Abstract
The current study evaluated the effects of tokens delivered on differential reinforcement of zero-rate behavior (DRO) schedules or noncontingently on tic suppression in 4 children with tics. Tic frequency was lower in 3 of 4 children when tokens were delivered contingent on the absence of tics than when tokens were delivered noncontingently.
Keywords: differential reinforcement of other behavior, noncontingent reinforcement, suppression, tic disorder, tokens, Tourette syndrome
Motor and vocal tics are typically conceptualized as being involuntary (i.e., not learned; Leckman, Bloch, Scahill, & King, 2006). Nevertheless, research has shown that tics can be modified, at least temporarily, by environmental events. Woods and Himle (2004) and Himle and Woods (2005) demonstrated that tics were temporarily reduced when children were instructed to suppress their tics and token reinforcers were delivered for tic-free periods (i.e., differential reinforcement of zero-rate behavior, DRO). Furthermore, it was shown that suppression was greater during DRO than when only instructions to suppress tics were provided. Results from these studies suggest that tics are potentially modifiable by environmental consequences. Although it seems plausible (if not likely) that the omission contingency was responsible for tic reduction in these studies, it is also possible that some other feature of the experimental paradigm was responsible. For example, tokens delivered during DRO may have served as discriminative stimuli (i.e., prompts) for verbally mediated suppression strategies.
Understanding the influence of reinforcement on tic frequency is interesting for several reasons. For example, tics often wax and wane in response to specific contextual stimuli (e.g., social situations; Silva, Munoz, Barickman, & Friedhoff, 1995). A history of differential reinforcement for tic suppression across different settings is one possible explanation for this variation. However, an important first step in evaluating this hypothesis is to demonstrate that tics are generally responsive to reinforcement contingencies.
The current study examined the role of the reinforcement contingency in tic suppression by comparing tic frequency during conditions of no suppression (baseline), suppression instructions plus DRO, and suppression instructions plus noncontingent reinforcement (NCR). The rationale was that if the omission contingency was indeed responsible for tic suppression, tic frequency during DRO should be lower than during NCR and baseline.
Method
Participants
Four typically developing children (Alex, Ben, Carl, and Dawn) who met criteria for Tourette syndrome or chronic motor/vocal tic disorder of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000) and who exhibited tics at a rate of at least 1 per minute participated in the study. Alex was an 8-year-old boy whose tics included exaggerated eye blinking, head jerking, arm and hand tensing, symmetrical touching (i.e., touching an object with one hand followed immediately by touching the same object with the other hand), throat clearing, whistling, various vocalizations (e.g., saying “sucker”), and shouting an obscene sentence. Ben was a 9-year-old boy whose tics included shoulder shrugging, head rolling, patterned head jerking (side to side), and exaggerated mouth movements (smiling). Carl was an 8-year-old boy whose tics included eye darting, exaggerated blinking, eyebrow raising, head jerking (backward extension) that was accompanied by tongue protrusions, lip “popping,” throat clearing, and sniffing. Dawn was a 10-year-old girl whose tics included mouth movements, eyebrow raising, eye rolling, and throat clearing.
Procedure
Each child participated in repeated sessions of three experimental conditions: baseline, DRO, and NCR. Following the protocol of Woods and Himle (2004), each child was seated alone in a small room facing a token dispenser and were told that the token machine was capable of monitoring and counting their tics, although it was not. They were also told that at various times during the study they could earn tokens that could later be exchanged for small prizes. The experimental conditions (including detailed descriptions of the various contingencies) were explained to the child. The child was told that during the DRO condition tokens would be delivered only after 10-s tic-free periods and that during the NCR condition, although they were expected to suppress their tics, they would receive tokens regardless of whether tics were exhibited.
A multielement design was used to evaluate the effects of DRO and NCR on tic reduction, and different-colored tokens were used in each condition to facilitate differential responding. Each session was 5 min in duration, and all sessions were conducted consecutively on the same day. During baseline, children were instructed to relax and to tic freely while being monitored by the token machine. During DRO, children were instructed to suppress all tics and received a token delivered on a resetting 10-s DRO schedule. During NCR, children were instructed to suppress all tics and received a token delivered on a fixed-time (FT) schedule regardless of whether or not a tic was observed. The number of tokens delivered during each NCR session was yoked to the previous DRO session such that the schedule value was determined by dividing the length of the session (300 s) by the number of tokens that were delivered during the previous DRO session. To yolk the NCR schedules, a DRO session was always administered first. Following the initial DRO session, all subsequent sessions were conducted in random order. Each condition was repeated four times for a total of 12 sessions. Ben asked to discontinue the study after nine sessions (three repetitions of each condition), and Alex is missing one of the DRO conditions due to equipment failure. When multiple NCR sessions were administered consecutively, each session was yoked to the most recently administered DRO session.
A researcher covertly observed and videotaped the child from an adjacent control room that was separated from the experimental room by a one-way mirror. The token dispenser was activated manually. Immediately following each session, the child was asked to repeat the instructions and was asked to indicate (yes or no) whether he or she had attempted to suppress tics. All children consistently responded in a manner consistent with the expectations of the respective session. After completion of the experiment, children exchanged their tokens for a preferred prize, regardless of the number of tokens earned.
Measurement and Interobserver Agreement
After completion of the study, videotapes were scored for tic frequency (the dependent variable) using the videotape scoring procedures outlined by Himle et al. (2006). Two independent observers, naive to the purpose of the study but not necessarily blind to condition, coded each of the videotapes. Interobserver agreement was calculated for all sessions using a frequency-within-interval method (see Himle et al.). Mean interobserver agreement was 74% (range, 66% to 90%) for Alex, 92% (range, 87% to 100%) for Ben, 86% (range, 82% to 100%) for Carl, and 78% (range, 74% to 94%) for Dawn.
Integrity of The Independent Variable
A successful token delivery during DRO was defined as a token that was delivered within 10 ± 1.5 s of either the previous tic or the previous token. A successful token delivery during NCR was defined as a token that was delivered within ±1.5 s of the corresponding FT schedule value. Mean successful token delivery was 92% during DRO (range, 80% to 96%) and 98% during NCR (range, 96% to 100%).
Results and Discussion
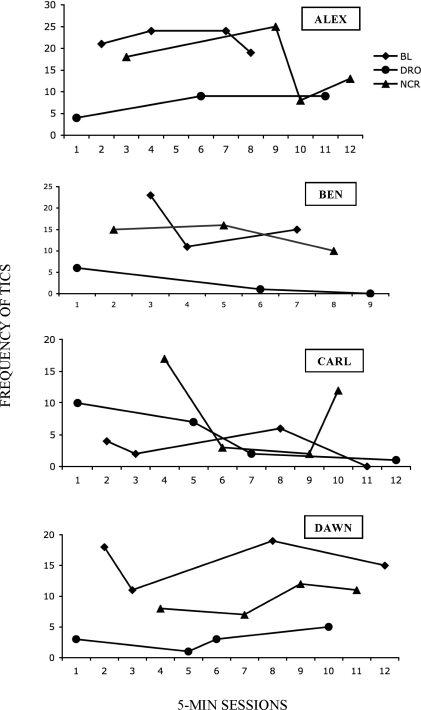
Figure 1 shows the results for all participants; 3 of the 4 children (Alex, Ben, and Dawn) demonstrated suppression of tics during DRO compared to baseline. Alex's mean tic frequency was 22 during baseline and 7 during DRO, and no data points overlapped between the two conditions. Ben's mean tic frequency was 16 during baseline and 2 during DRO, with no overlapping data points. Dawn's mean tic frequency was 16 during baseline and 3 during DRO, with no overlapping data points. Of the 3 children who achieved suppression under DRO, only Dawn demonstrated tic suppression during NCR. Her mean tic frequency of 9 during NCR was lower than baseline (16) and higher than during DRO (3). Alex and Ben did not show reliable tic suppression during NCR.
Figure 1.
Number of tics during baseline (BL), differential reinforcement of zero-rate behavior (DRO), and noncontingent reinforcement (NCR) conditions for Alex, Ben, Carl, and Dawn.
Carl's tic frequencies were undifferentiated across experimental conditions and fluctuated over the course of the evaluation. It is possible that this pattern is a result of multiple-treatment interference within the multielement design. A more extensive evaluation might have produced schedule control. An alternative explanation for Carl's data, however, is that his tics were insensitive to the instructions and contingencies that operated during the experiment.
The results are consistent with several recent studies showing that tic frequency can be reduced when reinforcers are provided for tic-free periods. Furthermore, the fact that the noncontingent delivery of tokens did not reliably suppress tics strongly suggests that the contingency within DRO was responsible for its effects.
These findings are important for several reasons. First, recent studies have shown that tics are worsened (or attenuated) by a variety of environmental factors (e.g., social settings; Silva et al., 1995). The results of the present study suggest that some of this contextual variability may perhaps be explained by reinforcement. For example, a common clinical phenomenon is that children emit tics more at home than they do at school. Perhaps it is the case that children display fewer tics in the classroom because tic absence has been reinforced by the avoidance of teasing from peers. The results of the present study may also have implications for refining behavioral treatments for tics. Behavioral treatments, such as habit reversal training (HRT), are typically delivered as a treatment package that includes awareness training, competing response training, and social support. The social support component of HRT typically uses a support person to prompt and reinforce the appropriate use of the competing response. The results of the current study highlight the importance of reinforcement for achieving suppression. Future research should evaluate whether formal reinforcement arrangements, such as DRO, enhance the effectiveness of procedures such as HRT for teaching children to suppress tics, especially in the early stages of treatment or for those children who have difficulty learning to use a competing response.
There are limitations to the study that also warrant mention. First, children were not taught specific behaviors to use during the suppression task (e.g., competing or antagonistic behaviors). Thus, it is unclear exactly what specific behavior was being strengthened during DRO. It is possible that the participants had already learned tic-suppressing repertoires and that the DRO acted on these strategies. Interestingly, recent research has shown that tics are often preceded by aversive “premonitory urges” that worsen when tics are suppressed and are assuaged by performance of the tic (i.e., tics are negatively reinforced; Woods, Piacentini, Himle, & Chang, 2005). If this is the case, then perhaps DRO provided reinforcers that were capable of competing with the reinforcement obtained by tics. Future research should examine the specific strategies that children use to suppress their tics during DRO and how these behaviors are influenced by specific reinforcement contingencies. It is also notable that although the reinforcement contingency was an important factor in suppressing tics, the fact that children were informed a priori of the contingency and tic reduction under DRO was observed with very little exposure suggest that tic reductions may have been rule governed. Future research should isolate the effects of instructions from those of the omission contingency.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.) Washington, DC: Author; 2000. [Google Scholar]
- Himle M.B, Chang S, Woods D.W, Pearlman A, Buzzella B, Bunaciu L, et al. Establishing the feasibility of direct observation in the assessment of tics in children with chronic tic disorders. Journal of Applied Behavior Analysis. 2006;39:429–440. doi: 10.1901/jaba.2006.63-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himle M.B, Woods D.W. An experimental evaluation of tic suppression and the rebound effect. Behaviour Research and Therapy. 2005;43:1443–1451. doi: 10.1016/j.brat.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Leckman J.F, Bloch M.H, Scahill L, King R.A. Tourette syndrome: The self under siege. Journal of Child Neurology. 2006;21:642–649. doi: 10.1177/08830738060210081001. [DOI] [PubMed] [Google Scholar]
- Silva R.R, Munoz D.M, Barickman J, Friedhoff A.J. Environmental factors and related fluctuation of symptoms in children and adolescents with Tourette's disorder. Journal of Child Psychology and Psychiatry. 1995;36:305–312. doi: 10.1111/j.1469-7610.1995.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Woods D.W, Himle M.B. Creating tic suppression: Comparing the effects of verbal instruction to differential reinforcement. Journal of Applied Behavior Analysis. 2004;37:417–420. doi: 10.1901/jaba.2004.37-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.W, Piacentini J.C, Himle M.B, Chang S. Initial development and psychometric properties of the premonitory urge for tics scale (PUTS) in children with Tourette syndrome. Journal of Developmental and Behavioral Pediatrics. 2005;26:1–7. doi: 10.1097/00004703-200512000-00001. [DOI] [PubMed] [Google Scholar]