Abstract
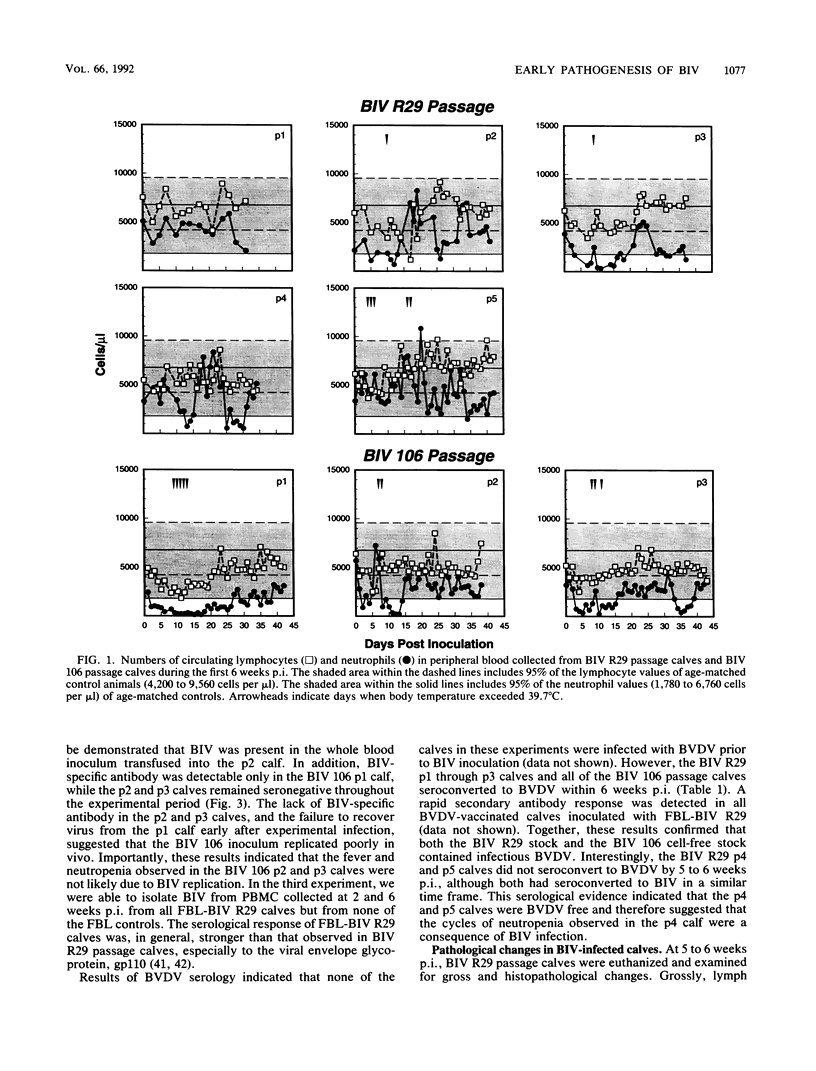
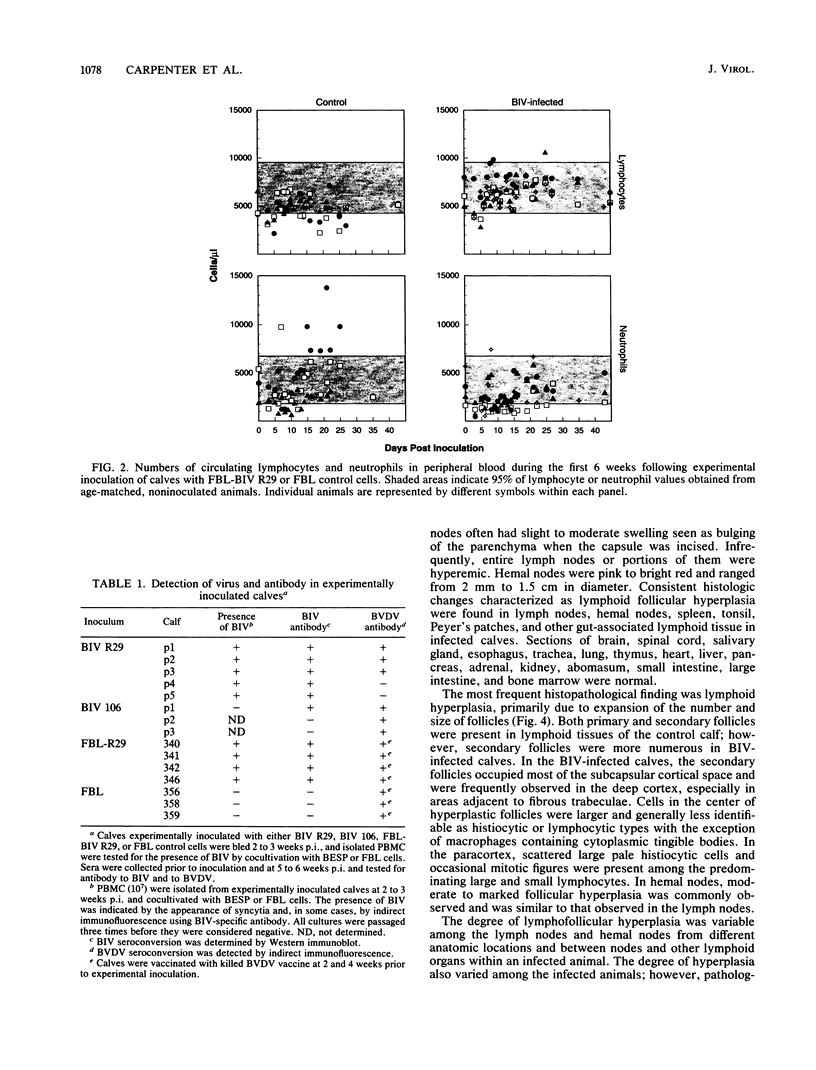
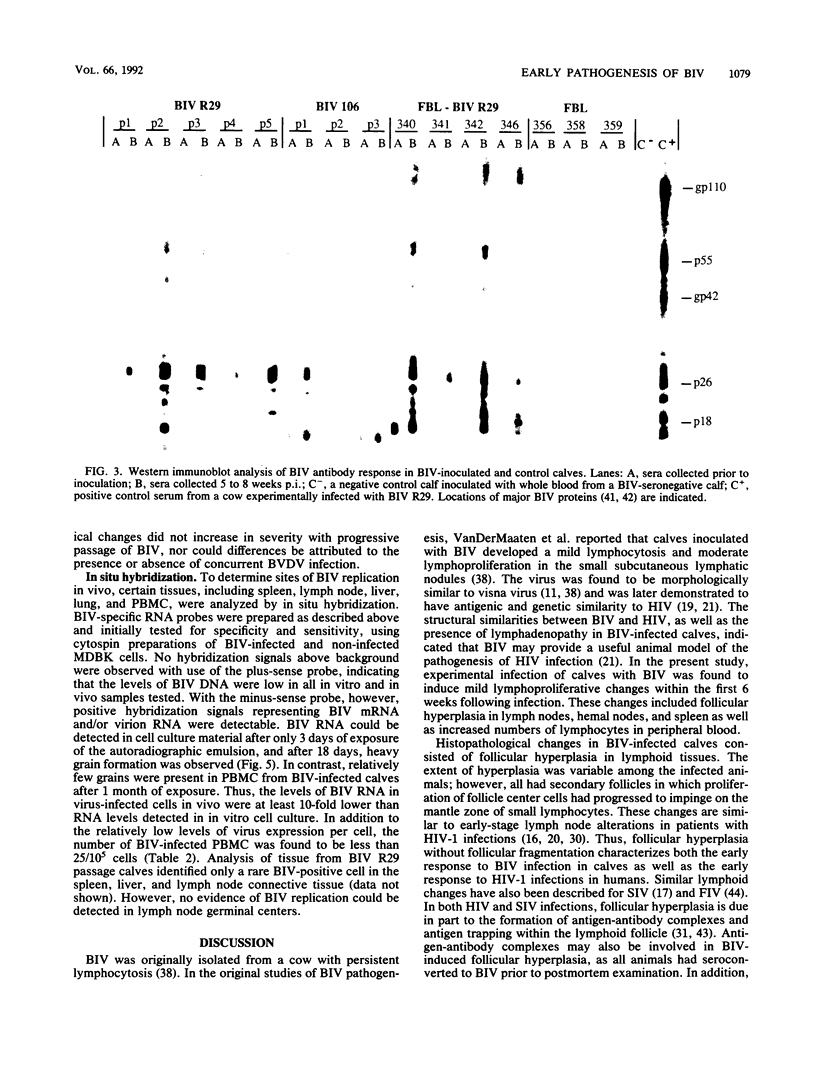
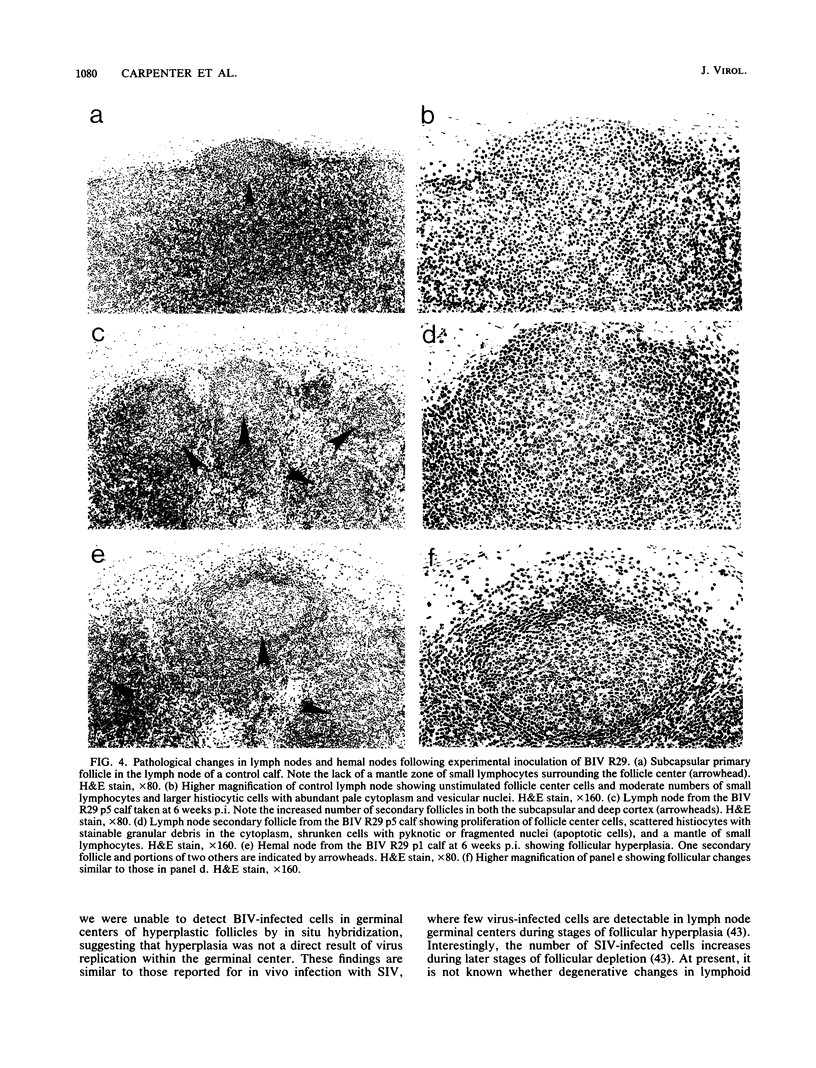
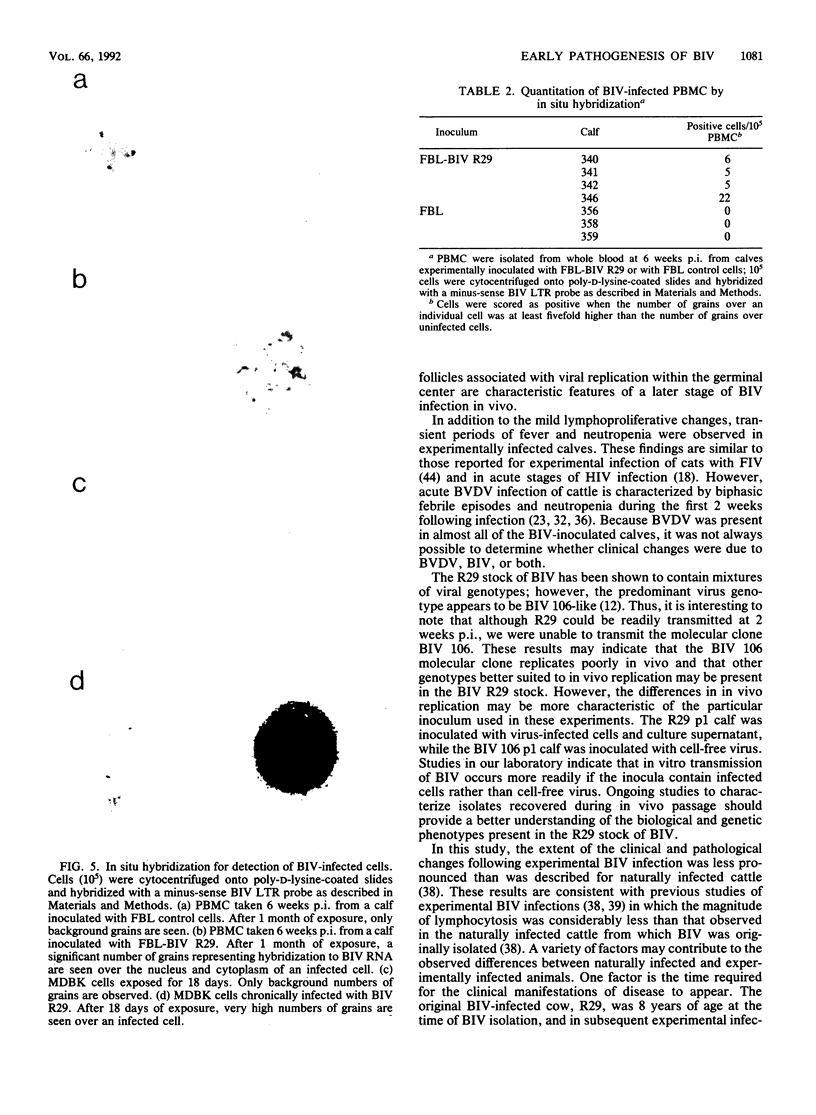
The early pathogenic effects of bovine immunodeficiency-like virus (BIV) were studied in calves experimentally inoculated with BIV. All animals inoculated with BIV R29-infected cells seroconverted by 6 weeks postinoculation, and BIV was recoverable from each animal at 2 weeks postinoculation. However, levels of BIV replication in vivo appeared to be low. In situ hybridization studies indicated that during peak periods of viral replication in vivo, less than 0.03% of peripheral blood mononuclear cells were expressing detectable levels of viral RNA. Moreover, the levels of viral RNA in these cells in vivo were less than 1/10 the levels observed in persistently infected cells in vitro. BIV-inoculated calves had significantly higher numbers of circulating lymphocytes, and follicular hyperplasia was observed in lymph nodes, hemal nodes, and spleen. The histopathological changes observed in BIV-infected calves were similar to changes found early after infection with the immunosuppressive lentiviruses, including human immunodeficiency virus type 1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Carpenter S. Characterization of variable regions in the envelope and S3 open reading frame of equine infectious anemia virus. J Virol. 1991 Aug;65(8):4255–4262. doi: 10.1128/jvi.65.8.4255-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter G. D., Collins R. J., Harmon B. V., Kumar S., Prentice R. L., Smith P. J., Lavin M. F. Cell death by apoptosis in acute leukaemia. J Pathol. 1989 Jun;158(2):123–129. doi: 10.1002/path.1711580207. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Mori S., Wolfinbarger J. B. Analysis of parvovirus infections using strand-specific hybridization probes. Virus Res. 1989 Sep;14(1):1–25. doi: 10.1016/0168-1702(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., Matthews P. J., Ridpath J. F. Methods for detection and frequency of contamination of fetal calf serum with bovine viral diarrhea virus and antibodies against bovine viral diarrhea virus. J Vet Diagn Invest. 1991 Jul;3(3):199–203. doi: 10.1177/104063879100300302. [DOI] [PubMed] [Google Scholar]
- Boothe A. D., Van der Maaten A. J. Ultrastructural studies of a visna-like syncytia-producing virus from cattle with lymphocytosis. J Virol. 1974 Jan;13(1):197–204. doi: 10.1128/jvi.13.1.197-204.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. J., Lahn S., Boyd A. L., Kost T. A., Nagashima K., Gonda M. A. Molecular cloning of biologically active proviruses of bovine immunodeficiency-like virus. Virology. 1988 Dec;167(2):515–523. [PubMed] [Google Scholar]
- Carpenter S., Alexandersen S., Long M. J., Perryman S., Chesebro B. Identification of a hypervariable region in the long terminal repeat of equine infectious anemia virus. J Virol. 1991 Mar;65(3):1605–1610. doi: 10.1128/jvi.65.3.1605-1610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Chesebro B. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J Virol. 1989 Jun;63(6):2492–2496. doi: 10.1128/jvi.63.6.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Evans L. H., Sevoian M., Chesebro B. Role of the host immune response in selection of equine infectious anemia virus variants. J Virol. 1987 Dec;61(12):3783–3789. doi: 10.1128/jvi.61.12.3783-3789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadburn A., Metroka C., Mouradian J. Progressive lymph node histology and its prognostic value in patients with acquired immunodeficiency syndrome and AIDS-related complex. Hum Pathol. 1989 Jun;20(6):579–587. doi: 10.1016/0046-8177(89)90247-5. [DOI] [PubMed] [Google Scholar]
- Chalifoux L. V., Ringler D. J., King N. W., Sehgal P. K., Desrosiers R. C., Daniel M. D., Letvin N. L. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus (SIV). Am J Pathol. 1987 Jul;128(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Cooper D. A., Gold J., Maclean P., Donovan B., Finlayson R., Barnes T. G., Michelmore H. M., Brooke P., Penny R. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985 Mar 9;1(8428):537–540. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- Garvey K. J., Oberste M. S., Elser J. E., Braun M. J., Gonda M. A. Nucleotide sequence and genome organization of biologically active proviruses of the bovine immunodeficiency-like virus. Virology. 1990 Apr;175(2):391–409. doi: 10.1016/0042-6822(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Gerstoft J., Pallesen G., Mathiesen L. R., Pedersen C., Gaub J., Lindhardt B. O. The value of lymph node histology in human immunodeficiency virus related persistent generalized lymphadenopathy. APMIS Suppl. 1989;8:24–27. [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Konno S., Yamamoto H. Pathology of equine infectious anemia. Proposed classification of pathological types of disease. Cornell Vet. 1970 Jul;60(3):393–449. [PubMed] [Google Scholar]
- Malmquist W. A., Van der Maaten M. J., Boothe A. D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969 Jan;29(1):188–200. [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989 Jul;70(Pt 7):1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis P., Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984 Jul;170(3):421–435. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- Orrego A., Issel C. J., Montelaro R. C., Adams W. V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982 Sep;43(9):1556–1560. [PubMed] [Google Scholar]
- Ost A., Baroni C. D., Biberfeld P., Diebold J., Moragas A., Noël H., Pallesen G., Rácz P., Schipper M., Tenner-Rácz K. Lymphadenopathy in HIV infection: histological classification and staging. APMIS Suppl. 1989;8:7–15. [PubMed] [Google Scholar]
- Racz P., Tenner-Racz K., Schmidt H. Follicular dendritic cells in HIV-induced lymphadenopathy and AIDS. APMIS Suppl. 1989;8:16–23. [PubMed] [Google Scholar]
- Roth J. A., Bolin S. R., Frank D. E. Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Am J Vet Res. 1986 May;47(5):1139–1141. [PubMed] [Google Scholar]
- Roth J. A., Kaeberle M. L., Griffith R. W. Effects of bovine viral diarrhea virus infection on bovine polymorphonuclear leukocyte function. Am J Vet Res. 1981 Feb;42(2):244–250. [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Lodmell D., Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985 Feb;141(1):110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- Tyler D. E., Ramsey F. K. Comparative pathologic, immunologic, and clinical responses produced by selected agents of the bovine mucosal disease-virus diarrhea complex. Am J Vet Res. 1965 Jul;26(113):903–913. [PubMed] [Google Scholar]
- Uttenthal A., Larsen S., Lund E., Bloom M. E., Storgård T., Alexandersen S. Analysis of experimental mink enteritis virus infection in mink: in situ hybridization, serology, and histopathology. J Virol. 1990 Jun;64(6):2768–2779. doi: 10.1128/jvi.64.6.2768-2779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Van der Maaten M. J., Whetstone C. A., Khramtsov V. V., Miller J. M. Experimentally-induced infections with bovine immunodeficiency-like virus, a bovine lentivirus. Dev Biol Stand. 1990;72:91–95. [PubMed] [Google Scholar]
- Whetstone C. A., VanDerMaaten M. J., Black J. W. Humoral immune response to the bovine immunodeficiency-like virus in experimentally and naturally infected cattle. J Virol. 1990 Jul;64(7):3557–3561. doi: 10.1128/jvi.64.7.3557-3561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstone C. A., VanDerMaaten M. J., Miller J. M. A western blot assay for the detection of antibodies to bovine immunodeficiency-like virus in experimentally inoculated cattle, sheep, and goats. Arch Virol. 1991;116(1-4):119–131. doi: 10.1007/BF01319236. [DOI] [PubMed] [Google Scholar]
- Wyand M. S., Ringler D. J., Naidu Y. M., Mattmuller M., Chalifoux L. V., Sehgal P. K., Daniel M. D., Desrosiers R. C., King N. W. Cellular localization of simian immunodeficiency virus in lymphoid tissues. II. In situ hybridization. Am J Pathol. 1989 Feb;134(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]
- van der Valk P., Meijer C. J. The histology of reactive lymph nodes. Am J Surg Pathol. 1987 Nov;11(11):866–882. doi: 10.1097/00000478-198711000-00005. [DOI] [PubMed] [Google Scholar]