Abstract
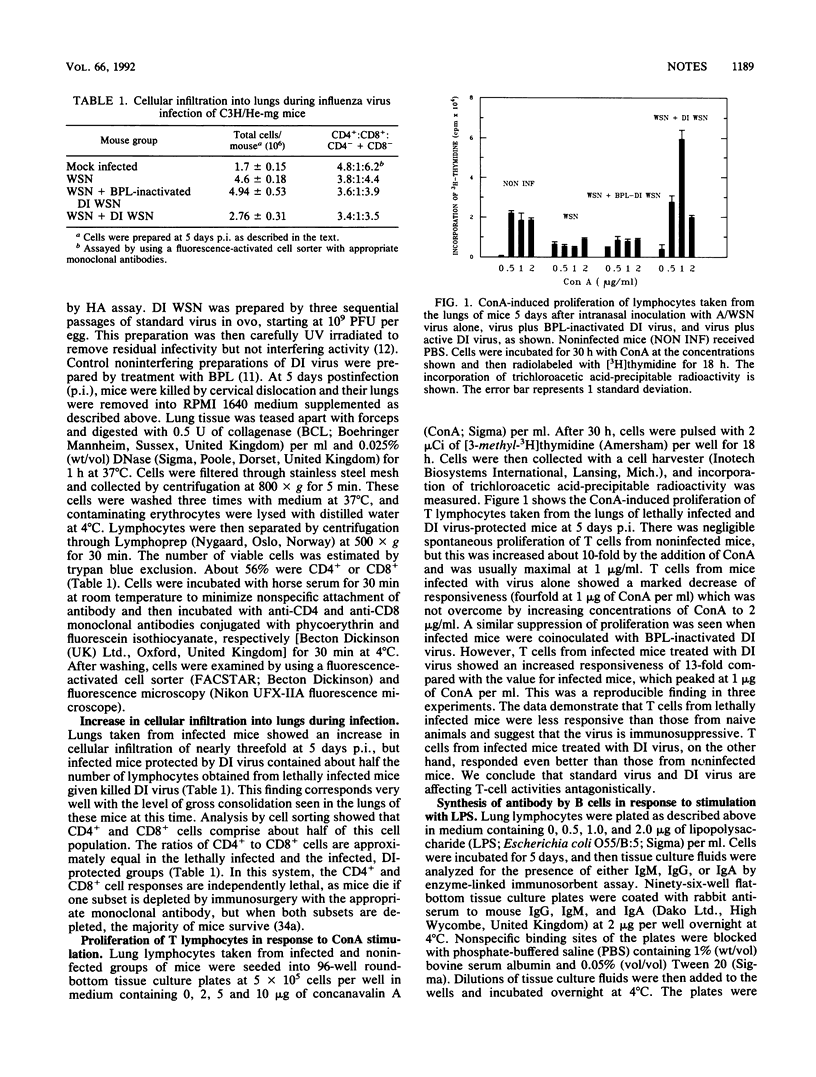
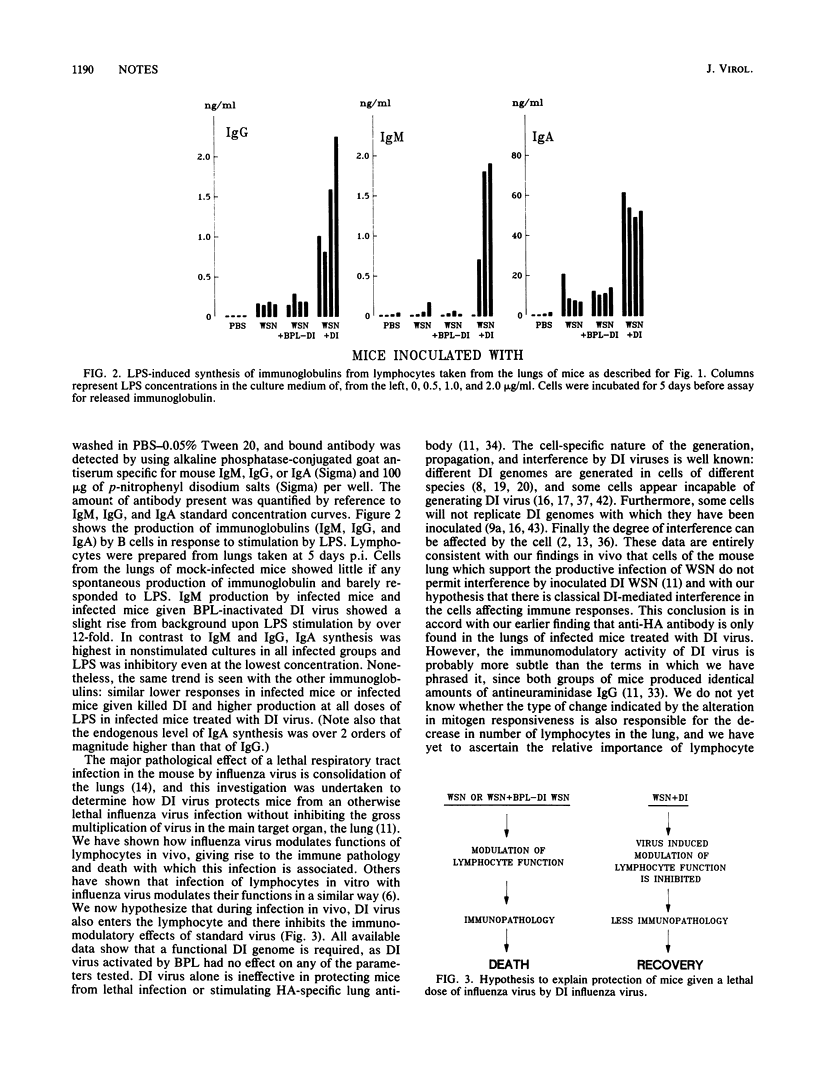
Mice inoculated intranasally with a lethal dose of standard influenza virus die with an immune-mediated pneumonia but are protected by coinoculation with defective interfering (DI) virus. Here we show that recruitment of immune cells into the infected lung is halved by treatment with DI virus although the CD4+/CD8+ cell ratio is not affected. Responsiveness of lung T and B cells to lectins is inhibited by standard virus, but coinoculation of mice with DI virus causes a 13-fold increase in T-cell proliferation and up to a 100-fold increase in immunoglobulin production. This effect appears to be due to lymphocyte-specific DI virus-mediated interference, since there is no inhibition of virus multiplication in the lungs. The net result is a shift from a lethal to a beneficial immune response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Taylor P. M., Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Crouch C. F., Dimmock N. J. Assay of defective-interfering semliki forest virus by the inhibition of synthesis of virus-specified RNAs. J Gen Virol. 1981 Jun;54(Pt 2):273–280. doi: 10.1099/0022-1317-54-2-273. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Dimmock N. J. Defective interfering viruses and infections of animals. Curr Top Microbiol Immunol. 1986;128:55–84. doi: 10.1007/978-3-642-71272-2_2. [DOI] [PubMed] [Google Scholar]
- Brownson J. M., Mahy B. W., Hazleman B. L. Interaction of influenza A virus with human peripheral blood lymphocytes. Infect Immun. 1979 Aug;25(2):749–756. doi: 10.1128/iai.25.2.749-756.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton W. M., Avery R. J., Dimmock N. J. Influence of the host cell on the genomic and subgenomic RNA content of defective-interfering influenza virus. J Gen Virol. 1981 Mar;53(Pt 1):173–177. doi: 10.1099/0022-1317-53-1-173. [DOI] [PubMed] [Google Scholar]
- Daniels C. A., Marbrook J. Influenza A2 inhibits murine in vitro antibody synthesis. J Immunol. 1981 May;126(5):1737–1741. [PubMed] [Google Scholar]
- Del Gobbo V., Villani N., Marini S., Balestra E., Caliò R. Suppressor cells induced by influenza virus inhibit interleukin-2 production in mice. Immunology. 1990 Mar;69(3):454–459. [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J., Beck S., McLain L. Protection of mice from lethal influenza: evidence that defective interfering virus modulates the immune response and not virus multiplication. J Gen Virol. 1986 May;67(Pt 5):839–850. doi: 10.1099/0022-1317-67-5-839. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J., Kennedy S. I. Prevention of death in Semliki Forest virus-infected mice by administration of defective-interfering Semliki Forest virus. J Gen Virol. 1978 May;39(2):231–242. doi: 10.1099/0022-1317-39-2-231. [DOI] [PubMed] [Google Scholar]
- Eaton B. T. Defective interfering particles of Semliki Forest virus generated in BHK cells do not interfere with viral RNA synthesis in Aedes albopictus cells. Virology. 1975 Dec;68(2):534–538. doi: 10.1016/0042-6822(75)90293-7. [DOI] [PubMed] [Google Scholar]
- HERS J. F., MUDLER J., MASUREL N., vd KUIP L., TYRRELL D. A. Studies on the pathogenesis of influenza virus pneumonia in mice. J Pathol Bacteriol. 1962 Jan;83:207–217. [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantzler G. B., Lauteria S. F., Cusumano C. L., Lee J. D., Ganguly R., Waldman R. H. Immunosuppression during influenza virus infection. Infect Immun. 1974 Nov;10(5):996–1002. doi: 10.1128/iai.10.5.996-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Snyderman R., Daniels C. A. Depressed monocyte chemotaxis during acute influenza infection. Lancet. 1975 Nov 29;2(7944):1063–1066. doi: 10.1016/s0140-6736(75)90432-8. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Cells mediating delayed-type hypersensitivity in the lungs of mice infected with an influenza A virus. Scand J Immunol. 1980;12(5):393–400. doi: 10.1111/j.1365-3083.1980.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Different functions of subsets of effector T cells in murine influenza virus infection. Cell Immunol. 1982 Mar 1;67(2):312–324. doi: 10.1016/0008-8749(82)90223-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Delayed-type hypersensitivity to influenza virus. Induction of antigen-specific suppressor T cells for delayed-type hypersensitivity to hemagglutinin during influenza virus infection in mice. J Exp Med. 1980 Apr 1;151(4):799–814. doi: 10.1084/jem.151.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Inhibition of pathogenic effect of effector T cells by specific suppressor T cells during influenza virus infection in mice. Nature. 1983 Aug 11;304(5926):541–543. doi: 10.1038/304541a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. D., Taylor P. M., Askonas B. A. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology. 1989 Jul;67(3):375–381. [PMC free article] [PubMed] [Google Scholar]
- Mak N. K., Zhang Y. H., Ada G. L., Tannock G. A. Humoral and cellular responses of mice to infection with a cold-adapted influenza A virus variant. Infect Immun. 1982 Oct;38(1):218–225. doi: 10.1128/iai.38.1.218-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. R., Couch R. B., Greenberg S. B., Cate T. R., Warr G. A. Effects of infection with influenza virus on the function of polymorphonuclear leukocytes. J Infect Dis. 1981 Sep;144(3):279–280. doi: 10.1093/infdis/144.3.279. [DOI] [PubMed] [Google Scholar]
- McLain L., Dimmock N. J. An influenza haemagglutinin-specific IgG enhances class I MHC-restricted CTL killing in vitro. Immunology. 1991 May;73(1):12–18. [PMC free article] [PubMed] [Google Scholar]
- McLain L., Dimmock N. J. Protection of mice from lethal influenza by adoptive transfer of non-neutralizing haemagglutination-inhibiting IgG obtained from the lungs of infected animals treated with defective interfering virus. J Gen Virol. 1989 Oct;70(Pt 10):2615–2624. doi: 10.1099/0022-1317-70-10-2615. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Lundquist R. E., Maizel J. V., Jr Absence of subviral particles and assembly activity in HeLa cells infected with defective-interfering (DI) particles of poliovirus. Virology. 1980 Jan 15;100(1):116–124. doi: 10.1016/0042-6822(80)90557-7. [DOI] [PubMed] [Google Scholar]
- Reed W. P., Olds J. W., Kisch A. L. Decreased skin-test reactivity associated with influenza. J Infect Dis. 1972 Apr;125(4):398–402. doi: 10.1093/infdis/125.4.398. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Steigbigel R. T. Effect of in vitro virus infection on response of human monocytes and lymphocytes to mitogen stimulation. J Immunol. 1978 Sep;121(3):1052–1058. [PubMed] [Google Scholar]
- Scheinberg M., Blacklow N. R., Goldstein A. L., Parrino T. A., Rose F. B., Cathcart E. S. Influenza: response of T-cell lymphopenia to thymosin. N Engl J Med. 1976 May 27;294(22):1208–1211. doi: 10.1056/NEJM197605272942204. [DOI] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Steacie A. D., Eaton B. T. Properties of defective interfering particles of Sindbis virus generated in vertebrate and mosquito cells. J Gen Virol. 1984 Feb;65(Pt 2):333–341. doi: 10.1099/0022-1317-65-2-333. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Daniel S., Djeu J. Y., Kiley S. C., Ennis F. A. Recovery from a viral respiratory tract infection. IV. Specificity of protection by cytotoxic T lymphocytes. J Immunol. 1983 Jun;130(6):2908–2914. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]


