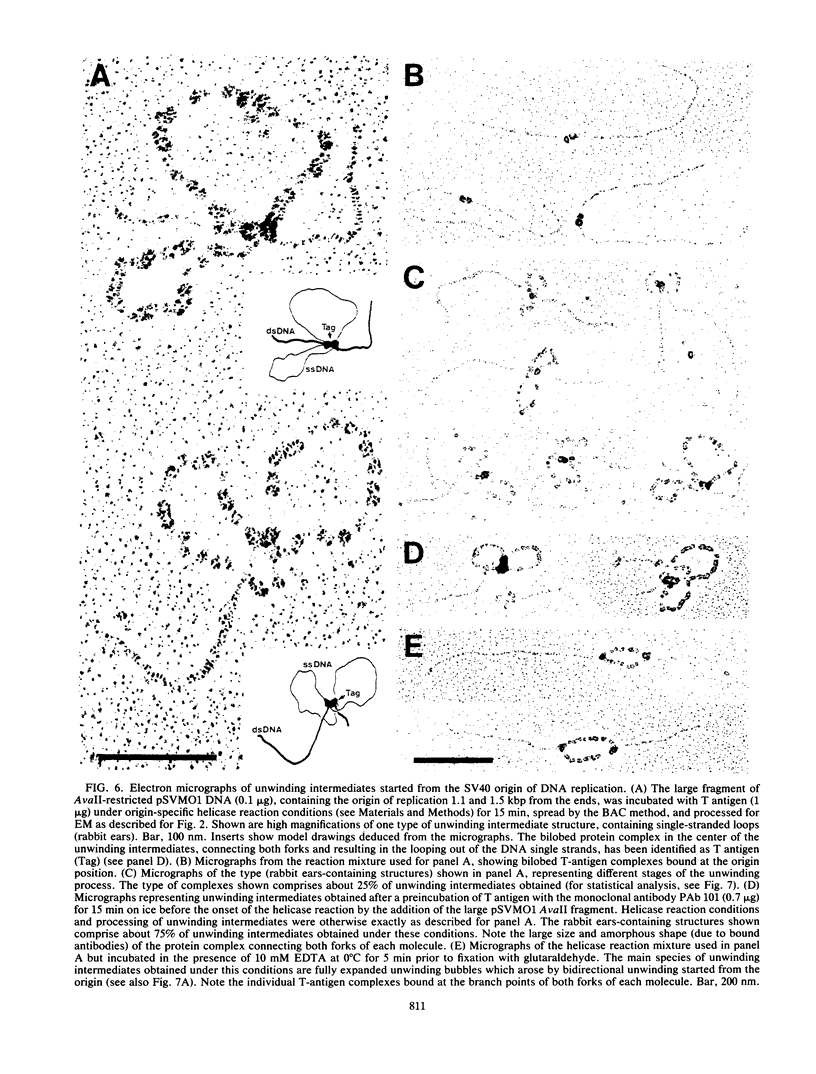
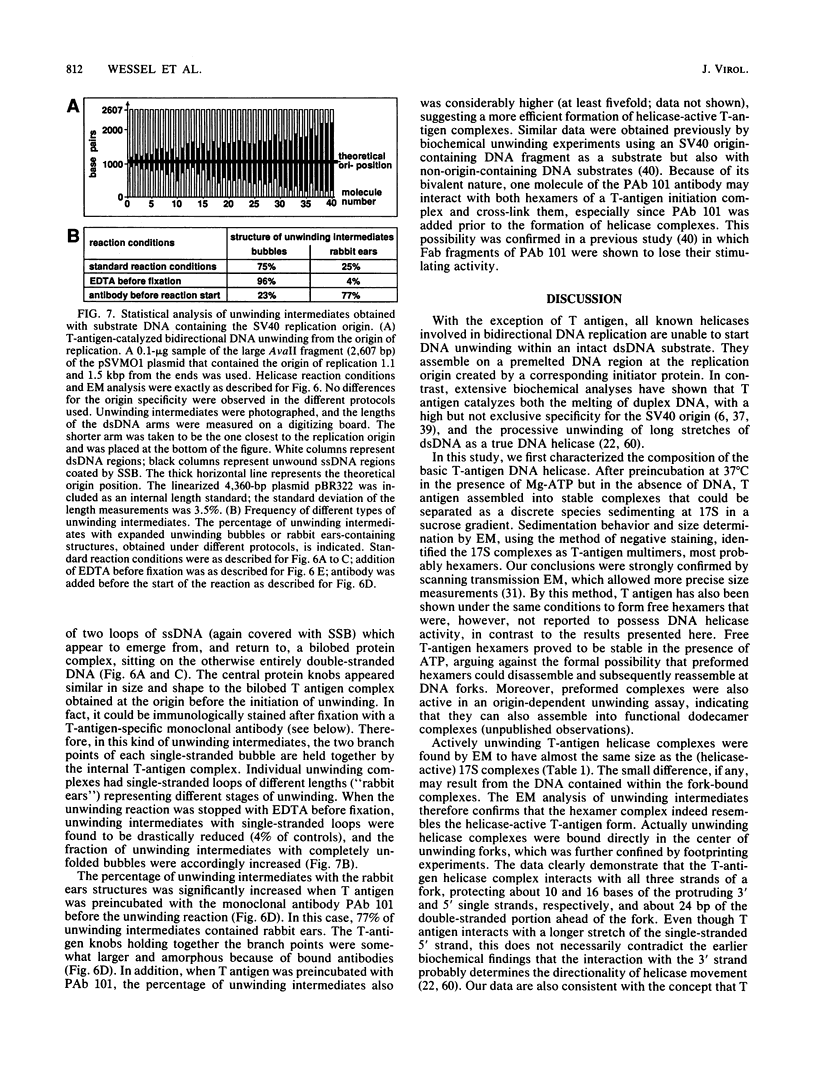
Abstract
The role of simian virus 40 (SV40) large tumor antigen (T antigen) as a DNA helicase at the replication fork was studied. We found that a T-antigen hexamer complex acts during the unidirectional unwinding of appropriate DNA substrates and is localized directly in the center of the fork, contacting the adjacent double strand as well as the emerging single strands. When bidirectional DNA unwinding, initiated at the viral origin of DNA replication, was analyzed, a larger T-antigen complex that is simultaneously active at both branch points of an unwinding bubble was observed. The size and shape of this helicase complex imply that the T-antigen dodecamer complex, assembled at the origin and active in the localized melting of duplex DNA, is subsequently also used to continue DNA unwinding bidirectionally. Then, however, the dodecamer complex does not split into two hexamer subunits that track along the DNA; rather, the DNA is threaded through the intact complex, with the concomitant extrusion of single-stranded loops.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati B. B., Gasser S. M. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell. 1988 Sep 23;54(7):967–978. doi: 10.1016/0092-8674(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Amati B., Gasser S. M. Drosophila scaffold-attached regions bind nuclear scaffolds and can function as ARS elements in both budding and fission yeasts. Mol Cell Biol. 1990 Oct;10(10):5442–5454. doi: 10.1128/mcb.10.10.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati B., Pick L., Laroche T., Gasser S. M. Nuclear scaffold attachment stimulates, but is not essential for ARS activity in Saccharomyces cerevisiae: analysis of the Drosophila ftz SAR. EMBO J. 1990 Dec;9(12):4007–4016. doi: 10.1002/j.1460-2075.1990.tb07622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Yasuda S., Kornberg A. Mechanism of dnaB protein action. I. Crystallization and properties of dnaB protein, an essential replication protein in Escherichia coli. J Biol Chem. 1981 May 25;256(10):5247–5252. [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. K. Activation of ATPase activity of simian virus 40 large T antigen by the covalent affinity analog of ATP, fluorosulfonylbenzoyl 5'-adenosine. J Virol. 1990 Oct;64(10):4939–4947. doi: 10.1128/jvi.64.10.4939-4947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Dombroski A. J., Platt T. Transcription termination factor rho is an RNA-DNA helicase. Cell. 1987 Mar 27;48(6):945–952. doi: 10.1016/0092-8674(87)90703-3. [DOI] [PubMed] [Google Scholar]
- Brun C., Dang Q., Miassod R. Studies of an 800-kilobase DNA stretch of the Drosophila X chromosome: comapping of a subclass of scaffold-attached regions with sequences able to replicate autonomously in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Oct;10(10):5455–5463. doi: 10.1128/mcb.10.10.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Dodson M., Echols H., Hurwitz J. ATP-dependent formation of a specialized nucleoprotein structure by simian virus 40 (SV40) large tumor antigen at the SV40 replication origin. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8981–8985. doi: 10.1073/pnas.84.24.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S. P., Tegtmeyer P. ATP enhances the binding of simian virus 40 large T antigen to the origin of replication. J Virol. 1987 Dec;61(12):3649–3654. doi: 10.1128/jvi.61.12.3649-3654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Baur C. P., Koff A., Tegtmeyer P. Domain structure of the simian virus 40 core origin of replication. Mol Cell Biol. 1986 May;6(5):1663–1670. doi: 10.1128/mcb.6.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., Dean F. B., Bullock P., Echols H., Hurwitz J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science. 1987 Nov 13;238(4829):964–967. doi: 10.1126/science.2823389. [DOI] [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache G., Razin S. V., Rzeszowska-Wolny J., Moreau J., Targa F. R., Scherrer K. Mapping of structural and transcription-related matrix attachment sites in the alpha-globin gene domain of avian erythroblasts and erythrocytes. Mol Cell Biol. 1990 Oct;10(10):5349–5358. doi: 10.1128/mcb.10.10.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger L. R., Richardson J. P. Stabilization of the hexameric form of Escherichia coli protein rho under ATP hydrolysis conditions. J Mol Biol. 1982 Mar 25;156(1):203–219. doi: 10.1016/0022-2836(82)90467-3. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gayama S., Kataoka T., Wachi M., Tamura G., Nagai K. Periodic formation of the oriC complex of Escherichia coli. EMBO J. 1990 Nov;9(11):3761–3765. doi: 10.1002/j.1460-2075.1990.tb07589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz G. S., Dean F. B., Hurwitz J., Matson S. W. The unwinding of duplex regions in DNA by the simian virus 40 large tumor antigen-associated DNA helicase activity. J Biol Chem. 1988 Jan 5;263(1):383–392. [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper F., Florentin Y., Puvion E. Large T antigen-rich viral DNA replication loci in SV40-infected monkey kidney cells. Exp Cell Res. 1985 Dec;161(2):434–444. doi: 10.1016/0014-4827(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Hough P. V., Mastrangelo I. A., Wall J. S., Hainfeld J. F., Sawadogo M., Roeder R. G. The gene-specific initiation factor USF (upstream stimulatory factor) bound at the adenovirus type 2 major late promoter: mass and three-dimensional structure. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4826–4830. doi: 10.1073/pnas.84.14.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Su R. T. Association of viral and plasmid DNA with the nuclear matrix during productive infection. Biochim Biophys Acta. 1987 Oct 9;910(1):52–62. doi: 10.1016/0167-4781(87)90094-7. [DOI] [PubMed] [Google Scholar]
- Kornberg A. DNA replication. J Biol Chem. 1988 Jan 5;263(1):1–4. [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber G., Parsons R., Tegtmeyer P. The zinc finger region of simian virus 40 large T antigen. J Virol. 1989 Jan;63(1):94–100. doi: 10.1128/jvi.63.1.94-100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu H., Berezney R. Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol. 1989 Jan;108(1):1–11. doi: 10.1083/jcb.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R., Anderson M. E., Tegtmeyer P. Three domains in the simian virus 40 core origin orchestrate the binding, melting, and DNA helicase activities of T antigen. J Virol. 1990 Feb;64(2):509–518. doi: 10.1128/jvi.64.2.509-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Gill S. J. Stability of protein structure and hydrophobic interaction. Adv Protein Chem. 1988;39:191–234. doi: 10.1016/s0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. M. Simian virus 40 (SV40) large tumor antigen causes stepwise changes in SV40 origin structure during initiation of DNA replication. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3939–3943. doi: 10.1073/pnas.86.11.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Wessel R., Stahl H. SV40 T antigen catalyzed duplex DNA unwinding. Curr Top Microbiol Immunol. 1989;144:37–45. doi: 10.1007/978-3-642-74578-2_5. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Wessel R., Stahl H. Sequence independent duplex DNA opening reaction catalysed by SV40 large tumor antigen. Nucleic Acids Res. 1989 Jan 11;17(1):93–106. doi: 10.1093/nar/17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedner G., Wessel R., Scheffner M., Stahl H. Renaturation and DNA looping promoted by the SV40 large tumour antigen. EMBO J. 1990 Sep;9(9):2937–2943. doi: 10.1002/j.1460-2075.1990.tb07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J Virol. 1987 Nov;61(11):3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Structural topography of simian virus 40 DNA replication. J Virol. 1991 May;65(5):2578–2588. doi: 10.1128/jvi.65.5.2578-2588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Bauer M., Knippers R. The simian-virus-40 large-tumor antigen in replicating viral chromatin. A salt-resistant protein-DNA interaction. Eur J Biochem. 1983 Jul 15;134(1):55–61. doi: 10.1111/j.1432-1033.1983.tb07530.x. [DOI] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Zentgraf H., Knippers R. A large-tumor-antigen-specific monoclonal antibody inhibits DNA replication of simian virus 40 minichromosomes in an in vitro elongation system. J Virol. 1985 May;54(2):473–482. doi: 10.1128/jvi.54.2.473-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Proctor G. N. Two major replicating simian virus 40 chromosome classes. Synchronous replication fork movement is associated with bound large T antigen during elongation. J Biol Chem. 1987 May 5;262(13):6339–6349. [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. B., Gralla J. D. Simian virus 40 associates with nuclear superstructures at early times of infection. J Virol. 1987 Mar;61(3):748–754. doi: 10.1128/jvi.61.3.748-754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel R., Müller H., Hoffmann-Berling H. Electron microscopy of DNA.helicase-I complexes in the act of strand separation. Eur J Biochem. 1990 Apr 30;189(2):277–285. doi: 10.1111/j.1432-1033.1990.tb15487.x. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Dröge P., Stahl H. Monoclonal antibodies as probes for a function of large T antigen during the elongation process of simian virus 40 DNA replication. J Virol. 1987 Feb;61(2):411–418. doi: 10.1128/jvi.61.2.411-418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiekowski M., Schwarz M. W., Stahl H. Simian virus 40 large T antigen DNA helicase. Characterization of the ATPase-dependent DNA unwinding activity and its substrate requirements. J Biol Chem. 1988 Jan 5;263(1):436–442. [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- van der Velden H. M., Wanka F. The nuclear matrix--its role in the spatial organization and replication of eukaryotic DNA. Mol Biol Rep. 1987;12(2):69–77. doi: 10.1007/BF00368873. [DOI] [PubMed] [Google Scholar]