Abstract
Growth of mouse neural crest cultures in the presence of glial cell line-derived neurotrophic factor (GDNF) resulted in a dramatic dose-dependent increase in the number of tyrosine hydroxylase (TH)-positive cells that developed when 5% chicken embryo extract was present in the medium. In contrast, growth in the presence of bone morphogenetic protein (BMP)-2, BMP-4, BMP-6, transforming growth factor (TGF) β1, TGF-β2, and TGF-β3 elicited no increase in the number of TH-positive cells. The TH-positive cells that developed in the presence of GDNF had neuronal morphology and contained the middle and low molecular weight neurofilament proteins. Numerous TH-negative cells with the morphology of neurons also were observed in GDNF-treated cultures. Analysis revealed that the period from 6 to 12 days in vitro was the critical time for exposure to GDNF to generate the increase in TH-positive cell number. The growth factors neurotrophin-3 and fibroblast growth factor-2 elicited increases in the number of TH-positive cells similar to that seen in response to GDNF. In contrast, nerve growth factor was unable to substitute for GDNF. These findings extend the previously reported biological activities of GDNF by showing that it can act on mouse neural crest cultures to promote the development of neurons.
Keywords: tyrosine hydroxylase, adrenergic development, transforming growth factor β superfamily, neurogenesis
In vertebrates the neural crest is a transient embryonic structure that is the source of many adult cell types including neurons and glia of the dorsal root, sympathetic, parasympathetic, and enteric ganglia, melanocytes of the skin and irides, chromaffin cells of the adrenal medulla, connective tissue cells of the head and face, and cells that make up part of the aorticopulmonary septum of the heart (1–5). As such, the neural crest is an excellent model system in which to investigate the cellular and molecular mechanisms that control the generation of cellular diversity during development. Neural crest cells initially form on the dorsal neural tube and subsequently migrate to many locations in the embryo where they undergo differentiation into the adult cell types noted above. Evidence from studies of avian embryos indicates that molecules in the embryonic environment encountered by neural crest cells either during or after migration are important determinants of cell fate and phenotype (3, 6). Analysis of the mechanisms controlling the development of neural crest cells in the mouse is of particular interest because of the existence of several natural mutations involving neural crest derivatives and the ability to engineer mice with either specific genes deleted or overexpressed (7–9). In vivo studies have shown that while neural crest migration in the mouse is similar to that observed in the avian embryo, there are differences with respect to the timing of neural crest migration (10–12). Like avian, amphibian, and rat neural crest cells, at least some mouse neural crest cells are multipotential with respect to their developmental fate (6, 13–15).
Differentiation of mouse neural crest cells into neurons in vitro has been observed in medium containing chicken or rat embryo extract and in defined medium (16–21). Studies indicate that growth factors play a central role in the establishment of specific phenotypes in mouse neural crest cultures (22). Fibroblast growth factor 2 (FGF-2) can stimulate the proliferation of mouse trunk neural crest cells in vitro and promote neuronal differentiation (23). Also, the neuropoietic cytokines leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) can promote the differentiation of mouse neural crest cells into sensory neurons (19, 23).
The transforming growth factor β (TGF-β) superfamily of growth factors has been found to exert a wide variety of effects on developing and mature tissues (24, 25). Glial cell line- derived neurotrophic factor (GDNF) is a disulfide-bridge-linked homodimer of two 134-amino acid peptide chains and is a distant member of the TGF-β superfamily (26). GDNF was originally identified as an activity in glial-cell-conditioned medium that stimulated the uptake of dopamine in primary cultures of neurons of the substantia nigra. Subsequent studies have shown that GDNF can promote the survival and process outgrowth of a wide spectrum of central nervous system neurons (27–33). In addition, GDNF can promote the survival of some classes of neurons in the peripheral nervous system (34–36).
Given the activity of GDNF on differentiated neurons, it is also of interest to determine if it can act on populations of neuronal progenitors. In the present study, we have focused on the development of adrenergic cells from the mouse trunk neural crest in vitro. We show herein that GDNF stimulates the development of adrenergic and other neurons from mouse neural crest cultures grown in medium containing chicken embryo extract. In contrast, other TGF-β family members including bone morphogenetic protein (BMP)—2, BMP-4, BMP-6, TGF-β1, TGF-β2, and TGF-β3 did not promote adrenergic development.
MATERIALS AND METHODS
Neural Crest Cultures.
Neural tubes containing the neural crest caudal to the heart, but excluding the primitive streak, were dissected from the trunk region of embryonic day 9 (E9) CBA mice (Walter and Eliza Hall Institute) as described (19). One neural tube was plated per fibronectin-coated (50 μg/ml, Boehringer Mannheim) well of four-well culture dishes (Greiner, Nurtingen, F.R.G.) in 75 μl of Monomed medium (Commonwealth Serum Laboratories, Melbourne) containing 10% fetal bovine serum (Commonwealth Serum Laboratories). After 20 hr, the cultures were fed with 2 ml of either Monomed with 10% fetal bovine serum or Monomed with 10% fetal bovine serum and 5% chicken embryo extract (CEE) prepared from 9-day chicken embryos (CEE medium) (37). Neural tubes remained present throughout the culture period. The following growth factors were added to the cultures where indicated: BMP-2, BMP-4, and BMP-6 (Genetics Institute, Cambridge, MA); TGF-β1, TGF-β2, and TGF-β3 (R & D Systems); GDNF and neurotrophin (NT-3) (PeproTech, Rocky Hill, NJ); and nerve growth factor (NGF) and FGF-2 (Boehringer Mannheim).
Immunocytochemistry.
Cultures were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4) for 40 min and washed three times in mouse tonicity PBS (19). Cells that were immunoreactive for tyrosine hydroxylase (TH), neurofilament, peripherin, and SCG10 were visualized by standard indirect immunofluorescence (38). For TH immunocytochemistry, both a mouse monoclonal antibody (39) and a rabbit antibody (40) were used. For neurofilament staining, a rabbit antibody to the 150-kDa neurofilament protein (Chemicon) and a mouse monoclonal antibody to the 67-kDa neurofilament protein (Amersham) were used. For peripherin (Chemicon) and SCG10 (41) staining, rabbit antibodies were used. Detailed staining protocols are available on request from the authors.
Total Cell Number.
To determine total cell number neural tubes were removed from the cultures with fine tungsten needles after 12 days in vitro followed by addition of 0.1% trypsin to remove the cells in the neural crest outgrowths from the substrate. After the cells had detached from the substrate, as determined by microscopic examination, an equal volume of Monomed medium with 10% fetal bovine serum was added and the number of cells in an aliquot of a known volume of cell suspension was determined by hemacytometer counting.
Statistical Analysis.
Differences among multiple treatment groups were analyzed by one-way analysis of variance followed by the Tukey post hoc test.
RESULTS
GDNF Promotes Adrenergic Development When Cultures Are Grown in Medium Containing Embryo Extract.
As shown in Fig. 1, addition of GDNF at 10 ng/ml to neural crest cultures grown in CEE-containing medium resulted in a greater than 50-fold increase in the number of TH-positive cells that were present when the cultures were assayed at 12 days in vitro. However, addition of GDNF to cultures grown in medium without CEE had little effect on the number of TH-positive cells that developed. In marked contrast to the effect of GDNF, several other TGF-β superfamily members tested did not increase the number of TH-positive cells irrespective of the presence or absence of CEE in the medium. Cultures treated with BMP-2, BMP-4, BMP-6, TGF-β1, TGF-β2, and TGF-β3 showed no increase in TH-positive cell number relative to controls. The dose–response profile to GDNF in the presence of CEE is shown in Fig. 2. The number of TH-positive cells that developed rose rapidly with GDNF between 1 and 10 ng/ml, with 10 ng/ml being a maximal dose.
Figure 1.

Development of TH-positive cells in mouse trunk neural crest cultures in response to selected growth factors in the TGF-β superfamily. Cultures were grown for 12 days either in medium containing 10% fetal bovine serum and 5% CEE (solid bars) or in medium containing 10% fetal bovine serum (open bars) in the presence of the growth factors indicated. Concentrations of the growth factors were as follows: GDNF, BMP-2, BMP-4, and BMP-6, 10 ng/ml; TGF-β1, TGF-β2, and TGF-β3, 2 ng/ml. The number of TH-positive cells per culture is expressed as the mean ± SEM of three to eight cultures analyzed per condition.
Figure 2.

Dose–response profile of the development of TH-positive cells to GDNF. Neural crest cultures were grown in medium containing 10% fetal bovine serum and 5% CEE with the concentrations of GDNF indicated for 12 days in vitro. They were then processed to reveal TH-positive cells. The number of TH cells is expressed as the mean ± SEM with four cultures analyzed per condition. The 0, 0.1, and 1 ng/ml values are statistically different from the 10 and 50 ng/ml values with P < 0.05.
When total cell number was determined in cultures grown in CEE medium, we found that GDNF at 10 ng/ml stimulated a 5-fold increase compared with control cultures after 12 days in vitro. In the presence of GDNF, 5.6 × 105 ± 0.3 × 105 cells were present per culture (mean ± SEM, n = 5) compared with 1.1 × 105 ± 0.1 × 105 (mean ± SEM, n = 5) cells per control culture. Thus, the magnitude of increase in the number of TH-positive cells was about 10-fold greater than the increase in total cell number.
As shown in Fig. 3, round TH-positive fluorescent cell bodies with neuronal morphology were observed in both the control and GDNF-treated conditions. Fluorescent processes connected to TH-positive cell bodies were observed in both the control and GDNF-treated cultures on some, but not all, cells. The TH-positive cells in both control and GDNF-treated conditions often appeared to develop on top of another cell layer. Another feature of both the control and the GDNF-treated cultures was that the TH-positive cells were intermingled with cells that were TH-negative but that had neuronal morphology under phase-contrast microscopy (Fig. 3).
Figure 3.

Appearance of mouse trunk neural crest cultures in the presence and absence of GDNF. (A) Fluorescence photograph of TH-positive cells in a cultures grown in CEE medium in the presence of GDNF at 10 ng/ml for 12 days in vitro. Numerous TH-immunoreactive cells bodies and some cellular processes are present. (B) Phase-contrast view of the same field in A, demonstrating the dense packing of the neuron-like cell bodies in these cultures. Many TH-negative cells are intermingled with the TH-positive cells. (C) Fluorescence photograph of a control culture grown in CEE medium in the absence of GDNF. Two TH-positive cells are present. (D) Phase-contrast view of the same field as in C, showing the presence of numerous TH-negative cells with both neuronal and nonneuronal morphologies. (Bar = 30 μm.)
Staining of GDNF-treated cultures with antibodies to TH and the middle molecular weight neurofilament protein showed that the TH-positive cells also had neurofilaments that were labeled (Fig. 4). Similar results were obtained when the cultures were stained for TH and the low molecular weight neurofilament protein (data not shown). In addition, in GDNF-treated cultures, there was also extensive neurofilament staining that was not associated with TH-positive cells, indicating the presence of many nonadrenergic cells in the neuronal lineage. The presence of neurons that were TH-negative was further supported by examination of the neuronal markers SCG10 and peripherin. Numerous SCG10-positive cell bodies and peripherin-positive fibers were present in GDNF-treated cultures (data not shown). These SCG10-positive and peripherin-positive cells were not TH-positive. The complexity of the SCG10 and peripherin staining patterns precluded quantitation of the number of cells with these phenotypes.
Figure 4.

TH-positive cells that develop in mouse trunk neural crest are also neurofilament-positive. Cultures were prepared as described and grown in medium with 10% fetal bovine serum, 5% CEE, and GDNF (10 ng/ml) for 12 days in vitro. Confocal image of a culture that was double labeled for TH and the middle neurofilament protein after 12 days in vitro. (A) Fluorescent image of TH-positive cells. (B) Fluorescent image of neurofilament-positive cells. Neurofilament-positive processes emanating from other cells in the culture are also observed. (Bar = 5 μm.)
Temporal Requirement for Exposure to GDNF.
We examined the temporal response to GDNF (Fig. 5A). The results of these experiments showed that there were few TH-positive cells present after 6 days in vitro under either control or GDNF-treated conditions. In contrast, by 12 days in vitro the number of TH-positive cells was dramatically increased in the presence of GDNF compared with control cultures grown in CEE medium without GDNF. Although the increase in TH-positive cell number was observed after 12 days in vitro, these experiments did not define when GDNF was acting during this period. Accordingly, we performed experiments in which we varied the time period when GDNF was present over the 12 days in vitro. As shown in Fig. 5B, these experiments clearly indicated that 6–12 days in vitro was the critical period required for GDNF to be present to affect an increase in TH-positive cell number. This relatively late action of GDNF indicates that the precursors of the TH-positive cells are able to survive for 6 days in vitro in the absence of GDNF. GDNF may then act to promote their further differentiation into TH-positive cells. Alternatively, the TH-positive cells may differentiate in the absence of GDNF but require GDNF for their survival once they assume the TH-positive phenotype.
Figure 5.
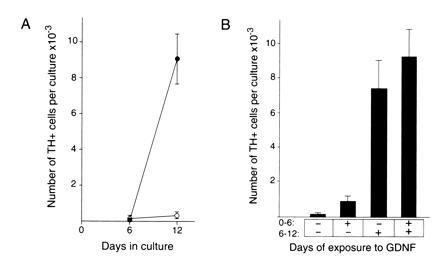
(A) Time course of development of TH-positive cells in the presence and absence of GDNF. Cultures were grown in the presence (•) or absence (○) of GDNF at 10 ng/ml in medium containing 10% fetal bovine serum and 5% CEE and assayed at the times indicated for number of TH-positive cells. The number of TH-positive cells is expressed as the mean ± SEM with three cultures analyzed per condition. The day 12 GDNF and control values are statistically significant with P < 0.05. (B) Analysis of the temporal requirement for GDNF. Cultures were grown for a total of 12 days in vitro in either the presence (+) or absence (−) of GDNF at 10 ng/ml for the time period indicated. TH-positive cell number per culture is expressed as the mean ± SEM with 12 cultures analyzed per condition. These results indicate the presence of GDNF in the period from 6 to 12 days in vitro is required for a robust increase in TH-positive cell number. The −/− and +/− values are statistically different from the −/+ and the +/+ values with P < 0.05.
Comparison of the Activity of GDNF with NGF, FGF-2, and NT-3.
Several other growth factors including NGF, NT-3, and FGF-2 have been reported to act on various stages of sympathetic neuron development (42–45). Accordingly, we have compared the action of these factors to that of GDNF. Few TH-positive cells developed in the presence of NGF. Furthermore, NGF with GDNF did not potentiate the effect of GDNF (Fig. 6). These results argue that the action of GDNF is distinct from that of NGF. In contrast to the lack of effect of NGF, FGF-2 was effective at promoting the development of TH-positive cells to an extent similar to that of GDNF, while NT-3 was also active but somewhat less potent (Fig. 6). Simultaneous addition of GDNF, NT-3, and FGF-2 did not increase the number of TH-positive cells above that seen with GDNF alone, indicating that these factors do not act synergistically on the generation of adrenergic cells in the mouse trunk neural crest cultures.
Figure 6.
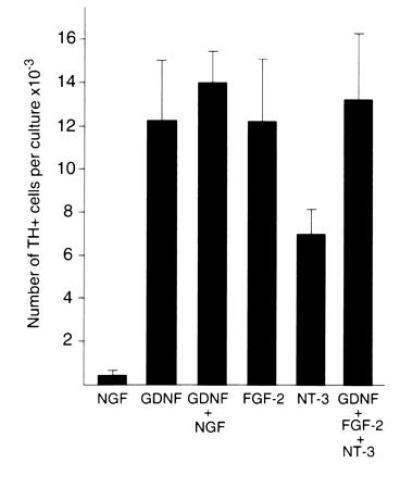
Comparison of the response to GDNF to that of NGF, FGF-2, and NT-3. Cultures were grown for 12 days in medium containing 10% fetal bovine serum and 5% CEE in the presence of the factors indicated. NGF, FGF-2, and NT-3 were present at 50 ng/ml and GDNF was present at 10 ng/ml. The values are expressed as the mean ± SEM of four to six cultures per condition. The NGF value is statistically different from the GDNF, NT-3, and FGF-2 values with P < 0.05.
DISCUSSION
Previous work has shown that GDNF can act on both central and peripheral nervous system neurons to promote survival and process outgrowth. Populations affected by GDNF include substantia nigra and locus coeruleus neurons, motor neurons, Purkinje cells, and peripheral autonomic neurons (26–36). Given the wide range of neuronal types affected by GDNF, it was of interest to learn if GDNF could also act on neuronal precursor cell populations of the neural crest. Our present work extends the range of GDNF activity to an earlier stage of development by showing that it can promote the appearance of adrenergic and other neurons in mouse neural crest cultures.
The activity of GDNF is apparent when neural crest cells are grown in medium containing 5% CEE and 10% fetal bovine serum but not in medium without CEE. The dose–response profile to GDNF is comparable to that seen in other systems (27, 32, 34). One possible explanation for the requirement for CEE is that a factor or factors present in CEE are required to allow neural crest cells to reach a developmental state where they can respond to GDNF. A second possibility is that the CEE factors work with GDNF to promote the development of the adrenergic and other neuronal phenotypes. The requirement for the presence of CEE for the development of adrenergic cells is consistent with the previous report of Ito and Takeuchi (16) that small numbers of catecholamine-positive cells developed in mouse neural crest cultures in the presence of CEE-containing medium. In addition, Matsumoto (20) has reported that TH-positive neurons can develop in mouse neural crest cultures in the presence of rat embryo extract.
The TH-positive cells in these cultures stain for the middle and low molecular weight neurofilament proteins. In addition, many of these TH-positive cells possessed rounded cell bodies and long cellular processes, some of which were more than 100 μm long. These immunocytochemical and morphological traits indicate that these cells are in the neuronal lineage. The TH-positive cells were not stained with SCG10 or peripherin antibodies, suggesting that perhaps that these cells represent an intermediate stage of neuronal differentiation (21). Additional evidence for the neuronal nature of these cells comes from the lack of staining with fluorescently labeled peanut agglutinin. In the rat, it has been reported that peanut agglutinin is a marker for neuroendocrine cells and not for neurons (46). We found that the TH-positive cells in our cultures were negative for peanut agglutinin, further suggesting that these TH-positive cells are in the neuronal lineage (data not shown).
Our results are consistent with a model in which neural crest progenitors survive and proliferate in the presence of CEE medium but do not require GDNF. This is shown by the fact that GDNF is not required until at least day 6 in vitro. Differentiation of these progenitors into TH-positive cells would then require the presence of GDNF. An alternative model is that the differentiation of TH-positive cells is independent of GDNF, but these cells require GDNF for their survival once they differentiate. It is also possible that GDNF acts indirectly by stimulating production of other growth factors required for adrenergic development. Regardless of which of these mechanisms is operative, it is clear that GDNF must do more than simply support the survival of fully differentiated adrenergic neurons. This is the case since one can wait until 6 days in vitro, a time when few TH-positive cells are present, to add GDNF and still generate large numbers of TH-positive cells.
The dramatic increase in TH-positive cells in the presence of GDNF is reminiscent of the increased numbers of TH-positive cells that developed in avian neural crest cultures in the presence of BMP-2, BMP-4, and OP-1/BMP-7 (47–49). Like GDNF, the activity of the BMPs in avian neural crest cultures also requires the presence of CEE in the growth medium (48). In contrast to the dramatic increase in TH-positive cells in quail trunk neural crest cultures in response to BMP-2 and BMP-4, we observed no increase in TH-positive cells in mouse trunk neural crest cultures in response to either BMP-2 or BMP-4. In addition, Shah et al. (50) have recently shown that in rat neural crest cultures BMP-2 and BMP-4 can stimulate the development of neurons in the sympathetic lineage, but these neurons do not express TH. GDNF has been shown to increase the survival of avian and mouse sympathetic neurons (34, 35). In the case of chicken sympathetic neuron cultures, younger developmental stages are more responsive to GDNF than are neurons from older embryos. However, GDNF does not increase the number of TH-positive cells that develop in quail neural crest cultures (47). Thus, these findings suggest that either there are either species differences in the response to members of the TGF-β superfamily or that relatively subtle differences in cultures conditions may have major effects on the way neural crest cells respond to this family of molecules.
Postulating a role for GDNF in the generation of TH-positive cells suggests that it should be present in the periphery of the developing embryo. In situ hybridization, Northern blot analysis, and polymerase chain reaction studies indicate that GDNF mRNA is present in the periphery of both rat and mouse embryos in a number of sites including the developing gut, skin, whisker pads, kidney, stomach, and testis (35, 51–54). Thus, the distribution of GDNF mRNA is consistent with it playing a role in the generation of peripheral adrenergic neurons.
Several reports have appeared recently that described the phenotype of GDNF null mice and characterized the GDNF receptor complex (55–61). Interestingly, in GDNF null mice, some neural crest-derived sympathetic and sensory neurons and virtually all neural-crest-derived enteric neurons are absent (55–59). This deficit in neural-crest-derived neurons is consistent with our present findings that GDNF can act on neural crest populations in vitro and underscores the important role played by GDNF in neural crest development. Identification of the ret tyrosine kinase and the GDNFR-α receptors for GDNF will provide important new avenues for the exploration of the role of GDNF in neural crest development (58–61).
We found that GDNF, NT-3, and FGF-2 can all increase the numbers of TH-positive cells which develop. Previous work has shown that NT-3 can act as a survival factor for rat sympathetic neuroblasts isolated from ganglia (43, 44). Our results suggest that NT-3 may act at even earlier stages of development. This conclusion would be consistent with the report that NT-3 is mitogenic for avian neural crest cells (62). Similarly FGF-2 has previously been shown to be able to promote the neuronal differentiation of mouse neural crest cells and rat neural crest-derived cells (23, 45). The fact that GDNF null mice have reduced numbers of sympathetic neurons suggests that in vivo FGF-2 and NT-3 do not act precisely like GDNF and cannot compensate for the lack of GDNF. Thus, although GDNF, FGF-2, and NT-3 can increase the number of TH-positive cells, these TH-positive cells may not be identical to one another. For example, perhaps distinct neuropeptide-containing subsets of TH-cells are generated by each of these growth factors. The fact that NGF does not share this TH-cell promoting activity with GDNF, NT-3, or FGF-2 reinforces the notion that there is a stage-specific shift in neurotrophic factor dependence that occurs during the ontogeny of specific neuronal populations of neural crest cell origin (43, 44).
In conclusion, our findings indicate that GNDF acts on an intermediate stage of neural crest cultures to promote the development of adrenergic and other neuronal phenotypes. These findings extend the range of known activities of GDNF to an earlier stage of development than previously identified. They suggest that further analysis of the mechanism of GDNF action may prove relevant to biological contexts in which the production of new neurons is sought to replace neurons lost to conditions of disease or injury.
Acknowledgments
We thank David Anderson, John Furness, and Don Newgreen for their generosity in sharing reagents; Richard Kerr for his assistance in preparing chicken embryo extract; Drew Berry and Simon Olding for figure production; and Janice Coventry and Steven Potashner for statistical analysis. This work was supported by National Institutes of Health Grant NS16115 (G.D.M.), an National Institutes of Health Fogarty Senior International Fellowship (G.D.M.), and grants from the Windermere Foundation (P.F.B.) and the National Health and Medical Research Council of Australia (P.F.B., K.R., A.E., and M.M.).
Footnotes
Abbreviations: GDNF, glial cell line-derived neurotrophic factor; TGF-β, transforming growth factor; TH, tyrosine hydroxylase; FGF-2, fibroblast growth factor; CEE, chicken embryo extract; NGF, nerve growth factor; NT-3, neurotrophin 3; BMP, bone morphogenetic protein.
References
- 1.Hall B, Horstadius S. The Neural Crest. London: Oxford Univ. Press; 1988. [Google Scholar]
- 2.Kirby M L, Gale T F, Stewart D E. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 3.LeDouarin N M. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 4.Noden D M. In: The Specificity of Embryological Interactions. Garrod D, editor. London: Chapman & Hall; 1978. pp. 3–49. [Google Scholar]
- 5.Weston J A. Adv Morphogenesis. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- 6.Anderson D J. Neuron. 1989;3:1–12. doi: 10.1016/0896-6273(89)90110-4. [DOI] [PubMed] [Google Scholar]
- 7.Morrison-Graham K, Weston J A. Trens Genet. 1989;5:116–121. doi: 10.1016/0168-9525(89)90042-5. [DOI] [PubMed] [Google Scholar]
- 8.Marusich M F, Weston J A. Curr Opin Genet Dev. 1991;1:221–229. doi: 10.1016/s0959-437x(05)80074-7. [DOI] [PubMed] [Google Scholar]
- 9.Capecchi M. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 10.Erickson C A, Weston J A. J Embryol Exp Morphol. 1983;74:97–118. [PubMed] [Google Scholar]
- 11.Sternberg J, Kimber S J. J Embryol Exp Morphol. 1986;91:267–282. [PubMed] [Google Scholar]
- 12.Serbedzija G N, Fraser S E, Bronner F M. Development (Cambridge, UK) 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Collazo A, Bronner-Fraser M, Fraser SE. Development (Cambridge, UK) 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- 14.Serbedzija G N, Bronner-Fraser M, Fraser SE. Development (Cambridge, UK) 1994;120:1709–1718. doi: 10.1242/dev.120.7.1709. [DOI] [PubMed] [Google Scholar]
- 15.Stemple D L, Anderson D J. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Takeuchi T. J Embryol Exp Morphol. 1984;84:49–62. [PubMed] [Google Scholar]
- 17.Boisseau S, Simonneau M. Development (Cambridge, UK) 1989;106:665–674. doi: 10.1242/dev.106.4.665. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Morita T, Sieber-Blum M. Dev Biol. 1993;157:517–525. doi: 10.1006/dbio.1993.1154. [DOI] [PubMed] [Google Scholar]
- 19.Murphy M, Reid K, Hilton D J, Bartlett P F. Proc Natl Acad Sci USA. 1991;88:3498–3501. doi: 10.1073/pnas.88.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto S G. Dev Brain Res. 1994;83:1–16. doi: 10.1016/0165-3806(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 21.Sommer L, Shah N, Rao M, Anderson D J. Neuron. 1995;15:1245–1258. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 22.Murphy M, Bartlett P F. Mol Neurobiol. 1993;7:111–135. doi: 10.1007/BF02935639. [DOI] [PubMed] [Google Scholar]
- 23.Murphy M, Reid K, Ford M, Furness J B, Bartlett P F. Development (Cambridge, UK) 1994;120:3519–3528. doi: 10.1242/dev.120.12.3519. [DOI] [PubMed] [Google Scholar]
- 24.Lyons K M, Jones C M, Hogan B L M. Trends Genet. 1991;7:408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- 25.Roberts A B, Sporn M B. Mol Reprod Dev. 1992;32:91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 26.Lin L H, Doherty D H, Lile J D, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 27.Henderson C E, Phillips H S, Pollock R A, Davies A M, Lemeulle C, Armanini M, Simpson L C, Moffet B, Vandlen R A, Koliatsos V E, Rosenthal A. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 28.Arenas E, Trupp M, Akerud P, Ibáñez C F. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 29.Oppenheim R W, Houenou L J, Johnson J E, Lin L F H, Li L, Lo A C, Newsome A L, Prevette D M, Wang S. Nature (London) 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 30.Yan Q, Matheson C, Lopez O T. Nature (London) 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 31.Sauer H, Rosenblad C, Bjorklund A. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mount H T J, Dean D O, Alberch J, Dreyfus C F, Black I B. Proc Natl. Acad Sci USA. 1995;92:9092–9096. doi: 10.1073/pnas.92.20.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Wutian W, Lin L-F, H, Lei M, Oppenheim R W, Houenou L J. Proc Natl Acad Sci USA. 1995;92:9771–9775. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buj-Bello A, Buchman V L, Horton A, Rosenthal A, Davies A M. Neuron. 1995;15:1–20. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 35.Trupp M, RydJ, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibáñez C F. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebendal T, Tomac A, Hoffer B J, Olson L. J Neurosci Res. 1995;40:276–284. doi: 10.1002/jnr.490400217. [DOI] [PubMed] [Google Scholar]
- 37.Cahn R D, Coon H G, Cahn M B. In: Methods in Developmental Biology. Wilt F H, Wessells N K, editors. New York: Crowell; 1967. pp. 493–530. [Google Scholar]
- 38.Maxwell G D, Forbes M E. Dev Biol. 1990;141:233–237. doi: 10.1016/0012-1606(90)90118-3. [DOI] [PubMed] [Google Scholar]
- 39.Hatanaka H, Arimatsu Y. Neurosci Res. 1984;1:253–263. doi: 10.1016/s0168-0102(84)80004-8. [DOI] [PubMed] [Google Scholar]
- 40.Landis S C, Jackson P C, Fredieu J R, Thibault J. J Neurosci. 1987;7:3574–3587. doi: 10.1523/JNEUROSCI.07-11-03574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson D J, Axel R. Cell. 1985;42:649–662. doi: 10.1016/0092-8674(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 42.Coughlin M D, Collins M B. Dev Biol. 1985;110:392–401. doi: 10.1016/0012-1606(85)90098-3. [DOI] [PubMed] [Google Scholar]
- 43.Birren S J, Lo L, Anderson D J. Development (Cambridge, UK) 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- 44.DiCicco-Bloom E, Friedman W J, Black I B. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- 45.Stemple D L, Mahanthappa N K, Anderson D J. Neuron. 1988;1:517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 46.Katz D M, White M E, Hall A K. J Neurobiol. 1995;26(2):241–252. doi: 10.1002/neu.480260208. [DOI] [PubMed] [Google Scholar]
- 47.Reissmann E, Ernsberger U, Francis-West P H, Rueger D, Brickell P M, Rohrer H. Development (Cambridge, UK) 1996;122:2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- 48.Varley J E, Wehby R G, Rueger D C, Maxwell G D. Dev Dyn. 1995;203:434–447. doi: 10.1002/aja.1002030406. [DOI] [PubMed] [Google Scholar]
- 49.Varley J E, Maxwell G D. Exp Neurol. 1996;140:84–94. doi: 10.1006/exnr.1996.0118. [DOI] [PubMed] [Google Scholar]
- 50.Shah N M, Groves A K, Anderson D J. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 51.Suter-Crazzolara C, Unsicker K. NeuroReport. 1994;5:2486–2489. doi: 10.1097/00001756-199412000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Choi-Lundberg D L, Bohn M C. Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- 53.Hellmich H L, Kos L, Cho E S, Mahon K A, Zimmer A. Mech Dev. 1996;54:95–105. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 54.Suvanto P, Hiltunen J O, Arumae U, Moshnyakov M, Sariola H, Saino K, Saarma M. Eur J Neurosci. 1996;8:816–822. doi: 10.1111/j.1460-9568.1996.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez M P, Silos-Santiago I, Frisen J, He B, Lira S A, Barbacid M. Nature (London) 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 56.Pichel J G, Shen L, Sheng H Z, Granholm A-C, Drago J, Grinberg A, Lee E J, Huang S P, Saarma M, Hoffer B J, Sariola H, Westphal H. Nature (London) 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 57.Moore, M. W., Klein, R. D., Farinas, I., Sauer, H., Armanini, MN., Phillips, H., Reichardt, L. F., Ryan, A. M., Carver-Moore, K. & Rosenthal, A. Nature (London) 382, 76–79. [DOI] [PubMed]
- 58.Trupp M, Arenas E, Fainzilber M, Nilsson A-S, Sieber B-A, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U, Sariola H, Saarma M, Ibanez C F. Nature (London) 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- 59.Durbec P, Marcos-Gutierrez C V, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V. Nature (London) 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 60.Treanor J J S, Goodman L, de Sauvage F, Stone D M, Poulsen K T, Beck C D, Gray C, Armanini M P, Pollock R A, Hefti F, Phillips H S, Goddard A, Moore M W, Buj-Bello A, Davies A M, Asai N, Takahashi M, Vandlen R, Henderson C E, Rosenthal A. Nature (London) 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 61.Jing S, Wen D, Yu Y, Holst P L, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J-C, Hu S, Altrock B W, Fox G M. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 62.Kalcheim C, Carmeli C, Rosenthal A. Proc Natl Acad Sci USA. 1992;89:1661–1665. doi: 10.1073/pnas.89.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


