Abstract
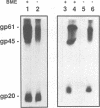
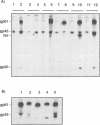
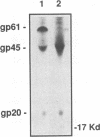
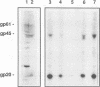
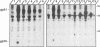
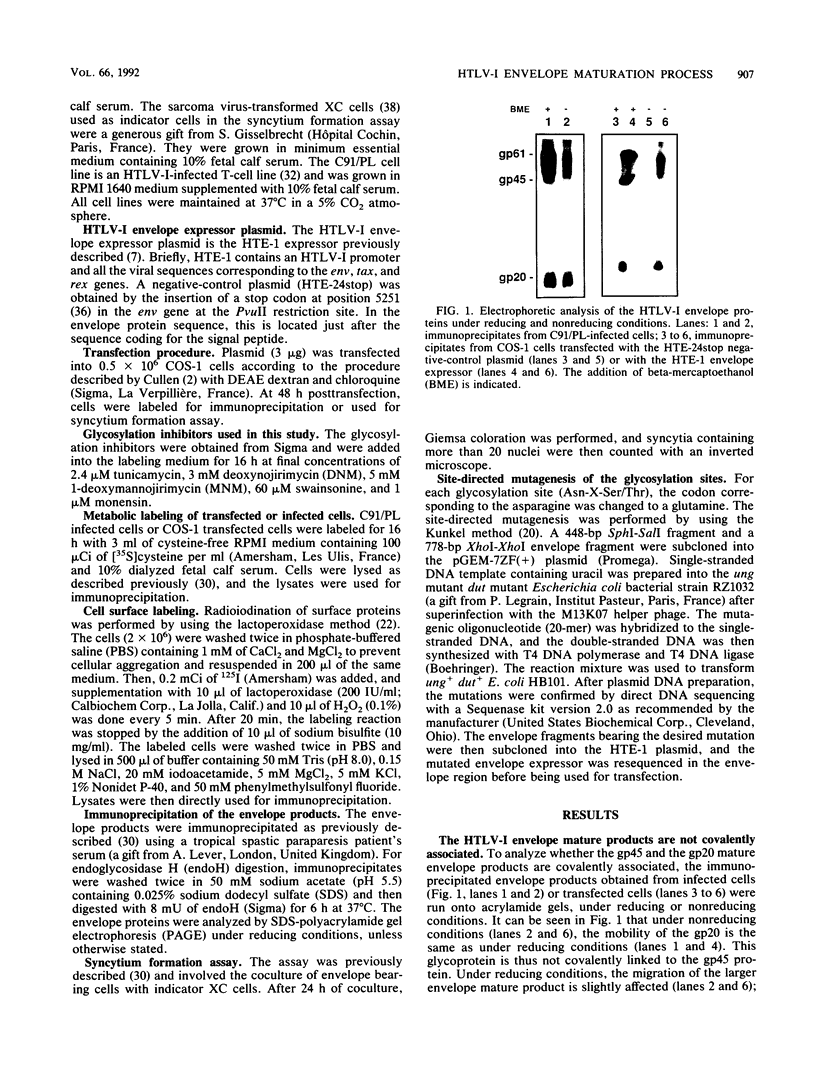
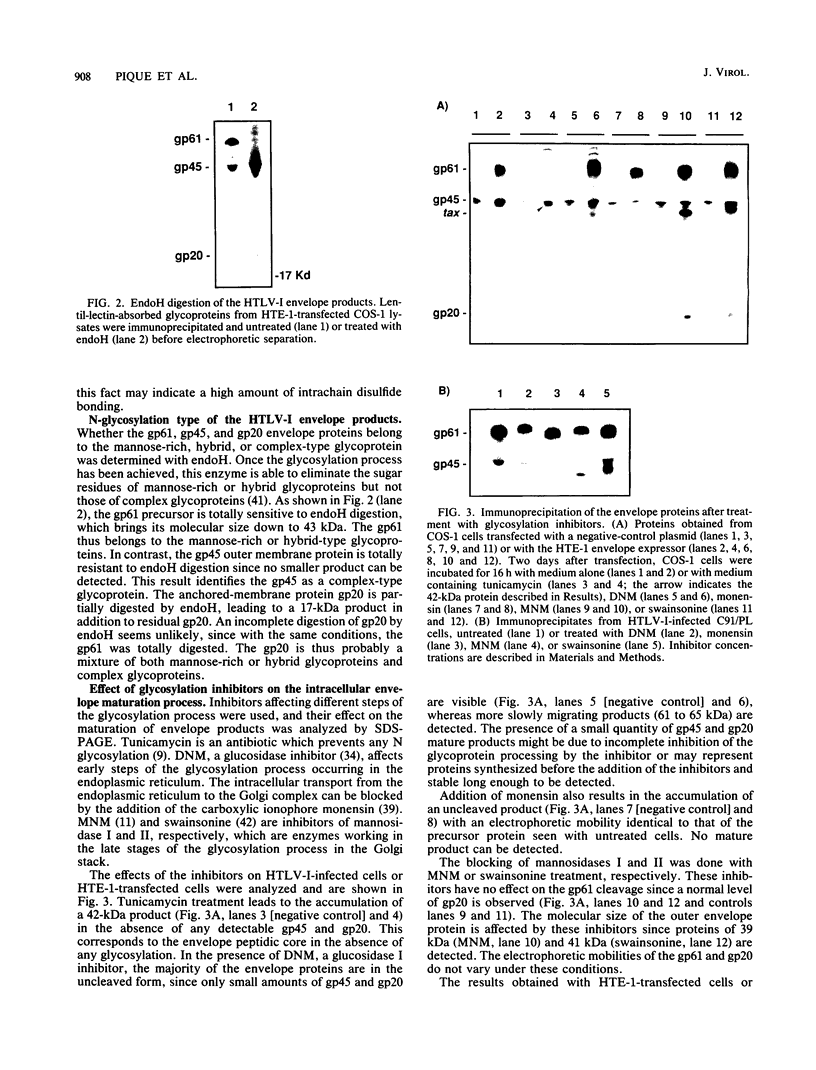
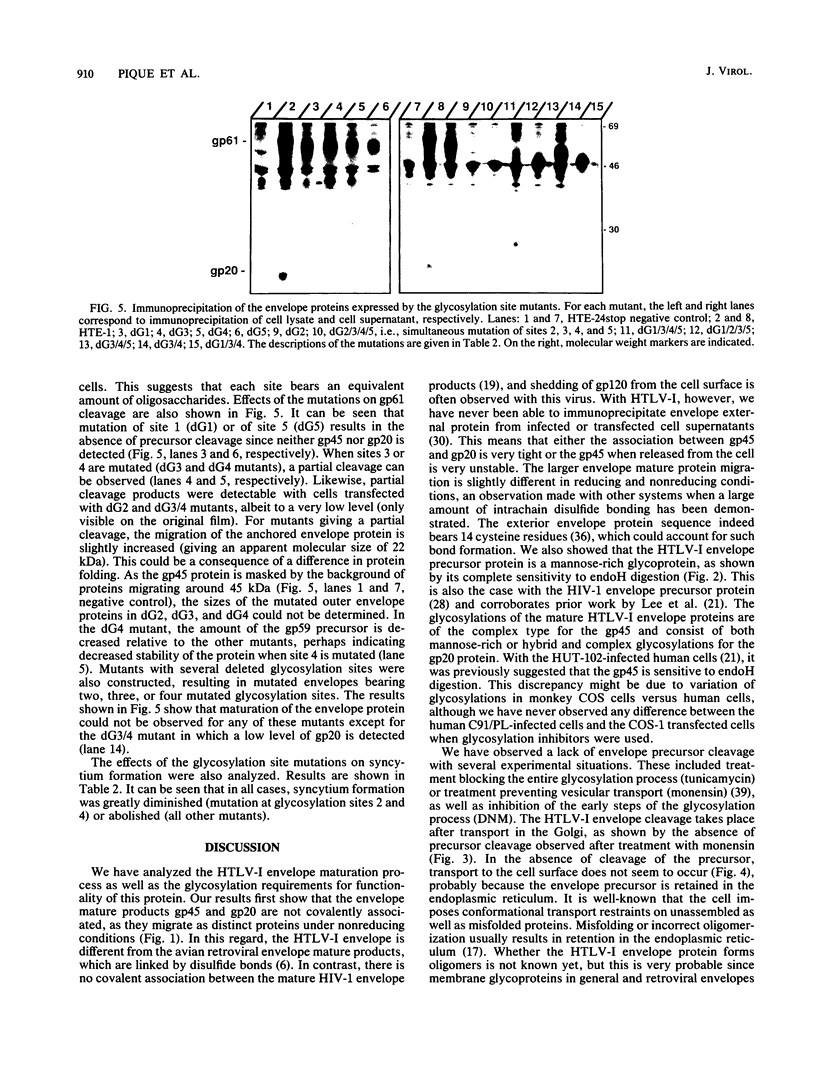
The human T-cell leukemia virus type I (HTLV-I) envelope protein is synthesized as a gp61 precursor product cleaved into two mature proteins, a gp45 exterior protein and a gp20 anchoring the envelope at the cell membrane. Using N-glycosylation inhibitors and site-directed mutagenesis of the potential glycosylation sites, we have studied the HTLV-I envelope intracellular maturation requirements for syncytium formation. We show here that experimental conditions resulting in the absence of precursor cleavage (tunicamycin, monensin treatments, and use of inhibitors of the reticulum steps of the N glycosylations) also result in no cell surface expression of envelope protein. The lack of syncytium formation observed in these cases is thus explained by incorrect intracellular transport. When the precursor is cleaved in the Golgi stack (no treatment or treatment with inhibitors of the Golgi steps of the N glycosylations), it is transported to the cell surface in all the cases examined. Syncytium formation is markedly reduced, however, when Golgi glycosylations are incorrect, which shows that the sugar moieties are involved in the envelope functions. Site-directed mutagenesis demonstrates that each of the five potential glycosylation sites is actually glycosylated. Glycosylation of sites 1 and 5 is required for normal maturation, whereas that of sites 2, 3, and 4 is dispensable. Glycosylation of each site, however, is required for normal syncytium formation. Altogether, the restraints exerted by the cell for the HTLV-I envelope to be transported and functional are very high, which might play a role in the observed conservation of the envelope amino acid sequence between various strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britt W. J., Vugler L. G. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J Virol. 1989 Jan;63(1):403–410. doi: 10.1128/jvi.63.1.403-410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Daenke S., Nightingale S., Cruickshank J. K., Bangham C. R. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990 Mar;64(3):1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. K., Lairmore M. D., Griffis K., Williams L. J., Villinger F., Quinn T. C., Brown C., Nzilambi, Sugimoto M., Araki S. Comparative analysis of nucleotide sequences of the partial envelope gene (5' domain) among human T lymphotropic virus type I (HTLV-I) isolates. Virology. 1991 May;182(1):413–419. doi: 10.1016/0042-6822(91)90692-5. [DOI] [PubMed] [Google Scholar]
- Dewar R. L., Vasudevachari M. B., Natarajan V., Salzman N. P. Biosynthesis and processing of human immunodeficiency virus type 1 envelope glycoproteins: effects of monensin on glycosylation and transport. J Virol. 1989 Jun;63(6):2452–2456. doi: 10.1128/jvi.63.6.2452-2456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokhelar M. C., Pickford H., Sodroski J., Haseltine W. A. HTLV-I p27rex regulates gag and env protein expression. J Acquir Immune Defic Syndr. 1989;2(5):431–440. [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gray G. S., White M., Bartman T., Mann D. Envelope gene sequence of HTLV-1 isolate MT-2 and its comparison with other HTLV-1 isolates. Virology. 1990 Jul;177(1):391–395. doi: 10.1016/0042-6822(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Hattori S., Kiyokawa T., Imagawa K., Shimizu F., Hashimura E., Seiki M., Yoshida M. Identification of gag and env gene products of human T-cell leukemia virus (HTLV). Virology. 1984 Jul 30;136(2):338–347. doi: 10.1016/0042-6822(84)90170-3. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Tsujimoto A., Shimotohno K. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int J Cancer. 1991 Feb 20;47(4):491–495. doi: 10.1002/ijc.2910470403. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Homma T., McLane M. F., Tachibana N., Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D. Primary structure and processing of gag and env gene products of human T-cell leukemia viruses HTLV-ICR and HTLV-IATK. Curr Top Microbiol Immunol. 1985;115:221–233. doi: 10.1007/978-3-642-70113-9_14. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Paine E., Garcia J., Philpott T. C., Shaw G., Ratner L. Limited sequence variation in human T-lymphotropic virus type 1 isolates from North American and African patients. Virology. 1991 May;182(1):111–123. doi: 10.1016/0042-6822(91)90654-t. [DOI] [PubMed] [Google Scholar]
- Pal R., Hoke G. M., Sarngadharan M. G. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3384–3388. doi: 10.1073/pnas.86.9.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Kalyanaraman V. S., Hoke G. M., Sarngadharan M. G. Processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1 in the presence of trimming glucosidase inhibitor deoxynojirimycin. Intervirology. 1989;30(1):27–35. doi: 10.1159/000150073. [DOI] [PubMed] [Google Scholar]
- Pique C., Tursz T., Dokhelar M. C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 1990 Dec;9(13):4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Schulz T. F., Calabrò M. L., Hoad J. G., Carrington C. V., Matutes E., Catovsky D., Weiss R. A. HTLV-1 envelope sequences from Brazil, the Caribbean, and Romania: clustering of sequences according to geographic origin and variability in an antibody epitope. Virology. 1991 Oct;184(2):483–491. doi: 10.1016/0042-6822(91)90418-b. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Wall J. S., Hainfeld J. F., Kaczorek M., Booy F. P., Trus B. L., Eiserling F. A., Steven A. C. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991 Jul;65(7):3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. E., Spiro R. C., Burns J. W., Buchmeier M. J. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology. 1990 Jul;177(1):175–183. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M. T., Chen I. S. Modes of transformation by the human T-cell leukemia viruses. Mol Biol Med. 1990 Feb;7(1):33–44. [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]