Abstract
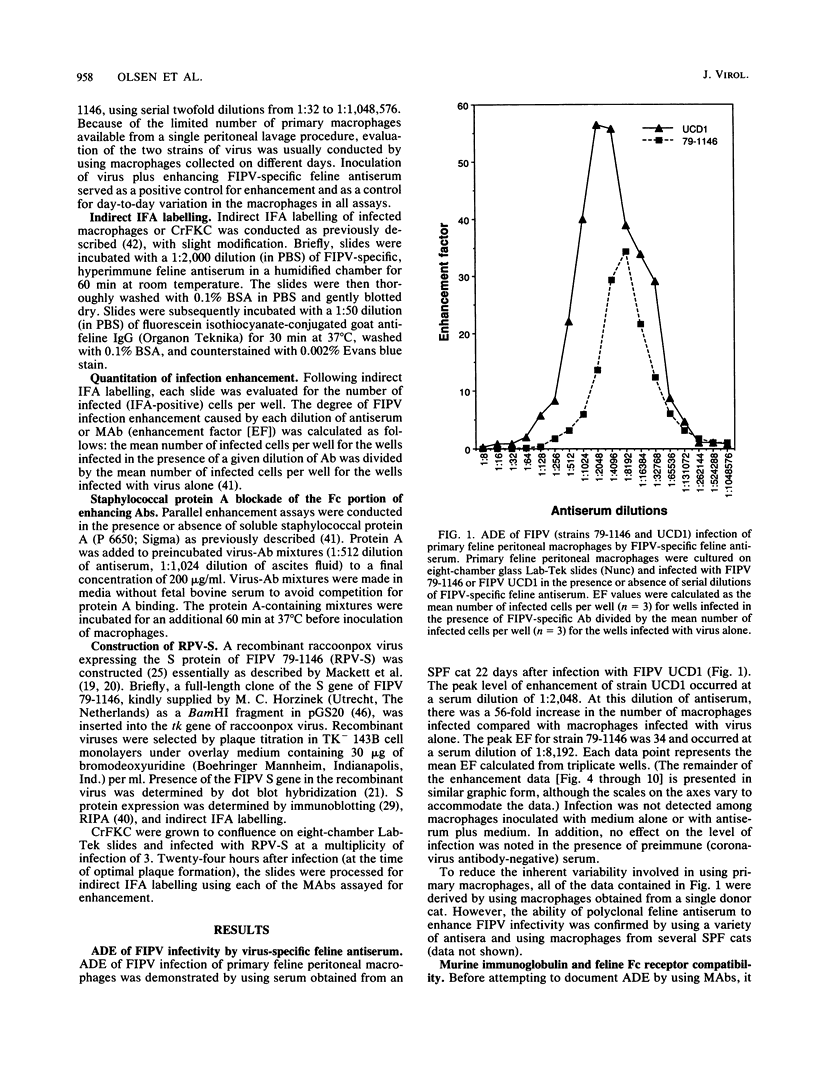
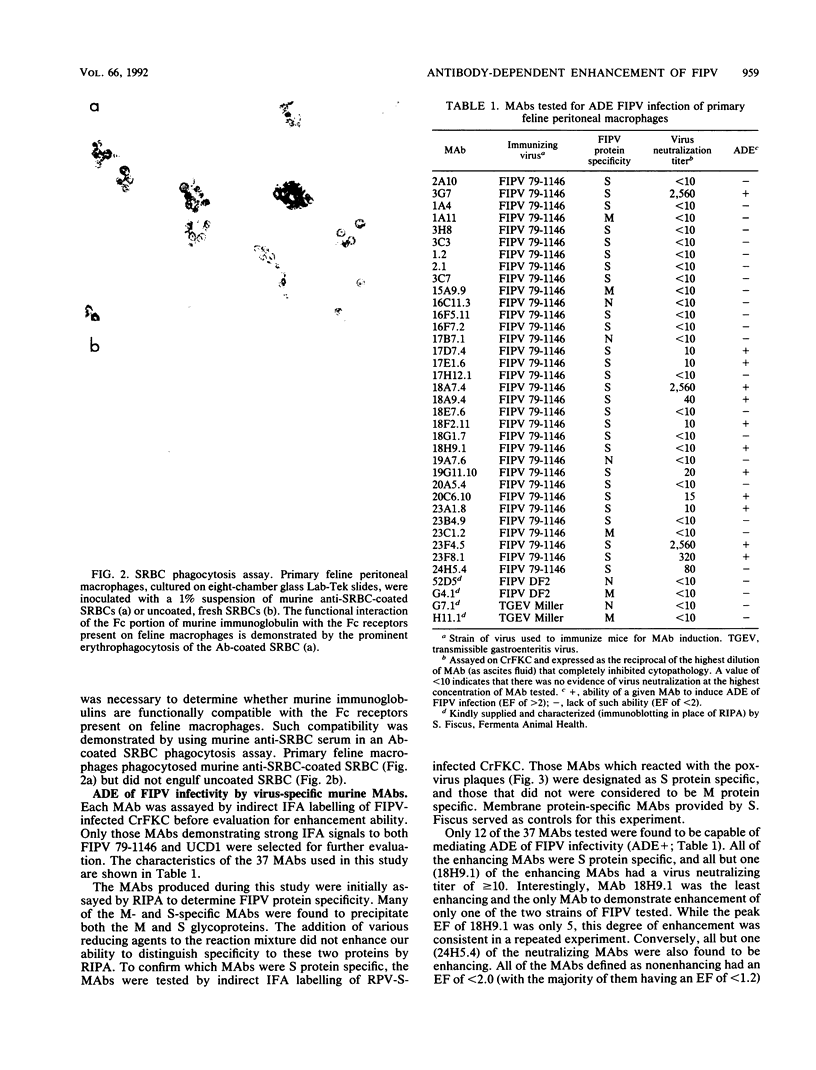
Antibody-dependent enhancement of virus infection is a process whereby virus-antibody complexes initiate infection of cells via Fc receptor-mediated endocytosis. We sought to investigate antibody-dependent enhancement of feline infectious peritonitis virus infection of primary feline peritoneal macrophages in vitro. Enhancement of infection was assessed, after indirect immunofluorescent-antibody labelling of infected cells, by determining the ratio between the number of cells infected in the presence and absence of virus-specific antibody. Infection enhancement was initially demonstrated by using heat-inactivated, virus-specific feline antiserum. Functional compatibility between murine immunoglobulin molecules and feline Fc receptors was demonstrated by using murine anti-sheep erythrocyte serum and an antibody-coated sheep erythrocyte phagocytosis assay. Thirty-seven murine monoclonal antibodies specific for the nucleocapsid, membrane, or spike proteins of feline infectious peritonitis virus or transmissible gastroenteritis virus were assayed for their ability to enhance the infectivity of feline infectious peritonitis virus. Infection enhancement was mediated by a subset of spike protein-specific monoclonal antibodies. A distinct correlation was seen between the ability of a monoclonal antibody to cause virus neutralization in a routine cell culture neutralization assay and its ability to mediate infection enhancement of macrophages. Infection enhancement was shown to be Fc receptor mediated by blockade of antibody-Fc receptor interaction using staphylococcal protein A. Our results are consistent with the hypothesis that antibody-dependent enhancement of feline infectious peritonitis virus infectivity is mediated by antibody directed against specific sites on the spike protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corapi W. V., Donis R. O., Dubovi E. J. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am J Vet Res. 1990 Sep;51(9):1388–1394. [PubMed] [Google Scholar]
- Fiscus S. A., Teramoto Y. A. Antigenic comparison of feline coronavirus isolates: evidence for markedly different peplomer glycoproteins. J Virol. 1987 Aug;61(8):2607–2613. doi: 10.1128/jvi.61.8.2607-2613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Keir H. M., Cash P. In vitro enhancement of respiratory syncytial virus infection of U937 cells by human sera. J Gen Virol. 1989 Jan;70(Pt 1):89–96. doi: 10.1099/0022-1317-70-1-89. [DOI] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature. 1986 May 15;321(6067):244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on viral fate. J Gen Virol. 1984 Aug;65(Pt 8):1261–1272. doi: 10.1099/0022-1317-65-8-1261. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Venkateshan C. N., Gentry M. K., Larsen L. K. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol. 1984 Mar;132(3):1529–1532. [PubMed] [Google Scholar]
- Henchal E. A., McCown J. M., Burke D. S., Seguin M. C., Brandt W. E. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985 Jan;34(1):162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- Homsy J., Meyer M., Levy J. A. Serum enhancement of human immunodeficiency virus (HIV) infection correlates with disease in HIV-infected individuals. J Virol. 1990 Apr;64(4):1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsy J., Meyer M., Tateno M., Clarkson S., Levy J. A. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science. 1989 Jun 16;244(4910):1357–1360. doi: 10.1126/science.2786647. [DOI] [PubMed] [Google Scholar]
- Hotta H., Wiharta A. S., Hotta S. Antibody-mediated enhancement of dengue virus infection in mouse macrophage cell lines, Mk1 and Mm1. Proc Soc Exp Biol Med. 1984 Mar;175(3):320–327. doi: 10.3181/00379727-175-41802. [DOI] [PubMed] [Google Scholar]
- Jacobse-Geels H. E., Daha M. R., Horzinek M. C. Antibody, immune complexes, and complement activity fluctuations in kittens with experimentally induced feline infectious peritonitis. Am J Vet Res. 1982 Apr;43(4):666–670. [PubMed] [Google Scholar]
- Jacobse-Geels H. E., Daha M. R., Horzinek M. C. Isolation and characterization of feline C3 and evidence for the immune complex pathogenesis of feline infectious peritonitis. J Immunol. 1980 Oct;125(4):1606–1610. [PubMed] [Google Scholar]
- Jolly P. E., Huso D. L., Sheffer D., Narayan O. Modulation of lentivirus replication by antibodies: Fc portion of immunoglobulin molecule is essential for enhancement of binding, internalization, and neutralization of visna virus in macrophages. J Virol. 1989 Apr;63(4):1811–1813. doi: 10.1128/jvi.63.4.1811-1813.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. A., Sands J. J., Porterfield J. S. Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1). J Gen Virol. 1984 Jun;65(Pt 6):1091–1093. doi: 10.1099/0022-1317-65-6-1091. [DOI] [PubMed] [Google Scholar]
- Krilov L. R., Anderson L. J., Marcoux L., Bonagura V. R., Wedgwood J. F. Antibody-mediated enhancement of respiratory syncytial virus infection in two monocyte/macrophage cell lines. J Infect Dis. 1989 Nov;160(5):777–782. doi: 10.1093/infdis/160.5.777. [DOI] [PubMed] [Google Scholar]
- Lai M. M. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D. M., Halstead S. B. Disease severity-related antigenic differences in dengue 2 strains detected by dengue 4 monoclonal antibodies. J Med Virol. 1987 Jun;22(2):169–174. doi: 10.1002/jmv.1890220208. [DOI] [PubMed] [Google Scholar]
- Morens D. M., Halstead S. B. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol. 1990 Dec;71(Pt 12):2909–2914. doi: 10.1099/0022-1317-71-12-2909. [DOI] [PubMed] [Google Scholar]
- Morens D. M., Venkateshan C. N., Halstead S. B. Dengue 4 virus monoclonal antibodies identify epitopes that mediate immune infection enhancement of dengue 2 viruses. J Gen Virol. 1987 Jan;68(Pt 1):91–98. doi: 10.1099/0022-1317-68-1-91. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Kurokawa M., Hayashi K., Niwayama S. Antibody-mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J Virol. 1988 Jan;62(1):20–26. doi: 10.1128/jvi.62.1.20-26.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H., Kurokawa M., Kuroki Y., Niwayama S. Infection enhancement of influenza A H1 subtype viruses in macrophage-like P388D1 cells by cross-reactive antibodies. J Med Virol. 1990 Apr;30(4):258–265. doi: 10.1002/jmv.1890300406. [DOI] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Weinberg R. L., Paoletti E. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5364–5368. doi: 10.1073/pnas.80.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J. S., Gordon S., Unkeless J. C., Porterfield J. S. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature. 1981 Jan 15;289(5794):189–191. doi: 10.1038/289189a0. [DOI] [PubMed] [Google Scholar]
- Petersen N. C., Boyle J. F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am J Vet Res. 1980 Jun;41(6):868–876. [PubMed] [Google Scholar]
- Porterfield J. S. Antibody-dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Kawamura T., Gorny M. K., Lake D., Xu J. Y., Matsumoto Y., Sugano T., Masuho Y., Mitchell W. M., Hersh E. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3185–3189. doi: 10.1073/pnas.87.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W. 17D yellow fever virus infection of P388D1 cells mediated by monoclonal antibodies: properties of the macrophage Fc receptor. J Gen Virol. 1983 Jun;64(Pt 6):1255–1262. doi: 10.1099/0022-1317-64-6-1255. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W. Antibody-mediated infection of macrophages and macrophage-like cell lines with 17D-yellow fever virus. J Med Virol. 1981;8(2):103–117. doi: 10.1002/jmv.1890080204. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W. Growth of 17D yellow fever virus in a macrophage-like cell line, U937: role of Fc and viral receptors in antibody-mediated infection. J Immunol. 1981 Aug;127(2):659–665. [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart C. A., Scott F. W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence. J Virol. 1989 Jan;63(1):436–440. doi: 10.1128/jvi.63.1.436-440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart C. A., Scott F. W. Isolation and identification of feline peritoneal macrophages for in vitro studies of coronavirus-macrophage interactions. J Leukoc Biol. 1988 Nov;44(5):319–328. doi: 10.1002/jlb.44.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Tuazon C. U., Ennis F. A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988 Oct 28;242(4878):580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- Vennema H., Heijnen L., Zijderveld A., Horzinek M. C., Spaan W. J. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J Virol. 1990 Jan;64(1):339–346. doi: 10.1128/jvi.64.1.339-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Groot R. J., Harbour D. A., Dalderup M., Gruffydd-Jones T., Horzinek M. C., Spaan W. J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990 Mar;64(3):1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. C., Scott F. W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4(2):175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. C., Scott F. W. Pathogenesis of feline infectious peritonitis: nature and development of viremia. Am J Vet Res. 1981 Mar;42(3):382–390. [PubMed] [Google Scholar]
- Weiss R. C., Scott F. W. Pathogenesis of feline infetious peritonitis: pathologic changes and immunofluorescence. Am J Vet Res. 1981 Dec;42(12):2036–2048. [PubMed] [Google Scholar]