Abstract
Periodic travelling waves have been reported in a number of recent spatio-temporal field studies of populations undergoing multi-year cycles. Mathematical modelling has a major role to play in understanding these results and informing future empirical studies. We review the relevant field data and summarize the statistical methods used to detect periodic waves. We then discuss the mathematical theory of periodic travelling waves in oscillatory reaction–diffusion equations. We describe the notion of a wave family, and various ecologically relevant scenarios in which periodic travelling waves occur. We also discuss wave stability, including recent computational developments. Although we focus on oscillatory reaction–diffusion equations, a brief discussion of other types of model in which periodic travelling waves have been demonstrated is also included. We end by proposing 10 research challenges in this area, five mathematical and five empirical.
Keywords: ecological modelling, reaction–diffusion, wavetrains, spatio-temporal patterns
1. Introduction
Over the last decade, a number of spatio-temporal field studies have reported periodic travelling waves in populations undergoing multi-year cycles (table 1). The same period has seen significant advances in the mathematical understanding of periodic travelling wave solutions of oscillatory reaction–diffusion equations. In this article, we review these developments, highlighting existing and unrealized synergies between ecology and mathematics, and suggesting key research challenges for the future.
Table 1.
The most extensively studied populations exhibiting population cycles, their associated spatial dynamics and example hypotheses for the mechanism of cycle generation. We conducted a thorough literature review in the process of constructing this table; however, it may not present all possible hypotheses for the population cycles of the selected taxa, or their associated spatial dynamics. A large number of hypotheses have been proposed and tested for the generation of cycles in different populations and we refer the reader to recent reviews by Berryman (2002) and Turchin (2003) for details of the controversies and debates. Note that not all populations of the selected taxa are cyclic.
| taxon | cycle period | examples of interactions hypothesized to be generating the cycles | spatial dynamics (TW=travelling waves) | possible hypothesis for spatial dynamics |
|---|---|---|---|---|
| larch budmoth, Zeiraphera diniana | 8–10 years (Turchin 2003) | plant–moth–parasitoid (Turchin 2003), plant–herbivore (Selås 2006a) | TW (Bjørnstad et al. 2002; Johnson et al. 2004) | gradients in habitat connectivity and site productivity (Johnson et al. 2004, 2006) |
| southern pine beetle, Dendroctonus frontalis | 6–9 years (Turchin 2003) | predator–prey (Turchin 2003; Turchin et al. 1999), plant–herbivore (Selås 2006a) | isolated patchy outbreaks (Turchin et al. 1998; Okland et al. 2005) | diffusion-driven instability by predator –prey interaction (Turchin et al. 1998) |
| red grouse, Lagopus lagopus scoticus | 6–11 years (Turchin 2003) | parasite–grouse (Hudson et al. 1998; Lambin et al. 1999; Turchin 2003; Redpath et al. 2006), kin selection (Moss et al. 1996; Matthiopoulos et al. 2003, 2005; Turchin 2003; Mougeot et al. 2005), plant–herbivore (Selås 2006a) | regional synchrony in some years (Cattadori et al. 2005), TW (Mougeot et al. 2005) | seasonal forcing (Cattadori et al. 2005), habitat boundary (Sherratt et al. 2003), productivity gradient (Johnson et al. 2006) |
| Fennoscandian voles, Microtus spp. and Clethrionomys spp. | 3–5 years (Turchin 2003) | predator–prey (Klemola et al. 1997; Korpimäki & Norrdahl 1998; Turchin 2003), plant–herbivore (Selås 1997, 2006a,b) | landscape-scale synchrony, TW (Ranta & Kaitala 1997) | nomadic generalist predators (Ims & Andreassen 2000) |
| Fennoscandian lemmings, Lemmus spp. | 3–4 years (Turchin 2003) | plant–herbivore (Turchin 2003), plant–herbivore–predator (Pitelka & Batzli 2007) | wide-scale synchrony (Angerbjörn et al. 2001) | climatic forcing (Angerbjörn et al. 2001) |
| Kielder Forest field voles, Microtus agrestis | 3–5 years (Turchin 2003) | local environment (Ergon et al. 2001), not predator–prey (Graham & Lambin 2002) | TW (Lambin et al. 1998; Mackinnon et al. 2001; Bierman et al. 2006) | landscape obstacle (reservoir) (Sherratt et al. 2003) |
| grey-sided voles, Clethrionomys rufocanus | 2–5 years (Stenseth et al. 1996) | predator–prey (Bjørnstad et al. 1999; Stenseth et al. 2002) | regional synchrony (Bjørnstad et al. 1999; Stenseth et al. 2002; Haydon et al. 2003) | seasonal forcing (Stenseth et al. 2002; Haydon et al. 2003), mobile predators (Bjørnstad et al. 1999) |
| Canadian lynx, Lynx canadensis | 9–11 years (Turchin 2003) | plant–herbivore–predator (Krebs et al. 2001; Turchin 2003), plant–herbivore (Selås 2006a) | large-scale synchrony (Stenseth et al. 1999; Schwartz et al. 2002; Rueness et al. 2003; Selås 2006a), TW (Ranta et al. 1997) | climatic forcing (Stenseth et al. 1999; Selås 2006a), widespread movement by lynx (Schwartz et al. 2002; Rueness et al. 2003) |
| spruce needleminer, Epinotia tedella | 6–7 years (Münster-Swendsen 2002) | host–parasitoid (Münster-Swendsen & Berryman 2005), plant–herbivore (Selås (2006a) | regional synchrony (Münster-Swendsen 2002) | unknown |
| autumnal moth, Epirrita autumnata | 9–10 years (Tanhuanpää et al. 2002) | moth–parasite (Tanhuanpää et al. 2002; Turchin 2003) plant–herbivore (Selås 2006a; Selås et al. 2001, 2004; Selås 1997; Yang et al. 2007) | TW (Tenow et al. 2007; Nilssen et al. 2007), regional synchrony (Tanhuanpää et al. 2002; Klemola et al. 2006) | masting by trees (Selås et al. 2001), sunspots (Selås et al. 2004) |
Although cyclic populations could oscillate uniformly across their habitat, data indicate that this is often not the case (e.g. table 1). Rather, the cycles have different phases at different locations, so that a peak in density at one location occurs simultaneously with a trough at another. The term ‘periodic travelling wave’ refers to a particular type of non-uniform distribution, in which the population density varies periodically in one spatial direction, as well as in time, with the spatial and temporal oscillations combining to give the appearance of a wave in population density. However, there is no net propagation of individuals with this wave; the wave speed is simply the ratio of the space and time periods. Note that the time period is the number of years for one complete population cycle at a fixed point in space, and space period is more commonly called wavelength. The significance of a periodic travelling wave is the correlated spatial and temporal density variations that it implies. A useful analogy is the ‘Mexican wave’ seen in sports stadia. Here each spectator raises and lowers their arms in a manner that is slightly out of phase with the oscillations of their neighbours' arms. The result is that a wave appears to run around the stadium, although none of the spectators is actually moving. Similarly, phase differences in population cycles at nearby locations generate a periodic wave travelling across the domain.
Detection of periodic travelling waves in field studies requires extensive spatio-temporal data and specific methods of statistical analysis. In §2 we give an overview of these methods, and review the ecological systems for which they have demonstrated periodic travelling waves. Many different types of mathematical model for cyclic populations show periodic travelling waves when simulated numerically, but there is a significant body of mathematical theory for such waves in only one class of model: oscillatory reaction–diffusion equations. This review will concentrate on this type of model, and in §3 we will describe the basic theory focusing on the notion of a wave family. Periodic travelling waves are certainly not a feature of all populations undergoing multi-year cycles, and in §4 we describe model predictions of the various scenarios in which periodic travelling waves will occur, and their ecological relevance. In §5 we discuss the important issue of the stability of periodic travelling waves. Finally, in §6 we give some examples of model types other than oscillatory reaction–diffusion models in which periodic travelling waves have been demonstrated, before listing the questions that we see as the key drivers for future research in this area.
2. Field data on periodic travelling waves
Spatio-temporal patterns in cyclic populations are characterized by the way in which the synchrony in population dynamics changes across the landscape (Bjørnstad et al. 1999; Koenig 1999; Liebhold et al. 2004). This synchrony can occur through both biotic and abiotic mechanisms (Liebhold et al. 2004). The biotic mechanisms may be the dispersal of individuals belonging to the cyclic populations themselves (Bjørnstad et al. 2002), or they could be the dispersal of some other organism that plays an important role in the dynamics of the cyclic populations (Ims & Andreassen 2000). The abiotic mechanisms could be large-scale stochastic perturbations in the environment that set all cyclic populations to the same cycle phase (the Moran effect (Moran 1953): see Koenig (2002) and Royama (2005) for details, and Cattadori et al. (2005) for a recent example) or could be multi-year oscillations in some important environmental factor, such as the North Atlantic Oscillation (Stenseth et al. 2004) or 10-year sunspot cycles (Selås 2006a). In recent decades, analyses of the dynamics of cyclic populations have revealed a variety of spatio-temporal behaviours (table 1). We will first give a brief overview of the techniques used to distinguish periodic travelling waves from other spatio-temporal patterns, before discussing the systems in which they have been found and possible reasons for their occurrence.
2.1 Methods for detecting travelling waves in empirical data
The raw material required to detect periodic travelling waves in the field is estimates of population size, at different sites in space, over time. Such datasets are rare due to the considerable investment of time and money required for data collection. Some of the best long-term datasets on the spatial dynamics of cyclic populations were, at least initially, collected for purposes other than ecological research (Krebs et al. 2001; Shaw et al. 2004). For example, the legendary 10-year cycles in Canadian lynx were originally inferred from the fur trading records kept by the Hudson's Bay Company (Krebs et al. 2001). Targeted ecological surveys commonly collect indices of abundance, such as signs of activity, as well as more direct, but more resource intensive, measures of abundance such as can be obtained through capture–mark–recapture methods. A variety of statistical techniques have been developed to allow potential bias and uncertainty to be taken into account when estimating abundance from these different sources of data (Greenwood & Robinson 2006).
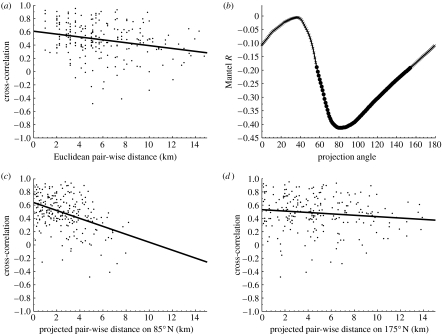
Prior to analysis, a dataset is usually modified in some way to remove spurious trends that are not of interest (Koenig 1999; Turchin 2003, ch. 7). For instance, time series of population growth rates rather than population densities are typically used in subsequent analyses because it is the synchrony in the change in population sizes, rather than in absolute abundance, which is of primary interest. Sometimes it may be possible to detect spatio-temporal patterns by simply arranging the temporal datasets of each site or location according to its spatial position and comparing the dynamics by eye (Moss et al. 2000; Mackinnon et al. 2001). Usually the first quantitative analysis of the spatio-temporal patterns in a dataset is to measure how the correlation between the dynamics of paired populations changes with the distance (Liebhold et al. 2004). If population oscillations are synchronous across the study area, then the oscillations at any two sites will be significantly positively correlated, regardless of their distances apart. More typically, however, cross-site synchrony tends to decrease with distance (Liebhold et al. 2004). For example, for field vole populations in Kielder Forest (northern UK), figure 1a illustrates how cross-site synchrony declines with the Euclidean distance between the sites.
Figure 1.
Spatio-temporal patterns can be partly revealed by measuring how the synchrony between the population dynamics of different sites varies with the distance between sites. To illustrate this we present analysis of data on the field vole populations in Kielder Forest (see Lambin et al. (1998, 2000), Mackinnon et al. (2001) and Bierman et al. (2006) for more details of this system and more detailed analysis). The raw data consist of average population density estimates from abundance indices (see Lambin et al. (2000) for a methodological description) for a range of sites, over the period 1984–1992. These are the years for which evidence of periodic travelling waves is the strongest: more recent data are not so indicative of a unidirectional wave (see Bierman et al. 2006 for details). We selected the spring (March–May) and autumn (September–November) population estimates for the Kielder Forest area only. We divided the map of Kielder Forest into 1 km squares and averaged the estimated population densities of sites if they occurred within the same 1 km square. We then used the centre of the 1 km squares as site locations. For each square we then calculated the time series for the rate of change of the population over the six-month interval between observations. We then analysed how the synchrony in the time series of population growth rates varies with distance. The degree of synchrony was indicated by the cross-correlation between the time series; we calculated this only between sites with six or more measurements made over the same time period. (a) The cross-correlation between the time series of population growth rates significantly declines with the Euclidean distance between sites. The Mantel test (see the main text for a description) indicates that the relationship between distance and correlation is significant in (a) (Mantel statistic R=−0.26, p<0.05). We have added a linear regression line to highlight the general trend in the dataset. Directionality in spatial pattern can be revealed by calculating the Mantel R statistic for projected distance: the distance between the perpendicular projections of the site locations onto a straight line. (b) The Mantel R statistic varies with projected distance between sites in Kielder Forest (crosses, n.s.; circles, p<0.05; the latter indicates the Mantel R statistic is significant). (c) Cross-correlation between population growth rate against site distance when projected at 85° N, the most significant projection angle (as indicated in (b); R=−0.41, p<0.001). In contrast, in (d), in which the angle of projection is perpendicular to that in (c) (175° N, R=−0.13, p=0.11), the cross-correlation between site time series does not decline significantly with projected distance.
Mantel tests are used to assess whether cross-site synchrony changes significantly with the distance (Legendre & Legendre 1998; Koenig 1999; Liebhold et al. 2004). In general, this technique tests for a significant correlation between two sets of data in matrix format. For the purpose of detecting spatio-temporal patterns in population data, one matrix is the distance between sites and the other is the correlation between the population dynamics of site pairs (usually Pearson's moment correlation; see Bjørnstad et al. (1999) for details). The Mantel test gives a value, similar to Pearson's correlation coefficient, which indicates the sign and strength of the correlation between the two matrices. The significance of the resulting statistic is tested by permutation. Rows or columns in one of the matrices are randomly shuffled and reanalysed to give a comparison with the original data (see Legendre & Legendre (1998) and Koenig (1999) for details). This is repeated a large number of times to enable the calculation of the probability that the original relationship is significantly different from random (see Koenig (1999) and Liebhold et al. (2004) for further discussion of this technique). For periodic travelling waves, one would expect cross-site synchrony to initially decline with distance relatively steeply in the direction of wave propagation, and then rise again as the site separation approaches one wavelength. In contrast, one would expect sites to remain relatively synchronous as their separation increases perpendicular to the direction in which the wave is travelling. Such directionally biased patterns in synchrony can be revealed by analysing how cross-site synchrony declines with projected distance: the distance between the perpendicular projections of the site locations onto a straight line. In figure 1b, we show the results of such an analysis for the field vole dataset. In this figure, cross-site synchrony only declines significantly with the projected distance for some projection angles. Such evidence is supportive of a unidirectional travelling wave; cross-site synchrony declines most strongly with projected distance in the direction of wave propagation (as shown in figure 1c), and does not significantly decline in the perpendicular direction (as shown in figure 1d). Note that, in some studies of other datasets, nonlinear relationships between synchrony and distance have been fitted (Ranta et al. 1997; Bjørnstad et al. 2002). This is appropriate since synchrony will oscillate with distance in the direction of wave propagation, with the same wavelength as the periodic wave. However, for field voles in Kielder Forest, the wavelength is significantly greater than the width of the habitat so that a linear relationship is anticipated. (Kielder Forest is approx. 30 km wide, while the wave speeds of 19 and 14 km yr−1, reported by Lambin et al. (1998) and Mackinnon et al. (2001), respectively, correspond to wavelengths of approx. 76 and 56 km, respectively.)
Once evidence of travelling waves has been obtained, a variety of statistical techniques can then be used to estimate the wavelength and speed of the waves. Analysis of the time-lagged synchrony between sites is a relatively straightforward extension to Mantel test-based techniques, enabling the estimation of wave characteristics. Here the dynamics at one of the sites is shifted in time prior to analysis (Bjørnstad et al. 2002). If there is a travelling wave, then the lagged cross-site synchrony will peak at a distance and direction corresponding to wave propagation, over the given period of time. Another method is to fit the statistical models of travelling waves to the data (Moss et al. 2000; Mackinnon et al. 2001). This has the additional advantage of allowing the estimation of the extent to which wave characteristics vary through space and time. Wavelet phase analysis is another recently developed and powerful technique for detecting travelling waves and estimating any variation in their properties through space and time (see Liebhold et al. (2004) for a description this technique and Johnson et al. (2004) for a recent application). This technique has advantages over the more traditional Mantel test-based techniques, especially for detecting spatio-temporal patterns in cyclic populations (as summarized in Liebhold et al. 2004). For example, Liebhold et al. (2004) argue that this method is unaffected by the relative amplitudes of the time series in question, and can be used to identify multiple periodic patterns within datasets, such as simultaneous seasonal and multi-year cycles.
2.2 Empirical evidence for travelling waves
Using the approaches outlined above, travelling waves have been detected in a variety of cyclic animal populations. Indeed, where such analyses have been conducted, travelling wave phenomena appear to be common, as illustrated in table 1.
The larch budmoth populations in the European Alps are one system for which there is strong evidence of periodic travelling waves. The almost metronomic multi-year dynamics of larch budmoth populations has fascinated ecologists for decades (Turchin et al. 2002, ch. 9). Recent studies have shown that these cycles are organized into travelling waves that move at approximately 250 km yr−1, although there is considerable variation in this estimate (Bjørnstad et al. 2002; Johnson et al. 2004). Johnson et al. (2004) also estimated how the speed and direction of the travelling waves change across space. This revealed that travelling waves appear to move away from epicentres of high-productivity habitat towards the surrounding lower quality habitat.
A study of the spatio-temporal dynamics of cyclic red grouse populations in one area of Scottish moorland (Moss et al. 2000) also revealed periodic travelling waves in abundance (moving at 2–3 km yr−1). In contrast, studies of cyclic red grouse populations in five different regions in northern England showed that climatic conditions in May and January can force the populations within each region to oscillate in synchrony (Cattadori et al. 2005). This study does not report any travelling wave phenomena and instead suggests that, in these red grouse populations, cycles are synchronized by a Moran effect (Koenig 2002; Royama 2005). The differences in the findings of these two studies may be due in part to differences in the scale and the type of analysis. However, the mechanisms driving the population cycles are also thought to differ in the Scottish and English populations (Turchin 2003, ch. 11).
Contrasting spatio-temporal dynamics have also been reported in the various cyclic rodent populations. Studies of vole and lemming populations in Scandinavia indicate wide-scale synchrony (Ims & Andreassen 2000; Angerbjörn et al. 2001), although there is some evidence that travelling waves may occur in cyclic vole populations in Finland (Ranta & Kaitala 1997). In contrast, data from the cyclic populations of field voles in Kielder Forest (northern UK) show that the 3–5 year cycles in population density are spatially organized into unidirectional periodic travelling waves (Lambin et al. 1998; Mackinnon et al. 2001). Again, there are differences in what is believed to be causing these cycles. In Scandinavia, there is evidence that some vole population cycles are caused by a specialist-predator–prey interaction (Oli 2003; see also Selås (2006b) for an alternative hypothesis, and Lambin et al. (2006) for a more general discussion). However, experiments in Kielder Forest have suggested that the cycles are not generated in this way (Graham & Lambin 2002; see also the related correspondence in Korpimäki et al. (2003) and Lambin & Graham (2003)).
Bierman et al. (2006) analysed the temporal trends in the spatial and temporal dynamics in the Kielder Forest field vole dataset. They restricted the dataset to particular time frames and studied what happened to their estimates as the time frame was shifted. They found that evidence for both population cycles and travelling waves was strong at the start of the dataset but absent at the end. Such changes were associated with dramatic reductions in the length and severity of the winter. Changes through time in spatial dynamics were also found recently for the cyclic autumnal moth populations in northern Scandinavia (Nilssen et al. 2007; Tenow et al. 2007). Studies by Selås et al. (2001, 2004) suggest that synchrony in the population cycles may be induced by region-wide synchrony in their food supply, which is possibly connected to sunspot activity. In contrast, Tenow et al. (2007) showed that the population cycles of the autumnal moth were sometimes organized into periodic travelling waves, and on other occasions the cycles occurred synchronously over wide areas. Nilssen et al. (2007) and Tenow et al. (2007) argue that, since the dispersal rates of the autumnal moth are relatively low in relation to the speed of movement of the periodic travelling wave, dispersal cannot account for the region-wide synchrony in the population dynamics. Instead they argue that the populations could become synchronized through the effects of the North Atlantic Oscillation.
Of all population datasets, the annual records of fur returns of the Canadian lynx (Lynx canadensis) populations, made by the Hudson's Bay Company in Canada, are unique for the length of time (over 100 years) and spatial scale (the whole of Canada) over which they have been collected (Krebs et al. 2001). Several studies of this dataset, and of more recent data collected by Statistics Canada (www.statcan.ca), have demonstrated population synchrony across large distances (Smith 1983; Ranta et al. 1997; Stenseth et al. 1999; Schwartz et al. 2002). In particular, Stenseth et al. (1999, 2004) showed that the populations could be grouped into three areas with broadly synchronous dynamics, according to three different climatic regions. Ranta et al. (1997) presented an analysis of this dataset showing significant U-shaped changes in cross-site synchrony with distance. These results have been cited as evidence of periodic travelling waves, although the authors did not mention periodic travelling waves in their paper, and did not extend their analysis to look at any directionality in the variation of cross-site synchrony with distance. A very approximate estimate from the graphs presented by Ranta et al. (1997) suggests that, if travelling waves are present, then they will have a wavelength of approximately 4000 km, which is close to the width of Canada! This highlights that periodic travelling waves may not be detected in some systems simply because the habitat is too small for the travelling waves that would arise or because the spatial scale required for analysis is too large. However, it seems plausible that the upturn in synchrony at large between-site distances reported by Ranta et al. (1997) may be an artefact of the fact that the Pacific and Atlantic regions, on either side of Canada and hence the furthest apart, tend to fluctuate more in synchrony with each other than with the populations in the central continental region (Stenseth et al. 2004).
The handful of studies that have now been undertaken show that travelling wave phenomena occur in some cyclic populations, while others show region-wide synchrony or patchy dynamics. Studies are also beginning to focus on why such dynamics occur, and why they might change through space and time. Theoretical studies are a crucial complement to such investigations owing to the time and expense of gathering the necessary field data, and because they enable testing of hypotheses in a way that is not possible in the field.
3. Mathematics of periodic travelling waves I: wave families
The simplest reaction–diffusion models for cyclic populations involve two interacting species, with densities u and v say, at different trophic levels
| (3.1a) |
| (3.1b) |
Thus, u and v may be predator and prey, host and parasite, herbivore and grazer, etc. Here x and y are spatial coordinates and t denotes time. Our focus on cyclic populations means that we assume that the local dynamics fu and fv are such that the spatially uniform equations , have a stable periodic solution (limit cycle), which oscillates either side of an unstable coexistence steady state. The theory of periodic travelling waves is essentially the same for models with three or more interacting species. A classic example of such a system is the 10 year cycles in snowshoe hare and lynx in North America. These are thought to be driven by a combination of hare predation by lynx, and the hare–vegetation interaction (see Turchin (2003, ch. 13) for review, and King & Schaffer (2001) and Stone & He (2007) for modelling).
Throughout this paper, we will use the Rosenzweig & MacArthur (1963) model for predator–prey interactions as a specific example of (3.1a) and (3.1b). When rescaled so that the parameters have no units, the model has the form
| (3.2a) |
| (3.2b) |
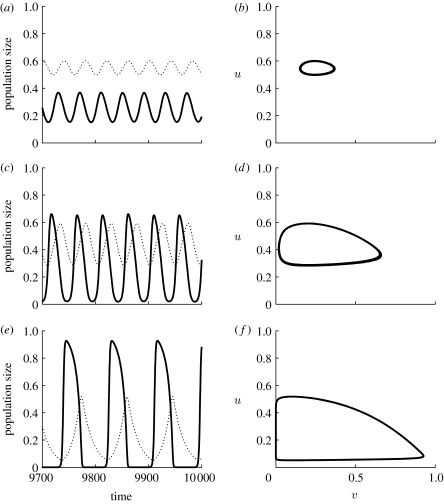
The local dynamics in this model are described in a number of textbooks (e.g. Murray 2002; Britton 2003; Turchin 2003). The variables u and v are the densities of predators and prey, respectively; α is the ratio of prey and predator dispersal coefficients; μ is the predator death rate; σ is the prey to predator conversion rate; and κ is the half-saturation constant in the rate of prey consumption by predators. There is a critical value of κ at which the kinetics have a Hopf bifurcation, with a stable limit cycle for values of κ below this critical value. This is illustrated in figure 2; note that the cycles are approximately sinusoidal and of low amplitude for κ close to the Hopf bifurcation value of 0.5; they increase in amplitude and become non-sinusoidal as κ is reduced. For consistency, all of the illustrative figures in this review are for equations (3.2a) and (3.2b), with small enough κ that the kinetics are cyclic.
Figure 2.
(a–f) An illustration of the local dynamics of the Rosenzweig–MacArthur predator–prey model (3.2a) and (3.2b). We take σ=0.15 and μ=0.05. In this case, the kinetics have a Hopf bifurcation at κ=0.5, with a stable limit cycle for smaller values of κ. We plot predator and prey densities u and v as functions of time and against one another, allowing a large solution time prior to plotting, to allow the solutions to settle on their long-term behaviour. (a,b) κ=0.49, (c,d) κ=0.40, (e,f) κ=0.20. (a,c,e) Solid curve, v; dotted curve, u.
The special relationship between the space and the time dependence of a periodic travelling wave means mathematically that the solution is a function of a single ‘travelling wave’ variable , where c is the wave speed. Thus, and , and (3.1a) and (3.1b) imply
| (3.3a) |
| (3.3b) |
A periodic travelling wave is a limit cycle solution of this ordinary differential equation system. The basic properties of such solutions were established in a landmark paper by Kopell & Howard (1973), and we now summarize their results. Kopell & Howard showed that, under certain conditions (detailed below), there is a one-parameter family of periodic travelling wave solutions of (3.1a) and (3.1b). This notion of a wave family is key to an understanding of periodic travelling waves and therefore merits discussion. For a given set of ecological parameters (α, μ, σ and κ in the case of (3.2a) and (3.2b)), there is a range of possible values for the speed, (spatial) wavelength, time period and amplitude of periodic travelling wave solutions. For example, the amplitude can take any value between zero and the amplitude of the spatially homogeneous oscillations implied by the kinetics. Fixing a value of the amplitude in this range then determines the speed, wavelength and time period. It is in this sense that the family is ‘one-parameter’: a contrasting example is nonlinear water waves, for which amplitude and wavelength can be specified independently, with time period then determined (i.e. a two-parameter wave family; Billingham & King 2000). For periodic travelling waves in (3.1a) and (3.1b), any one of amplitude, speed, wavelength and time period can typically be used to parametrize the family. Figure 3a illustrates the periodic travelling wave families for five different parameter sets in (3.2a) and (3.2b), via plots of the time period against wave speed. Note that the limiting case of infinite wave speed corresponds to spatially homogeneous oscillations. We also show (figure 3b) an example of a periodic travelling wave: this is one member of one of the families.
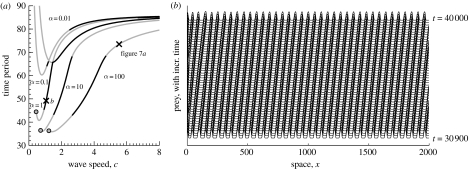
Figure 3.
(a) Comparison of travelling wave families for predator–prey equations (3.2a) and (3.2b) with different values of the parameter α (labelled). The other parameter values are σ=0.15, μ=0.05 and κ=0.2. Grey-filled circles indicate the position of the Hopf bifurcation in equations (3.3a) and (3.3b) from which the wave family emanates. This Hopf bifurcation does not exist for the lines with no filled circles. Grey lines denote unstable waves and black lines denote stable waves. These lines were drawn, and their stability profiles calculated, using the software package Auto (see text for an explanation). The Auto code is available at www.ma.hw.ac.uk/∼jas/supplements/ptwreview/index.html. The labelled crosses denote the periodic travelling waves selected in simulations of equations (3.2a) and (3.2b) that are illustrated in (b) and in figure 7a. In (b), equations (3.2a) and (3.2b) were solved numerically on a one-dimensional domain with u=v=0 at x=0 (zero Dirichlet boundary conditions, simulating a landscape obstacle) and ∂u/∂x=∂v/∂x=0 at x=2000 (zero Neumann boundary conditions), with randomly chosen initial values for u and v. The zero Dirichlet condition forces the system away from spatially uniform oscillations and generates a periodic travelling wave.
More specifically, Kopell & Howard proved two separate results on the existence of a periodic travelling wave family: (i) there is a family of large-amplitude waves in any oscillatory reaction–diffusion system and (ii) there is a family of small-amplitude waves provided that the dispersal coefficients Du and Dv are sufficiently close. Intuitive expectation, and extensive numerical evidence, indicates that these results characterize the two ends of a single wave family, although, to the best of our knowledge, this remains unproven for general reaction–diffusion equations. To prove (ii), Kopell & Howard showed that (3.3a) and (3.3b) has a Hopf bifurcation at some positive value of the wave speed c, and the Hopf theorem then implies a one-parameter family of periodic solutions of (3.3a) and (3.3b) as c increases above this Hopf bifurcation value. A reworking of this proof for the specific equations (3.2a) and (3.2b) is given by Huang et al. (2003), giving more explicit constraints on the diffusion coefficients (and kinetic parameters). More generally for (3.1a) and (3.1b), one requires closeness of Du and Dv because, when these are sufficiently different, there may not be a Hopf bifurcation as c is varied. Numerical computations for (3.2a) and (3.2b) indicate that, in such cases, there is again a one-parameter family of waves, with speeds taking any positive value. However, the generality of this finding remains an important open question.
Kopell & Howard (1973) were able to compute numerically some periodic travelling wave families, but this was a challenging numerical problem at the time. The basic difficulty is that the limit cycle corresponding to a periodic travelling wave is unstable as a solution of (3.3a) and (3.3b), irrespective of its stability as a solution of the partial differential equation (PDE) (3.1a) and (3.1b); therefore the limit cycle cannot be calculated directly. Note that we postpone discussion of PDE stability until §5. Fortunately, subsequent computational advances make the calculation of an unstable limit cycle relatively straightforward. The key tool is numerical bifurcation software, and we use the package Auto (indy.cs.concordia.ca/auto; Doedel 1981, 1997); other numerical packages such as Trilinos (trilinos.sandia.gov; Heroux et al. 2003) can also be used. The basic approach is to increase c from zero, and use Auto to detect the value of c at which (3.3a) and (3.3b) has a Hopf bifurcation. We then restart Auto at this Hopf bifurcation point, and use it to track the limit cycle (i.e. the periodic travelling waves) as c is increased. During this continuation, the detailed form of the periodic travelling wave can be output at any required value of the speed. This approach is effective provided that Du and Dv are such that (3.3a) and (3.3b) has a Hopf bifurcation at some c>0; this is guaranteed when Du and Dv are sufficiently close. Otherwise, we set Du=Dv and follow that wave family. Starting from a point on that family, we then use Auto to track the periodic travelling wave solution with time period fixed but c and Du/Dv varying, until the required ratio Du/Dv is reached. We can then determine the required wave family by fixing Du and Dv, and varying the time period (and c). An example Auto code that illustrates the use of these methods to determine the wave families shown in figure 3a is available at www.ma.hw.ac.uk/∼jas/supplements/ptwreview/index.html.
3.1 Behaviour close to Hopf bifurcation
For (3.2a) and (3.2b) and most other reaction–diffusion models, periodic travelling wave families can be found only via numerical computation. However, there are some particular systems with explicit wave solutions (Kopell & Howard 1973, §II.4; Cope 1979). By far the most important of these is the system
| (3.4a) |
| (3.4b) |
where ω0 and ω1 are parameters satisfying ω0(ω0−ω1)>0. This is a special case of the λ–ω class of equations, introduced by Kopell & Howard (1973). Crucially, the mathematical theory of normal forms (Hassard et al. 1981; Guckenheimer & Holmes 1983) implies that any standard1 oscillatory reaction–diffusion system (3.1a) and (3.1b) with Du=Dv can be transformed into (3.4a) and (3.4b) close to the Hopf bifurcation in the kinetics; p and q are functions of the population densities. Thus, when the spatially homogeneous population cycles are of low amplitude, and Du=Dv, all aspects of periodic travelling wave behaviour can be studied via (3.4a) and (3.4b). The mathematical technique of ‘reduction to normal form’ can be used to determine formulae for ω0 and ω1 in terms of ecological parameters. This is a rather cumbersome algebraic calculation that is greatly facilitated by computer algebra; it is described in detail in the appendices of Sherratt (2001) and Sherratt et al. (2003), with web addresses of computer programs that implement the calculation using the package Maple (www.maplesoft.com; Monagan et al. 2007).
For (3.4a) and (3.4b), the periodic travelling wave family has the simple form
| (3.5a) |
| (3.5b) |
where θ0 is an arbitrary constant. Here we take the x-axis as the direction of wave propagation. The wave amplitude A lies between 0 and 1, with
| (3.6) |
This shows clearly that as A→1−, the wavelength →∞ and the periodic travelling waves approach the spatially uniform cycles of the local dynamics. Taken together with the expressions for ω0 and ω1 obtained via reduction to normal form, these formulae can be used to determine how wave properties vary within the family, provided that Du=Dv and that the kinetics are sufficiently close to Hopf bifurcation.
4. Generation of periodic travelling waves
Kopell & Howard's (1973) paper initiated a large volume of mathematical research over the following decade. At the time there was no suggestion of periodic travelling waves in ecology, and authors had in mind applications in oscillatory chemical reactions (for reviews of this area see Scott (1994), Epstein & Showalter (1996) and Scott et al. (2000)). When these remarkable reactions occur in a very clean or well-stirred reaction vessel, the concentrations of the reactants oscillate periodically with time, and uniformly in space. However, in the presence of impurities, spatio-temporal oscillations develop, consisting of target or spiral patterns (movies illustrating this are available at heracles.chem.wvu.edu/gallery.html).
Periodic travelling waves are the one-dimensional analogue of these patterns, and this provided the motivation for research on periodic travelling waves in the 1970s and 1980s. Thus, there was a major focus on solutions that approach a periodic travelling wave as distance increases in either direction away from a central ‘core’; these are the one-dimensional equivalent of a spiral if the two periodic travelling waves move in the same direction and of a target pattern if the directions of motion are opposite (Ermentrout & Rinzel 1980; Hagan 1981; Kopell 1981; Kopell & Howard 1981). There was also extensive work on extending basic results such as those of Kopell & Howard (1973) to actual two-dimensional waves (Greenberg 1978, 1981; Kuramoto & Koga 1981; Hagan 1982; Koga 1982). The particular case of radially symmetric waves has been the subject of a more recent and very detailed study by Scheel (2003; for a general review of more recent work, see Fiedler & Scheel 2003).
Impurities in a chemical reaction vessel have a natural analogue in ecological applications, namely spatial noise in parameter values. One can expect this to be present in any ecological system and, if it is sufficiently strong, it can generate periodic travelling waves: results on this for the model (3.2a) and (3.2b) are given in Kay & Sherratt (2000). However, as in the chemical applications, the result is a series of bands of periodic travelling waves, each one relatively small in extent. The experimental precision possible in the chemistry laboratory means that this level of fine detail can easily be detected, but there is little prospect of detection in ecological field data. Rather, the fine-grained spatio-temporal patterns generated by spatial noise would just be perceived as spatially uniform population cycles.
There are, however, two other mechanisms that have been shown to generate periodic travelling waves in oscillatory reaction–diffusion systems: boundary effects and invasion. In contrast to spatial noise in the environment, both these mechanisms have the potential to generate large-scale regions with a single periodic travelling wave, and thus provide possible explanations for the waves seen in ecological field data.
4.1 Periodic travelling wave generation by boundaries with hostile environments
When an ecological habitat is surrounded by a hostile environment, the appropriate boundary condition is of Robin type, , say, where C is a positive constant whose size reflects the hostility of the surroundings (Ludwig et al. 1979; Cantrell et al. 1998). Intuitively, this boundary condition states that, in any given time interval, a fixed proportion of individuals located close to the boundary are lost to the hostile surroundings. Since C is typically very large, it is common to approximate the boundary condition by a simpler one of Dirichlet type: u=0.
Boundary conditions of these types may be appropriate at one or both ends of a habitat, or at the edge of an obstacle in the interior, and may apply to both interacting species in (3.1a) and (3.1b) or to just one. In any of these cases, numerical simulations of (3.2a) and (3.2b) and other equations of the form (3.1a) and (3.1b) show that periodic travelling waves develop. For the Dirichlet condition, this was first studied in an isolated paper of Auchmuty & Nicolis (1976) on a model of an oscillatory chemical reaction, and more recently by Sherratt et al. (2002, 2003) in the work on cyclic populations. Extension to the Robin boundary condition, which is more complicated mathematically, has been studied by Sherratt (submitted). A general classification of periodic travelling waves generated by boundary conditions is possible based on their group velocity far from the boundary; see Sandstede & Scheel (2004) for details of this, and Kollár & Scheel (2007, §1.3) for a brief summary.
Figure 3b illustrates the generation of a periodic travelling wave by Dirichlet boundary conditions for the predator–prey model (3.2a) and (3.2b). Except for a narrow region close to the boundaries, the entire domain contains a single periodic travelling wave solution. We have described the existence of a wave family for a given set of ecological parameters. The Dirichlet boundary condition acts to select a particular member of this family, thereby fixing wave speed, wavelength, time period and amplitude. Changes in initial conditions, for example, do not alter these wave properties. In figure 3b, the wave moves away from the boundary at x=0, on which the Dirichlet condition is applied; in fact this is always the case for the Rosenzweig–MacArthur model (3.2a) and (3.2b). However, for other models, the wave can move towards the boundary for some parameter values (see Sherratt 2003 for examples). Therefore, the direction of wave propagation relative to the boundary is not a test of this mechanism of wave generation, although it may be usable as a test if the underlying cause of the population cycles has been established and parameter values can be reliably estimated.
For the λ–ω system (3.4a) and (3.4b), a simple formula can be derived for the wave amplitude A that is selected by zero Dirichlet boundary conditions (Sherratt 2003). This in turn yields formulae for other wave properties, using (3.6). The reduction to normal form calculation, described in §3, enables these formulae to be extended to other reaction–diffusion systems (3.1a) and (3.1b) with Du=Dv close to Hopf bifurcation in the kinetics. However, there is a complication because a conversion must be applied to the boundary condition; this is discussed in Sherratt et al. (2003).
The solution shown in figure 3b is for a one-dimensional idealization, but in reality landscape obstacles in ecological domains are of course two dimensional. Target patterns then develop, which approach a one-dimensional periodic travelling wave far from the obstacle (figure 4). An important finding is that this wave varies according to the size of the obstacle, with wavelength and amplitude decreasing as obstacle size increases (Sherratt et al. 2003; Smith et al. 2008). Thus, different members of the periodic travelling wave family are selected by obstacles of different sizes. The particular case of very small obstacles has been studied in detail by Kollár & Scheel (2007).
Figure 4.
Solutions of the predator–prey model (3.2a) and (3.2b), showing the generation of periodic travelling waves by three separate obstacles. Each obstacle generates waves, but those from the largest obstacle dominate the solution. (This is a general finding: see Sherratt et al. (2003) and Smith et al. (2008).) We plot prey and predator density in space at a single time point. The boundary conditions are zero predator and prey densities at the edge of the obstacle, and zero flux at the edge of the domain. The domain is a square with side-length 336 dimensionless space units, and the solution is plotted at a dimensionless time of 1000. The parameter values were μ=0.46, σ=0.83, κ=0.204 and α=0.5. The scale bar uses a linear scale, with preymin=0.03, preymax=0.84, predmin=0.007 and predmax=0.6. The equations were solved numerically using an alternating direction-implicit Crank–Nicolson method. A movie clip corresponding to this figure is available at www.ma.hw.ac.uk/∼jas/supplements/ptwreview/index.html.
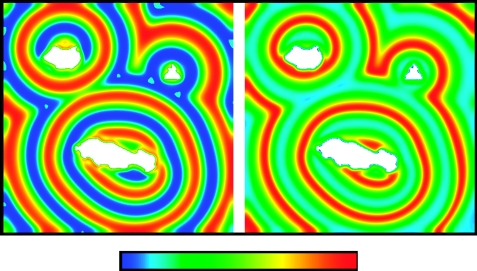
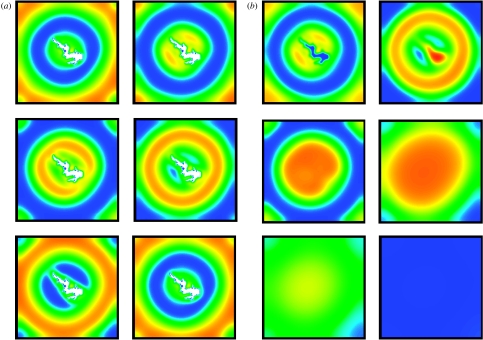
There is no conclusive evidence for any specific wave generation mechanism applying in a particular ecological system that exhibits periodic travelling waves. However, generation by a boundary with a hostile surrounding environment is a plausible candidate in two cases. Kielder Forest (northern UK) contains a large central reservoir that is likely to act as a hostile landscape obstacle for field voles owing to increased hunting by avian predators (mainly short-eared owls) around the reservoir edge. Hence, this boundary would tend to generate periodic travelling waves. Figure 5a illustrates this, via a solution of (3.2a) and (3.2b) on a two-dimensional square domain containing a large central obstacle in the shape of Kielder Water, on which a Dirichlet boundary condition has been imposed. To emphasize the key role of the obstacle in generating the waves, figure 5b shows their gradual disappearance when the reservoir is removed. Note that for this figure we use the model (3.2a) and (3.2b), for consistency with the other figures in this paper. However, the predation hypothesis is in fact considered an unlikely explanation for vole cycles in Kielder (see §2; Graham & Lambin 2002). Despite this, the simulations in figure 5 demonstrate that, if the reservoir is generating the waves, then one would expect that cross-site synchrony will change with distance in a direction-dependent manner, and furthermore that the waves travel in different directions on opposite sides of the reservoir.
Figure 5.
Numerical simulation of periodic travelling wave generation by a large obstacle in the shape of Kielder Water. In (a), we solved the predator–prey model (3.2a) and (3.2b) on a two-dimensional square domain intended as a crude representation of Kielder Forest, with zero Dirichlet boundary conditions applied at the edge of the central obstacle, which is based on a scanned image of Kielder Water. Target pattern waves, which are a simple two-dimensional analogue of periodic travelling waves, propagate outwards from the reservoir. In (b) we first solve as in (a) to allow this target pattern to develop, and we then remove the reservoir by replacing it with empty habitat. It is rapidly colonized, and the target pattern then gradually disappears, to be replaced by spatially uniform oscillations. This emphasizes the role of the reservoir, acting as a landscape obstacle, in generating the waves. The kinetic parameters are μ=0.46, σ=0.83 and κ=0.204; these are based on estimates for a weasel–vole interaction (see Sherratt 2001 for details), although we emphasize that the predation hypothesis is in fact considered an unlikely explanation for vole cycles in Kielder (see §2; Graham & Lambin 2002; Brandt & Lambin 2007). The diffusion ratio α=1, and the dimensionless side length of the domain is 107. In (a), the solutions are shown at six equally spaced times (with time increasing from left to right first; thus, for example, the panel in row 2, column 1, is third in the sequence), with a dimensionless time separation of 3.3; under the parameter estimates of Sherratt (2001) for the weasel–vole interaction, this corresponds to a dimensional separation of 1 year. The initial population densities were chosen randomly, and a dimensionless time of 300 was allowed for transients to disappear before the first solution was plotted. In (b), the solution times are separated more widely and unequally: they are chosen to best illustrate the disappearance of the target pattern. The colours indicate prey density (blue, low; red, high). The equations were solved numerically using an alternating direction-implicit Crank–Nicolson method. Results of this type could be used to compare model predictions with statistical analysis of field data, such as those presented in figure 1. However, for this to be of value, it would be necessary to have a fully parametrized model for vole cycles based on current ecological data, rather than the predation hypothesis, which is used here for consistency with other figures.
As discussed in §2, periodic travelling waves have been detected in the red grouse populations on Kerloch moor (NE Scotland). The grouse habitat is bordered on one side by farmland, which is known to be hostile to grouse; specifically, DNA testing shows that grouse almost never succeed in crossing the farmland (Piertney et al. 1998). Therefore, the boundary between moorland and farmland is a natural candidate for periodic travelling wave generation. Note that in this case the periodic travelling waves move towards the boundary; as discussed above, this does not exclude boundary-driven wave generation.
4.2 Periodic travelling wave generation by invasion
In their simplest forms, ecological invasions result in one uniform state being replaced by another. For example, a prey population may be invaded by predators, leading to a lower but constant and spatially uniform prey density that coexists with a constant and spatially uniform predator population. However, the temporal complexity of cyclic populations translates into spatio-temporal complexity following an invasion. Again, predator–prey interactions are an ideal example. A large body of theoretical work predicts that, when the parameters of the interaction are such that it generates population cycles, periodic travelling waves develop behind the invasion (Sherratt et al. 1995, 1997; Petrovskii et al. 1998; Petrovskii & Malchow 2000; Sherratt 2001; Garvie 2007). Figure 6 shows a typical example of this mechanism of wave generation, in which a small population of predators has been introduced (near x=0 at time t=0) into an otherwise uniform prey population. An invading front of predators and a corresponding receding front of prey are clearly visible. Behind this, the populations settle towards a coexistence state, even though this state is unstable; Petrovskii & Malchow (2000) have termed this phenomenon ‘dynamical stabilization’. Further from the invading front, the solutions move away from the coexistence state. One might expect spatially uniform oscillations to develop since these are a stable solution,2 but the oscillatory decay of the solutions behind the invasion front forces a spatial component in the behaviour, which consists instead of a band of periodic travelling waves; in figure 6 these move in the opposite direction to the invasion. As for boundary-driven wave generation, the direction of propagation is model dependent and parameter dependent: in some cases, the periodic travelling waves move in the same direction as the invasive front (see Smith & Sherratt 2007 for examples).
Figure 6.
Illustration of periodic travelling waves generated by the invasion of (a) a prey population by (b) predators in one space dimension. We plot population densities as a function of distance from the centre of the obstacle, with the vertical separation of the solutions proportional to the time intervals. There is a receding wavefront of prey, and a corresponding advancing wavefront of predators, behind which there is a periodic travelling wave. We solved equations (3.2a) and (3.2b) numerically for parameter values μ=0.083, σ=0.25, κ=0.33 and α=1, using the method of lines and Gear's method. The initial conditions corresponded to a prey-only state everywhere, except at x=0, where we introduced a small non-zero predator density.
Other invasion processes can also generate periodic travelling waves in cyclic populations. In particular, in the Rosenzweig–MacArthur model (3.2a) and (3.2b), a local disturbance of the coexistence steady state again induces an invasion with periodic travelling waves in its wake (Sherratt 1996a; Petrovskii & Malchow 1999, 2001). In this case, the system is in the coexistence rather than prey-only state ahead of the invading front. In applications, this would be relevant when a change in environmental conditions alters the local dynamics from non-cyclic to cyclic, so that the coexistence state changes stability.
In general, numerical simulations of invasion-driven waves are typically much more time consuming than for boundary-driven waves, because large domains and solution times are required. Efficient computational methods are discussed in detail by Garvie (2007), and corresponding computer codes are available at www.uoguelph.ca/∼mgarvie.
When the kinetics are close to Hopf bifurcation and the diffusion coefficients Du and Dv are equal, the reduction to normal form calculation (see §3) can again be used to obtain predictions of periodic travelling wave properties as a function of parameters. In practice this is straightforward, involving substitution of the normal form coefficients into the formula for wave amplitude derived by Sherratt (1994), namely
The mathematical basis of this formula is rather complicated. Immediately behind the invasion front, the solution decays towards the (unstable) predator–prey coexistence state via exponentially decaying oscillations whose rate can easily be calculated. The behaviour close to the coexistence state is much more complicated, involving a modulated travelling transition wave in phase gradient, with repeated singularities. This in turn implies the rate at which the solution grows away from the coexistence state. Finally, this rate feeds into a calculation of periodic travelling wave generation in systems of λ–ω form (3.4a) and (3.4b), to give the invasion amplitude. A full account of this theory is given in Sherratt (1998).
With the current level of data, attribution of invasion as a mechanism of wave generation in any specific ecological system is highly speculative. The most plausible case is the waves of the autumnal moth Epirrita autumnata in Fennoscandia (Tanhuanpää et al. 2002; Tenow et al. 2007) in which there are two invasion processes. Firstly, very severe winters kill the eggs, requiring de novo invasion of the mountain birch forests by the moths, and secondly, major outbreaks can cause total forest defoliation and even tree death, requiring a re-establishment of vegetation prior to invasion. One hypothesis for the population cycles in E. autumnata is that they are driven by larval parasitism (Tanhuanpää et al. 2002). It is unclear whether moth invasions would be followed by a later invasion of parasitoids or whether the two would occur simultaneously; comparative modelling of these two types of invasion scenario is reviewed by Fagan et al. (2002). Therefore, specific theoretical models, allied with new field data, will be required to clarify the role of invasion in establishing the periodic travelling waves in E. autumnata. In addition, we are aware of two examples of field data demonstrating spatio-temporal oscillations behind ecological invasions: the invasion of Daphnia by its crustacean predator Bythotrephes in Lake Michigan (Lehman & Caceres 1993), and the invasion of thistle populations by tephritid flies Urophora carudi (Jeltsch et al. 1992). However, neither dataset has been analysed for periodic travelling wave behaviour in the manner described in §2.
5. Mathematics of periodic travelling waves II: wave stability
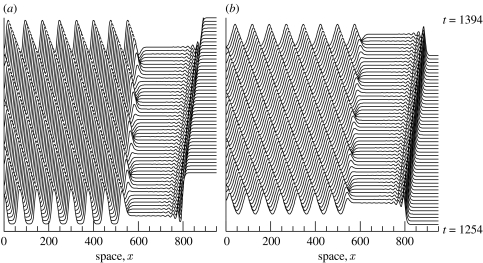
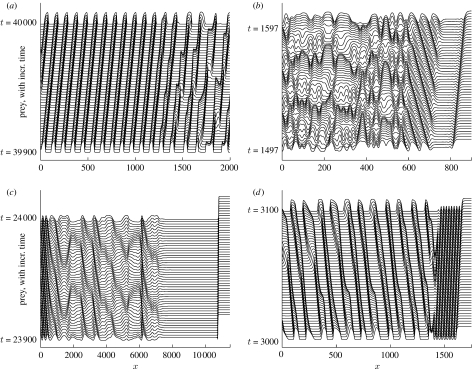
In §§3 and 4, we have deliberately avoided discussion of the very important issue of periodic travelling wave stability. We have described the existence of a family of periodic travelling wave solutions of (3.1a) and (3.1b) for any given set of ecological parameters. Some members of this family are in fact unstable as solutions of the model equations (3.1a) and (3.1b) (figure 3). Unstable waves cannot be a long-term solution, and when the theories of boundary- or invasion-based wave generation predict a periodic travelling wave that is unstable, numerical simulations indicate irregular spatio-temporal oscillations as the long-term behaviour (figure 7). Careful numerical study suggests that these oscillations are a genuine example of spatio-temporal chaos (Sherratt 1995; Petrovskii & Malchow 1999, 2001). In figure 7a,b, bands of periodic travelling waves are clearly visible close to the boundary/invasion front that is generating the waves; however, instabilities gradually grow and overwhelm these waves, leading to spatio-temporal irregularities further behind. In contrast, figure 7c shows a case in which the instability of the predicted waves is such that they are never seen, so that the invasion appears to lead directly to spatio-temporal irregularity. In figure 7d the invasive front has a different form, with low-amplitude periodic travelling waves immediately behind the invasion and moving at the invasion speed. Mathematically, this corresponds to invasion occurring via a ‘point-to-periodic’ transition wave; the existence of such transitions has been studied by Dunbar (1986) and Fraile & Sabina (1989). In figure 7d the low-amplitude waves are unstable, and further behind the invasion they break down into irregular spatio-temporal oscillations.
Figure 7.
Examples of the generation of unstable periodic travelling waves for the predator–prey model (3.2a) and (3.2b). (a) Wave generation by a zero Dirichlet boundary condition at x=0. (b–d) Wave generation by the invasion of predators into a uniform prey-only population. In (a,b,d), a band of periodic travelling waves is visible transiently, before breaking down into spatio-temporal irregularities. In (c), the instability of the predicted waves is such that they are never seen, so that the invasion appears to lead directly to irregular oscillations. In (b,c) the invasion occurs via a simple transition front, with the (unstable) coexistence steady state clearly visible immediately behind the front. In (d) the invasion occurs via a ‘point-to-periodic’ transition, with a band of (unstable) periodic travelling waves immediately behind the invasion, moving at the invasion speed. The parameter values are: (a) σ=0.15, μ=0.05, κ=0.2, α=100; (b) σ=0.833, μ=0.694, κ=0.077, α=1; (c) σ=0.15, μ=0.05, κ=0.2, α=0.004; (d) σ=0.15, μ=0.05, κ=0.2, α=0.63. In all cases we plot prey density as a function of space, with vertical separation corresponding to the time interval. The equations were solved numerically using a semi-implicit Crank–Nicolson method.
One important caveat to the irregular oscillations in figure 7 is that, when the population densities drop to very low levels, they recover via in situ growth rather than colonization. In some applications, this may be unrealistic, with low population densities resulting in local extinctions. This issue has been addressed in detail by Gurney et al. (1998) for equations (3.2a) and (3.2b). They imposed a threshold density for predators below which their mortality is increased to cause local extinction. The irregular oscillations are then lost, and instead the prey-only steady state is re-established behind the invasion front.
In §3, we described the use of numerical continuation software such as Auto to calculate periodic travelling wave families. The same software can be used to determine periodic travelling wave stability. However, this is a much more difficult numerical problem that was solved only recently (Sandstede & Scheel 2000; Bordiougov & Engel 2006; Rademacher et al. 2007). Rademacher et al. (2007) give a full but rather mathematically oriented account of the method; it involves applying Auto to a boundary-value problem for the eigenfunctions, and the theory underlying numerical continuation of such problems is reviewed by Champneys & Sandstede (2007). A less technical summary, including computer programs written in a tutorial style, is available at www.ma.hw.ac.uk/∼jas/supplements/ptwreview/index.html. These are for the system (3.2a) and (3.2b), but can easily be adapted to different equations.
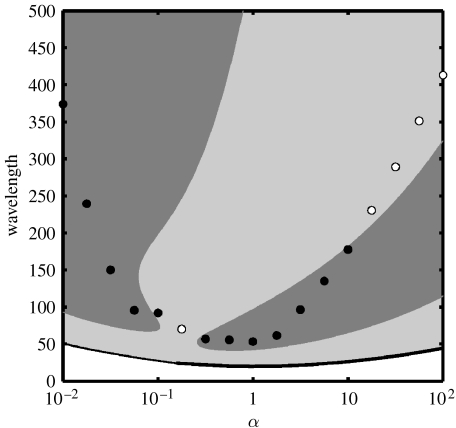
Smith & Sherratt (2007) have applied this new numerical method of calculating periodic travelling wave stability to the Rosenzweig–MacArthur predator–prey model (3.2a) and (3.2b), and typical results are illustrated in figure 8. Results of this type divide the wave family into stable and unstable parts, and are essential for accurate interpretation of numerical simulations of periodic travelling wave generation. For example, the circles in figure 8 indicate the wavelengths of the periodic travelling waves selected by zero Dirichlet boundary conditions, with filled/open circles corresponding, respectively, to stable waves and to waves that break down into irregular oscillations away from the boundary. Without the background shading to indicate stability, the pattern of filled and open circles appears rather arbitrary.
Figure 8.
The effect of α on the range of wavelengths in the travelling wave family for equations (3.2a) and (3.2b), and on the wavelength picked out by zero Dirichlet boundary conditions in numerical simulations. The values of the kinetic parameters are σ=0.15, μ=0.05 and κ=0.2. Light shaded areas denote the region of unstable travelling waves and dark shaded areas denote the region of stable travelling waves; in unshaded areas there are no travelling waves. The solid line corresponds to the position of the Hopf bifurcation of the wave family. Circles correspond to the values predicted by numerical simulations. Filled circles denote waves that showed no evidence of instability. Open circles denote waves that existed transiently before developing into irregular spatio-temporal behaviour. Spatio-temporal dynamics for two cases, α=1 and 100, are shown in figures 3b and 7a, respectively. Values reported for apparently unstable periodic travelling waves were measured from the region of waves that form directly behind the Dirichlet boundary, as is visible in figure 7a. Note the log axis for α.
A general review of the mathematical theory of wave stability is given by Sandstede (2002), and a detailed classification of instabilities is presented in Rademacher & Scheel (2007a). Analytical results on conditions for wave stability are relatively few. In their original paper, Kopell & Howard (1973) showed that waves of sufficiently low amplitude are unstable whenever the travelling wave equations (3.3a) and (3.3b) have a Hopf bifurcation. Over the following decade, a number of authors attempted to extend these results, with some success. In particular, Maginu (1981) showed that, for the special case of a finite domain with periodic boundary conditions, waves of sufficiently high amplitude are stable whenever the kinetics of (3.1a) and (3.1b) have a stable limit cycle. Recently, a number of papers have addressed the stability of high-amplitude waves on unbounded domains when the kinetic parameters are close to a bifurcation point (homoclinic or saddle-node) for the spatially uniform oscillations (Coullet et al. 2000; Risler 2001; Rademacher & Scheel 2007b). However, the crucial issue of the division of the wave family into stable and unstable parts remains completely open, with the single exception of λ–ω systems such as (3.4a) and (3.4b). In that case, an exact criterion for stability was derived by Kopell & Howard (1973); for (3.4a) and (3.4b), this implies that waves are stable if and only if their amplitude exceeds the critical value . Moreover, for some cases of periodic travelling waves in λ–ω systems, Kapitula (1994) has derived bounds on the decay rate of perturbations.
It is important to emphasize that boundary- or invasion-driven generation of an unstable periodic travelling wave is only one of many ways in which irregular spatio-temporal oscillations can arise in ecological systems. A full discussion of this is outside the scope of this review, and we mention just two examples of other mechanisms that have particular similarities to the results we have described. First, Pascual (1993) and Pascual & Caswell (1997) showed that, for the Rosenzweig–MacArthur model (3.2a) and (3.2b) with α=1, a spatial gradient in prey birth rate can result in a series of bifurcations in which chaos develops via quasi-periodicity as the domain size is increased. There is no suggestion of periodic travelling wave involvement in this behaviour. Secondly, Morozov et al. (2004) studied a predator–prey model without saturation in prey consumption by predators, but with an Allee effect in the prey-only dynamics. The Allee effect (Allee 1938) is that many natural populations shrink at very low densities because, on average, individuals cannot reproduce themselves (see Taylor & Hastings (2005) and Berec et al. (2007) for recent reviews). Morozov et al. (2004) showed that, with a sufficiently strong Allee effect, invasions can generate solutions that are regular in space but temporally chaotic. Again there is no suggestion of periodic travelling wave involvement in this process. Subsequent work by Petrovskii and co-workers has revealed the highly novel phenomenon of ‘patchy invasion’ in other predator–prey models that include the Allee effect (Petrovskii et al. 2002, 2005; Morozov et al. 2006). Here invasion occurs via the irregular motion and interaction of separate population patches, without a continuous invasion front.
Finally, we should mention that, when there are three or more interacting populations, the local dynamics themselves can be chaotic. (This is not possible for two populations when time is continuous.) Clearly, spatio-temporal chaos is then a natural possibility for long-term solutions. However in some cases, the spatial interactions act to stabilize the solution. For instance, in their work on a model for gypsy moth dynamics, Wilder et al. (1995) showed that the combination of spatial diffusion and chaotic local dynamics can cause periodic travelling waves to develop. This is a very different mechanism of periodic travelling wave generation from those we have discussed, and its mathematical basis is not understood to the best of our knowledge.
6. Discussion
The combination of recent mathematical advances in the theory of periodic travelling waves and the increasingly widespread identification of such waves in ecological field data makes this an important and exciting area of current research. In this review, we have retained a relatively narrow focus on reaction–diffusion models for systems with cyclic population dynamics. As such, we have excluded three classes of theoretical model exhibiting periodic travelling waves: integrodifferential equations; excitable systems; and discrete-time models. Comprehensive reviews of these are outside our scope, and we give only a brief description.
Integrodifferential equations have been in use as ecological models for more than 30 years (May 1976), but their recent study originates from the papers of Britton (1989, 1990). In this work, an integral is used in the term modelling intraspecific competition. Britton argues that, since individuals are moving, this term will in general depend on population levels across a local neighbourhood. Moreover, a temporal average is also appropriate, due to both the time taken for individuals to move and the time taken for resources to recover after consumption. Therefore Britton's (1989, 1990) model includes an integral over space and time. Temporal delays have a long history in ecological models, and can cause cycles in single, non-interacting populations. The extension to integrodifferential equations leads to periodic travelling wave solutions (Britton 1990; Gourley & Britton 1993; Duehring & Huang 2007). In fact, periodic travelling waves also occur in integrodifferential equations with non-local terms but no time delay (Billingham 2004). Of particular relevance to the present paper is work by Ashwin et al. (2002), who demonstrate the development of periodic travelling waves behind an invading wavefront in an integrodifferential equation model for a single population.
An excitable system is one in which sufficiently large (above a threshold) perturbations of a steady state induce a long transient away from the steady state, prior to return. In a spatial setting, local dispersal during the transient can act as an above-threshold perturbation of a neighbouring site, leading to a propagating ‘excitation’ wave. Such waves have been very well studied in the context of physiology (see Keener & Sneyd 1998 for review). The ecological interactions most studied via excitable system models are zooplankton–phytoplankton dynamics (Truscott & Brindley 1994). Spatial versions of these models often involve both diffusion and convection terms, with the latter representing pursuit/evasion behaviour (Tsyganov et al. 2004). A wide range of complex wave phenomena have been reported for such models, including spirals (Biktashev et al. 2004; Brindley et al. 2005). These are two-dimensional generalizations of periodic travelling waves, strongly suggesting the possibility of periodic travelling waves in ecologically relevant contexts; however, to the best of our knowledge these have yet to be explored.
Discrete-time models have been very widely used to study spatial dynamics in ecological interactions. Models may be continuous or discrete in space. The former typically represent dispersal via contact distributions, giving integrodifference equations (Kot & Schaffer 1986; Kot et al. 1996). In discrete-time/discrete-space models, population densities can be either taken as continuous (coupled map lattices) or restricted to a discrete set of values (cellular automata). All these models have a rich variety of spatio-temporal behaviours, including periodic travelling waves. Examples with particular similarities to the reaction–diffusion waves we have described include work of Kot (1992) on periodic travelling waves in an integrodifference predator–prey model, an application by Sherratt et al. (2000) of coupled map lattice models to periodic travelling waves of field voles in Kielder Forest, and mathematically oriented work on periodic travelling waves in cellular automata (Sherratt 1996b; Courbage 1997; Courbage & Yasmineh 2001).
We end this review by listing what we regard as the major research challenges for fieldwork on periodic travelling waves in cyclic populations, and their study via oscillatory reaction–diffusion equations. We propose five challenges for each community, beginning with mathematics.
What are the properties of periodic travelling waves far from Hopf bifurcation in the kinetics? For kinetics close to Hopf bifurcation, the basic existence and stability properties of periodic travelling waves were established in the 1970s. However, understanding behaviour far from bifurcation remains a major challenge for mathematicians. Numerical determination of travelling wave families is relatively straightforward (see §3), and wave stability can now also be computed, using new numerical methods (see §5). There is an urgent need for the application of these methods to a wide range of oscillatory reaction–diffusion systems. We hope that the detailed presentation of one implementation of this (available at www.ma.hw.ac.uk/∼jas/supplements/ptwreview/index.html) will help to facilitate such work. In addition, new analytical results on waves far from Hopf bifurcation would be a major advance. There are two special cases in which there has been recent progress. Firstly, when the ratio of diffusion coefficients is either zero or infinity, periodic travelling waves can sometimes be constructed via singular perturbation theory, with wave existence then extended to sufficiently small or large diffusion ratios using Conley index theory (Gardner & Smoller 1983; Gameiro et al. 2007). Secondly, when the kinetic parameters are close to a bifurcation point (homoclinic or saddle-node) for the spatially uniform oscillations, the stability of high-amplitude waves can be studied analytically (Coullet et al. 2000; Risler 2001; Rademacher & Scheel 2007b). However, for more general cases, appropriate mathematical machinery appears to be lacking at the present time.
Can one prove the existence of solutions corresponding to periodic travelling wave generation by obstacles and invasion? For the reaction–diffusion system (3.4a) and (3.4b), with kinetics in Hopf normal form and equal diffusion coefficients, periodic travelling wave generation by a zero Dirichlet boundary condition has a simple, rigorous mathematical basis: an exact solution of the PDEs, which approaches a periodic travelling wave as distance from the boundary tends to infinity. There is strong numerical evidence that there is a countably infinite family of other solutions satisfying the Dirichlet condition and tending to a periodic travelling wave (with a different amplitude in each case), but that all of these other solutions are unstable as PDE solutions (see Sherratt 2003 for details). Proof of this would provide a complete picture of this wave generation scenario. For other equations, even existence of the solution corresponding to wave generation by an obstacle boundary remains unproven: the case of (3.4a) and (3.4b) provides a natural springboard for a proof. Wave generation by invasion is more complex mathematically (see §4). Current understanding is built on an intuitive division of the solution into different regions, with the regions and their interfaces studied separately (Sherratt 1998; Petrovskii & Malchow 2000). A more global investigation of the solution would be a major advance. One particularly important question to be answered is: for what range of invasion speeds is there a solution corresponding to periodic travelling wave generation?
-
What are the effects of temporal forcing on periodic travelling waves? The parameters of ecological systems vary in time, which is not reflected by simple models of the form (3.1a) and (3.1b). The most ubiquitous cause of temporal forcing is seasonality, which is particularly important in the more northerly latitudes in which population cycles typically occur. Mathematically, it is straightforward to incorporate seasonal forcing into (3.1a) and (3.1b), by making parameter values vary explicitly with time. However, the implications of this for periodic travelling wave solutions have received almost no attention. The only exception that we are aware of is a preliminary study by Webb & Sherratt (2004) on small temporal forcing of the λ–ω system (3.4a) and (3.4b). They show that the periodic travelling wave amplitude oscillates, with the amplitude of these oscillations increasing with the period of the forcing. More generally, it seems probable that the relative values of the forcing period (1 year) and the period of the local population dynamics will be critical, with the possibility of resonances. Recent field data for Kielder Forest suggest that changes in seasonal forcing have profound effects on population cycles and periodic travelling waves (Bierman et al. 2006), and thus there is a pressing need to develop a mathematical theory of periodic travelling waves in seasonally forced systems. This theory would also enable exploration of the effects of less frequent but repeating external effects, such as the North Atlantic Oscillation (Stenseth et al. 2004) or 10-year sunspot cycles (Selås 2006a).
A different type of external forcing is occasional, pan-habitat synchronizing events, such as unusually severe winter. This Moran effect (Koenig 2002; Royama 2005) would tend to reset the phase of the population cycles, so that periodic travelling wave patterns would have to be re-established. This demands a detailed understanding of the transient dynamics during periodic travelling wave generation, which is currently lacking.
What is the effect of spatial heterogeneity on periodic travelling waves? Simple models of the form (3.1a) and (3.1b) also neglect the spatial heterogeneities that are a feature of all ecological habitats. For single (scalar) reaction–diffusion equations, there is now an established body of literature on the propagation of wavefronts in heterogeneous environments (reviewed comprehensively by Xin (2000)). Extension of these ideas to periodic travelling waves in reaction–diffusion systems would be a major advance, with significant ecological implications. Some old literature on models of oscillatory chemical reactions is relevant in this context (e.g. Hagan 1981; Kopell 1981), but there has been almost no recent work (an exception is Kay & Sherratt 2000). Strong spatial noise will of course dominate periodic travelling waves, and can in fact act as a generator of small localized bands of periodic travelling waves (see §4). However, weaker noise can be expected to alter periodic travelling wave behaviour while retaining the basic spatio-temporal pattern, so that periodic travelling waves would still be detectable in the field. A particularly important specific question is: how does weak spatial noise affect periodic travelling wave generation by obstacle boundaries and invasion?
Are there other ecologically relevant mechanisms of periodic travelling wave generation? We have described periodic travelling wave generation by obstacle boundaries and invasion. Strong spatial noise can also generate periodic travelling waves in reaction–diffusion systems, but this is probably less relevant to ecological applications (see §4). As field studies increasingly reveal periodic travelling waves, identification of other mechanisms of wave generation is urgently required. One approach to this is via simulation results from discrete-time models. For example, in a coupled map lattice model for larch budmoth dynamics, Johnson et al. (2004, 2006) demonstrated periodic travelling waves originating in regions with a high density of habitat patches aggregated around a single focus; the waves travel towards the surrounding and more isolated habitat patches. The natural analogue of this for reaction–diffusion models would be periodic travelling wave generation by spatial gradients in parameter values; to the best of our knowledge, this has not been investigated.
Our five proposed empirical research challenges are as follows.
Do periodic travelling waves occur through the mechanisms predicted by reaction–diffusion models? This review has highlighted a number of predictions from mathematical studies that require testing against empirical data. Some of the necessary data already exist. One example is the target pattern waves generated in simulations of models with landscape obstacles. This prediction implies that one should observe waves moving in different directions at different locations in space relative to the obstacle. For example, if the large reservoir in the centre of Kielder Forest is generating the periodic travelling waves in field vole abundance (see table 1; figure 5), then reaction–diffusion models predict that waves should travel in opposite directions on either side of the longest axis of the reservoir. This prediction could be investigated by re-analysing the data already gathered. For example, if travelling waves move in the same direction on both sides of the reservoir, then this would imply a different mechanism of wave generation.
Do periodic travelling waves occur in other populations involved in the cycles? Another consistent prediction from reaction–diffusion models is that if periodic travelling waves are predicted in oscillatory biological systems, then they should occur in all the component populations involved in generating the cycles. In principle, periodic travelling waves may occur in taxa that simply respond to the abundance of a cyclic population, rather than driving it; however, few ecological interactions are genuinely one directional. Therefore, the presence of travelling waves in another population component would provide evidence that it plays some role in generating the population cycles. Looking for such coincident travelling waves provides a means of identifying the key players in the population cycles and, to our knowledge, this avenue has never been explored. For example, plant–herbivore interactions have been proposed as a hypothesis for many of the population cycles detailed in table 1. If this is indeed the case then, for the systems exhibiting periodic travelling waves, we would expect travelling waves in the relevant properties of the vegetation (such as anti-herbivore defences). Testing such a hypothesis would be comparatively straightforward, since it is easier to collect the relevant data from plant communities than from animals.
How general are periodic travelling waves in cyclic populations? About half of the populations listed in table 1 show some evidence of periodic travelling waves. It would be informative to understand how common periodic travelling waves are in cyclic populations generally, in contrast to behaviours such as homogeneous oscillations, spatio-temporal irregularities or crystal-lattice patterns. Such information would provide valuable clues to the key factors that underlie differences in the spatio-temporal dynamics of different systems. For example, in Fennoscandian rodent populations, there are latitudinal gradients in the population cycles (Hanski et al. 1991); are there also gradients in characteristics of the spatio-temporal dynamics? In many systems, more information on the occurrence of periodic travelling waves would also be given by data at a higher spatial and temporal resolution than is currently available. The larch budmoth populations in the European Alps have been studied at a particularly high resolution, and statistical analysis has consequently been able to show changes in the speed and direction of the waves as they propagate (Bjørnstad et al. 2002; Johnson et al. 2004, 2006).
Is dispersal important in maintaining cycles in some populations? The paradigm adopted in most of the studies described in this review is that the populations exhibit cycles even in the absence of dispersal. However, mathematical studies of reaction–diffusion equations show that periodic travelling wave-like phenomena (and hence population cycles) can also occur in non-self-oscillatory models, such as excitable systems (see above). Do such mechanisms of periodic travelling wave generation occur in any populations that are currently labelled as ‘cyclic’? There is a clear case for experimental studies focused on whether dispersal is ever necessary for population cycles to occur. One example of such experimental manipulation is work on the ‘fence effect’ observed in some cyclic rodent populations (reviewed in Krebs 1996). Some studies have found that preventing rodent dispersal into and out of a cyclic population not only prevents the population cycles but also causes the population to increase to uncharacteristically high densities and then crash (the rodents over-exploit their food supply; see Krebs (1996) for review). This may be because density-dependent emigration has a regulating effect on the local population dynamics (Lidicker (1962), but see Ostfeld (1994) for counter-arguments), a feature not commonly incorporated into models of rodent cycles. While the generality of this phenomenon remains unclear, it does highlight that dispersal processes can play a crucial role in the local dynamics of cyclic populations.
What are the implications of dispersal for temporal behaviour in cyclic populations? One consistent message from the study of reaction–diffusion models of cyclic populations, and indeed of spatial models more generally, is that the incorporation of spatial dynamics can fundamentally change the temporal dynamics predicted by a non-spatial model. A considerable amount of effort has been put into accurately parametrizing non-spatial models of cyclic populations. However, even if these parameter estimates were perfect, the predictions of non-spatial models may be inaccurate if the effects of dispersal have not been incorporated. For example, in figure 3b the non-spatial model predicts a cycle period of approximately 85 time units, whereas the periodic travelling waves generated by a one-dimensional landscape obstacle in the same model have a period of approximately 48 time units. There is a compelling case for future empirical studies to estimate the dispersal properties of the interacting taxa thought to be important in population cycles; this would allow them to be incorporated into spatially extended versions of models, enabling a detailed assessment of their importance.
Our hope and expectation is that research targeted towards these 10 challenges will lead to a clear understanding of the mechanisms that cause periodic travelling waves in a range of specific ecological systems. In our view, such research will be most effective when it involves either mathematics that is rooted firmly in ecological debate, or fieldwork whose design is informed by mathematical theory. We expect the resulting insights into periodic travelling wave dynamics and their ecological contexts to significantly improve the understanding of both the mathematics of oscillatory reaction–diffusion systems and the ecology of cyclic populations.
Acknowledgments
This review constitutes the Adams Prize Essay, in connection with the award of the prize to J.A.S. in 2006. M.J.S. was supported by the NERC Environmental Mathematics and Statistics Programme. We thank Xavier Lambin (University of Aberdeen) for many helpful discussions. We are also grateful to many others who have helped our understanding of periodic travelling waves in recent years, in particular Gabriel Lord (Heriot-Watt University), Simon Malham (Heriot-Watt University), Jens Rademacher (CWI, Amsterdam) and Björn Sandstede (University of Surrey).
Footnotes
By this, we mean that the Hopf bifurcation is of a standard supercritical type. Periodic travelling waves in reaction–diffusion systems close to a subcritical Hopf bifurcation are considered by Ermentrout et al. (1997).
In equations of the form (3.1a) and (3.1b), the spatially homogeneous oscillations corresponding to the limit cycle solution of the kinetics are stable as a PDE solution when Du=Dv, but can be unstable when Du and Dv are sufficiently different (Kopell & Howard 1973; Ermentrout 1981). Numerical calculation of this stability is relatively straightforward, via a calculation of Floquet multipliers, and this confirms the stability for the parameter values used in figure 6.
References
- Allee W.C. Norton & Co; New York, NY: 1938. The social life of animals. [Google Scholar]
- Angerbjörn A, Tannerfeldt M, Lundberg H. Geographical and temporal patterns of lemming population dynamics in Fennoscandia. Ecography. 2001;24:298–308. doi: 10.1034/j.1600-0587.2001.240307.x. [DOI] [Google Scholar]
- Ashwin P, Bartuccelli M.V, Bridges T.J, Gourley S.A. Travelling fronts for the KPP equation with spatio-temporal delay. Z. Angew. Math. Phys. 2002;53:103–122. doi: 10.1007/s00033-002-8145-8. [DOI] [Google Scholar]
- Auchmuty J.F.G, Nicolis G. Bifurcation analysis of reaction–diffusion equations—III. Chemical oscillations. Bull. Math. Biol. 1976;38:325–350. [Google Scholar]
- Berec L, Angulo E, Courchamp F. Multiple Allee effects and population management. Trends Ecol. Evol. 2007;22:185–191. doi: 10.1016/j.tree.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Berryman A.A.Population cycles: the case for trophic interactions2002Oxford University Press; Oxford, UK [Google Scholar]
- Bierman S.M, Fairbairn J.P, Petty S.J, Elston D.A, Tidhar D, Lambin X. Changes over time in the spatiotemporal dynamics of cyclic populations of field voles (Microtus agrestis L.) Am. Nat. 2006;167:583–590. doi: 10.1086/501076. [DOI] [PubMed] [Google Scholar]
- Biktashev V.N, Brindley J, Holden A.V, Tsyganov M.A. Pursuit-evasion predator–prey waves in two spatial dimensions. Chaos. 2004;14:988–994. doi: 10.1063/1.1793751. [DOI] [PubMed] [Google Scholar]
- Billingham J. Dynamics of a strongly nonlocal reaction–diffusion population model. Nonlinearity. 2004;17:313–346. doi: 10.1088/0951-7715/17/1/018. [DOI] [Google Scholar]
- Billingham J, King A.C. Cambridge University Press; Cambridge, UK: 2000. Wave motion. [Google Scholar]
- Bjørnstad O.N, Ims R.A, Lambin X. Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol. Evol. 1999;14:427–432. doi: 10.1016/S0169-5347(99)01677-8. [DOI] [PubMed] [Google Scholar]
- Bjørnstad O.N, Peltonen M, Liebhold A.M, Baltensweiler W. Waves of larch budmoth outbreaks in the European Alps. Science. 2002;298:1020–1023. doi: 10.1126/science.1075182. [DOI] [PubMed] [Google Scholar]
- Bordiougov G, Engel H. From trigger to phase waves and back again. Physica D. 2006;215:25–37. doi: 10.1016/j.physd.2006.01.005. [DOI] [Google Scholar]
- Brandt M.J, Lambin X. Movement patterns of a specialist predator, the weasel Mustela nivalis exploiting asynchronous cyclic field vole Microtus agrestis populations. Acta Theriol. 2007;52:13–25. [Google Scholar]
- Brindley J, Biktashev V.N, Tsyganov M.A. Invasion waves in populations with excitable dynamics. Biol. Inv. 2005;7:807–816. doi: 10.1007/s10530-005-5207-9. [DOI] [Google Scholar]
- Britton N.F. Aggregation and the competitive exclusion principle. J. Theor. Biol. 1989;136:57–66. doi: 10.1016/S0022-5193(89)80189-4. [DOI] [PubMed] [Google Scholar]
- Britton N.F. Spatial structures and periodic traveling waves in an integrodifferential reaction–diffusion population model. SIAM J. Appl. Math. 1990;50:1663–1688. doi: 10.1137/0150099. [DOI] [Google Scholar]
- Britton N.F. Springer; New York, NY: 2003. Essential mathematical biology. [Google Scholar]
- Cantrell R.S, Cosner C, Fagan W. Competitive reversals inside ecological reserves: the role of external habitat degradation. J. Math. Biol. 1998;37:491–533. doi: 10.1007/s002850050139. [DOI] [PubMed] [Google Scholar]
- Cattadori I.M, Haydon D.T, Hudson P.J. Parasites and climate synchronize red grouse populations. Nature. 2005;433:737–741. doi: 10.1038/nature03276. [DOI] [PubMed] [Google Scholar]
- Champneys A.R, Sandstede B. Numerical computation of coherent structures. In: Krauskopf B, Osinga H.M, Galan-Vioque J, editors. Numerical continuation methods for dynamical systems. Springer; Berlin, Germany: 2007. pp. 331–358. [Google Scholar]
- Cope D. Reaction–diffusion systems with explicit travelling wave and transient solutions. SIAM J. Appl. Math. 1979;37:316–324. doi: 10.1137/0137023. [DOI] [Google Scholar]
- Coullet P, Risler E, Vandenberghe N. Spatial unfolding of elementary bifurcations. J. Stat. Phys. 2000;101:521–541. doi: 10.1023/A:1026415607690. [DOI] [Google Scholar]
- Courbage M. On the abundance of traveling waves in 1D infinite cellular automata. Physica D. 1997;103:133–144. doi: 10.1016/S0167-2789(96)00256-4. [DOI] [Google Scholar]
- Courbage M, Yasmineh S. Wavelength distribution of chaotic travelling waves in some cellular automata. Physica D. 2001;150:63–83. doi: 10.1016/S0167-2789(00)00213-X. [DOI] [Google Scholar]
- Doedel E.J. Auto, a program for the automatic bifurcation analysis of autonomous systems. Cong. Numer. 1981;30:265–384. [Google Scholar]
- Doedel E.J. Nonlinear numerics. J. Frankl. Inst. 1997;334:1049–1073. doi: 10.1016/S0016-0032(97)00027-6. [DOI] [Google Scholar]
- Duehring D, Huang W.Z. Periodic travelling waves for diffusion equations with time delayed and non-local responding reaction. J. Dyn. Differ. Eqns. 2007;19:457–477. doi: 10.1007/s10884-006-9048-8. [DOI] [Google Scholar]
- Dunbar S.R. Traveling waves in diffusive predator–prey equations: periodic orbits and point-to-periodic heteroclinic orbits. SIAM J. Appl. Math. 1986;46:1057–1078. doi: 10.1137/0146063. [DOI] [Google Scholar]
- Epstein I.R, Showalter K. Nonlinear chemical dynamics: oscillations, patterns and chaos. J. Phys. Chem. 1996;100:13 132–13 147. doi: 10.1021/jp953547m. [DOI] [Google Scholar]
- Ergon T, Lambin X, Stenseth N.C. Life-history traits of voles in a fluctuating population respond to the immediate environment. Nature. 2001;411:1043–1045. doi: 10.1038/35082553. [DOI] [PubMed] [Google Scholar]
- Ermentrout G.B. Stable small amplitude solutions in reaction–diffusion systems. Quart. Appl. Math. 1981;39:61–86. [Google Scholar]
- Ermentrout G.B, Rinzel J. One-dimensional λ–ω target patterns: empirical stability tests. J. Math. Biol. 1980;10:97–100. doi: 10.1007/BF00276399. [DOI] [Google Scholar]
- Ermentrout B, Chen X, Chen Z. Transition fronts and localized structures in bistable reation—diffusion equations. Physica D. 1997;108:147–167. doi: 10.1016/S0167-2789(97)82011-8. [DOI] [Google Scholar]
- Fagan W.F, Lewis M.A, Neubert M.G, van den Driessche P. Invasion theory and biological control. Ecol. Lett. 2002;5:148–157. doi: 10.1046/j.1461-0248.2002.0_285.x. [DOI] [Google Scholar]
- Fiedler B, Scheel A. Spatio-temporal dynamics of reaction–diffusion patterns. In: Kirkilionis M, Krömker S, Rannacher R, Tomi F, editors. Trends in nonlinear analysis. Springer; Berlin, Germany: 2003. pp. 21–150. [Google Scholar]
- Fraile J.M, Sabina J.C. General conditions for the existence of a critical point-periodic wave front connection for reaction–diffusion systems. Nonlin. Anal. Theory Methods Appl. 1989;13:767–786. doi: 10.1016/0362-546X(89)90071-0. [DOI] [Google Scholar]
- Gameiro M, Gedeon T, Kalies W, Kokubu H, Mischaikow K, Oka H. Topological horseshoes of traveling waves for a fast–slow predator–prey system. J. Dyn. Differ. Eqns. 2007;19:623–654. doi: 10.1007/s10884-006-9013-6. [DOI] [Google Scholar]
- Gardner R, Smoller J. The existence of periodic travelling waves for singularly perturbed predator–prey equations via the Conley index. J. Differ. Eqns. 1983;47:133–161. doi: 10.1016/0022-0396(83)90031-1. [DOI] [Google Scholar]
- Garvie M.R. Finite difference schemes for reaction–diffusion equations modeling predator–prey interactions in Matlab. Bull. Math. Biol. 2007;69:931–956. doi: 10.1007/s11538-006-9062-3. [DOI] [PubMed] [Google Scholar]
- Gourley S.A, Britton N.F. Instability of traveling wave solutions of a population model with nonlocal effects. IMA J. Appl. Math. 1993;51:299–310. doi: 10.1093/imamat/51.3.299. [DOI] [Google Scholar]
- Graham I.M, Lambin X. The impact of weasel predation on cyclic field-vole survival: the specialist predator hypothesis contradicted. J. Anim. Ecol. 2002;71:946–956. doi: 10.1046/j.1365-2656.2002.00657.x. [DOI] [Google Scholar]
- Greenberg J.M. Axi-symmetric, time-periodic solutions of reaction–diffusion equations. SIAM J. Appl. Math. 1978;34:391–397. doi: 10.1137/0134032. [DOI] [Google Scholar]
- Greenberg J.M. Spiral waves for λ–ω systems. Adv. Appl. Math. 1981;2:450–455. doi: 10.1016/0196-8858(81)90044-0. [DOI] [Google Scholar]
- Greenwood, J. J. D. & Robinson, R. A. 2006 General census methods. In: Ecological census techniques, vol. 2 (ed. W. J. Sutherland), pp. 87–185. Cambridge, UK: Cambridge University Press.
- Guckenheimer J, Holmes P. Springer; Berlin, Germany: 1983. Nonlinear oscillations, dynamical systems and bifurcations of vector fields. [Google Scholar]
- Gurney W.S.C, Veitch A.R, Cruickshank I, McGeachin G. Circles and spirals: population persistence in a spatially explicit predator–prey model. Ecology. 1998;79:2516–2530. [Google Scholar]
- Hagan P.S. Target patterns in reaction–diffusion systems. Adv. Appl. Math. 1981;2:400–416. doi: 10.1016/0196-8858(81)90042-7. [DOI] [Google Scholar]
- Hagan P.S. Spiral waves in reaction–diffusion equations. SIAM J. Appl. Math. 1982;42:762–786. doi: 10.1137/0142054. [DOI] [Google Scholar]
- Hanski I, Hansson L, Henttonen H. Specialist predators, generalist predators, and the microtine rodent cycle. J. Anim. Ecol. 1991;60:353–367. doi: 10.2307/5465. [DOI] [Google Scholar]
- Hassard B.D, Kazarinoff N.D, Wan Y.-H. Cambridge University Press; Cambridge, UK: 1981. Theory and applications of Hopf bifurcation. [Google Scholar]
- Haydon D.T, Greenwood P.E, Stenseth N.C, Saitoh T. Spatio-temporal dynamics of the grey-sided vole in Hokkaido: identifying coupling using state-based Markov-chain modelling. Proc. R. Soc. B. 2003;270:435–445. doi: 10.1098/rspb.2002.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux, M. et al. 2003 An overview of Trilinos. Technical report SAND2003-2952, Sandia National Laboratories, Albuquerque, NM.
- Huang J, Lu G, Ruan S. Existence of traveling wave solutions in a diffusive predator–prey model. J. Math. Biol. 2003;46:132–152. doi: 10.1007/s00285-002-0171-9. [DOI] [PubMed] [Google Scholar]
- Hudson P.J, Dobson A.P, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Ims R.A, Andreassen H.P. Spatial synchronization of vole population dynamics by predatory birds. Nature. 2000;408:194–196. doi: 10.1038/35041562. [DOI] [PubMed] [Google Scholar]
- Jeltsch F, Wissel Ch, Eber S, Brandl R. Oscillating dispersal patterns in tephritid fly populations. Ecol. Model. 1992;60:63–75. doi: 10.1016/0304-3800(92)90013-5. [DOI] [Google Scholar]
- Johnson D.M, Bjørnstad O.N, Liebhold A.M. Landscape geometry and travelling waves in the larch budmoth. Ecol. Lett. 2004;7:967–974. doi: 10.1111/j.1461-0248.2004.00659.x. [DOI] [Google Scholar]
- Johnson D.M, Bjørnstad O.N, Liebhold A.M. Landscape mosaic induces travelling waves of insect outbreaks. Oecologia. 2006;148:51–60. doi: 10.1007/s00442-005-0349-0. [DOI] [PubMed] [Google Scholar]
- Kapitula T. On the nonlinear stability of plane waves for the Ginzburg–Landau equation. Commun. Pure Appl. Math. 1994;47:831–841. doi: 10.1002/cpa.3160470603. [DOI] [Google Scholar]
- Kay A.L, Sherratt J.A. Spatial noise stabilizes periodic wave patterns in oscillatory systems on finite domains. SIAM J. Appl. Math. 2000;61:1013–1041. doi: 10.1137/S0036139999360696. [DOI] [Google Scholar]
- Keener J.P, Sneyd J. Springer; New York, NY: 1998. Mathematical physiology. [Google Scholar]
- King A.A, Schaffer W.M. The geometry of a population cycle: a mechanistic model of snowshoe hare demography. Ecology. 2001;82:814–830. [Google Scholar]
- Klemola T, Koivula M, Korpimäki E, Norrdahl K. Small mustelid predation slows population growth of Microtus voles: a predator reduction experiment. J. Anim. Ecol. 1997;66:607–614. doi: 10.2307/5914. [DOI] [Google Scholar]
- Klemola T, Huitu O, Ruohomaki K. Geographically partitioned spatial synchrony among cyclic moth populations. Oikos. 2006;114:349–359. doi: 10.1111/j.2006.0030-1299.14850.x. [DOI] [Google Scholar]
- Koenig W.D. Spatial autocorrelation of ecological phenomena. Trends Ecol. Evol. 1999;14:22–26. doi: 10.1016/S0169-5347(98)01533-X. [DOI] [PubMed] [Google Scholar]
- Koenig W.D. Global patterns of environmental synchrony and the Moran effect. Ecography. 2002;25:283–288. doi: 10.1034/j.1600-0587.2002.250304.x. [DOI] [Google Scholar]
- Koga S. Rotating spiral waves in reaction–diffusion systems—phase singularities of multi-armed waves. Prog. Theor. Phys. 1982;67:164–178. doi: 10.1143/PTP.67.164. [DOI] [Google Scholar]
- Kollár R, Scheel A. Coherent structures generated by inhomogeneities in oscillatory media. SIAM J. Appl. Dyn. Syst. 2007;6:236–262. doi: 10.1137/060666950. [DOI] [Google Scholar]
- Kopell N. Target pattern solutions to reaction–diffusion equations in the presence of impurities. Adv. Appl. Math. 1981;2:389–399. doi: 10.1016/0196-8858(81)90041-5. [DOI] [Google Scholar]
- Kopell N, Howard L.N. Plane wave solutions to reaction–diffusion equations. Stud. Appl. Math. 1973;52:291–328. [Google Scholar]
- Kopell N, Howard L.N. Target patterns and Horseshoes from a perturbed central force problem: some temporally periodic solutions for reaction–diffusion equations. Stud. Appl. Math. 1981;64:1–56. [Google Scholar]
- Korpimäki E, Norrdahl K. Experimental reduction of predators reverses the crash phase of small-rodent cycles. Ecology. 1998;79:2448–2455. [Google Scholar]
- Korpimäki E, Klemola T, Norrdahl K, Oksanen L, Oksanen T, Banks P.B, Batzli G.O, Henttonen H. Vole cycles and predation. Trends Ecol. Evol. 2003;18:494–495. doi: 10.1016/S0169-5347(03)00159-9. [DOI] [Google Scholar]
- Kot M. Discrete-time travelling waves: ecological examples. J. Math. Biol. 1992;30:413–436. doi: 10.1007/BF00173295. [DOI] [PubMed] [Google Scholar]
- Kot M, Schaffer W.M. Discrete-time growth-dispersal models. Math. Biosci. 1986;80:109–136. doi: 10.1016/0025-5564(86)90069-6. [DOI] [Google Scholar]
- Kot M, Lewis M.A, van den Driessche P. Dispersal data and the spread of invading organisms. Ecology. 1996;77:2027–2042. doi: 10.2307/2265698. [DOI] [Google Scholar]
- Krebs C.J. Population cycles revisited. J. Mammal. 1996;77:8–24. doi: 10.2307/1382705. [DOI] [Google Scholar]
- Krebs C.J, Boonstra R, Boutin S, Sinclair A.R.E. What drives the 10-year cycle of snowshoe hares? Bioscience. 2001;51:25–35. doi: 10.1641/0006-3568(2001)051[0025:WDTYCO]2.0.CO;2. [DOI] [Google Scholar]
- Kuramoto Y, Koga S. Turbulized rotating chemical waves. Prog. Theor. Phys. 1981;66:1081–1085. doi: 10.1143/PTP.66.1081. [DOI] [Google Scholar]
- Lambin X, Graham I.M. Testing the specialist predator hypothesis for vole cycles. Trends Ecol. Evol. 2003;18:493. doi: 10.1016/S0169-5347(03)00181-2. [DOI] [Google Scholar]
- Lambin X, Elston D.A, Petty S.J, MacKinnon J.L. Spatial asynchrony and periodic travelling waves in cyclic populations of field voles. Proc. R. Soc. B. 1998;265:1491–1496. doi: 10.1098/rspb.1998.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin X, Krebs C.J, Moss R, Stenseth N.C, Yoccoz N.G. Population cycles and parasitism. Science. 1999;286:2425. doi: 10.1126/science.286.5449.2425a. [DOI] [Google Scholar]
- Lambin X, Petty S.J, Mackinnon J.L. Cyclic dynamics in field vole populations and generalist predation. J. Anim. Ecol. 2000;69:106–118. doi: 10.1046/j.1365-2656.2000.00380.x. [DOI] [Google Scholar]
- Lambin X, Bretagnolle V, Yoccoz N.G. Vole population cycles in northern and southern Europe: is there a need for different explanations for single pattern? J. Anim. Ecol. 2006;75:340–349. doi: 10.1111/j.1365-2656.2006.01051.x. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Elsevier; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- Lehman J.T, Caceres C.E. Food-web responses to species invasion by a predatory invertebrate—Bythotrehes in Lake Michigan. Limnol. Oceanogr. 1993;38:879–891. [Google Scholar]
- Lidicker W.Z., Jr Emigration as a possible mechanism permitting the regulation of population density below carrying capacity. Am. Nat. 1962;96:29–33. doi: 10.1086/282204. [DOI] [Google Scholar]
- Liebhold A, Koenig W.D, Bjørnstad O.N. Spatial synchrony in population dynamics. Annu. Rev. Ecol. Evol. Syst. 2004;35:467–490. doi: 10.1146/annurev.ecolsys.34.011802.132516. [DOI] [Google Scholar]
- Ludwig D, Aronson D.G, Weinberger H.F. Spatial patterning of the spruce budworm. J. Math. Biol. 1979;8:217–258. [Google Scholar]
- Mackinnon J.L, Petty S.J, Elston D.A, Thomas C.J, Sherratt T.N, Lambin X. Scale invariant spatio-temporal patterns of field vole density. J. Anim. Ecol. 2001;70:101–111. doi: 10.1046/j.1365-2656.2001.00479.x. [DOI] [Google Scholar]
- Maginu K. Stability of periodic travelling wave solutions with large spatial periods in reaction–diffusion systems. J. Differ. Eqns. 1981;39:73–99. doi: 10.1016/0022-0396(81)90084-X. [DOI] [Google Scholar]
- Matthiopoulos J, Moss R, Mougeot F, Lambin X, Redpath S.M. Territorial behaviour and population dynamics in red grouse Lagopus lagopus scoticus. II. Population models. J. Anim. Ecol. 2003;72:1083–1096. doi: 10.1046/j.1365-2656.2003.00780.x. [DOI] [Google Scholar]
- Matthiopoulos J, Halley J.M, Moss R. Socially induced red grouse population cycles need abrupt transitions between tolerance and aggression. Ecology. 2005;86:1883–1893. doi: 10.1890/04-0253. [DOI] [Google Scholar]
- May R.M. Blackwell; Oxford, UK: 1976. Theoretical ecology: principles and applications. [Google Scholar]
- Monagan M.B, Geddes K.O, Heal K.M, Labahn H, Vorkoetter S.M, McCarron J, DeMarco P. Maplesoft; Waterloo, Canada: 2007. Maple introductory programming guide.www.maplesoft.com [Google Scholar]
- Moran P.A.P. The statistical analysis of the Canadian lynx cycle. 2. Synchronization and meteorology. Aust. J. Zool. 1953;1:291–298. doi: 10.1071/ZO9530291. [DOI] [Google Scholar]
- Morozov A.Y, Petrovskii S.V, Li B.-L. Bifurcations and chaos in a predator–prey system with the Allee effect. Proc. R. Soc. B. 2004;271:1407–1414. doi: 10.1098/rspb.2004.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A.Y, Petrovskii S.V, Li B.-L. Spatiotemporal complexity of patchy invasion in a predator–prey system with the Allee effect. J. Theor. Biol. 2006;238:18–35. doi: 10.1016/j.jtbi.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Moss R, Watson A, Parr R. Experimental prevention of a population cycle in red grouse. Ecology. 1996;77:1512–1530. doi: 10.2307/2265548. [DOI] [Google Scholar]
- Moss R, Elston D.A, Watson A. Spatial asynchrony and demographic travelling waves during red grouse population cycles. Ecology. 2000;81:981–989. [Google Scholar]
- Mougeot F, Piertney S.B, Leckie F, Evans S, Moss R, Redpath S.M, Hudson P.J. Experimentally increased aggressiveness reduces population kin structure and subsequent recruitment in red grouse Lagopus lagopus scoticus. J. Anim. Ecol. 2005;74:488–497. [Google Scholar]
- Münster-Swendsen M. The role of insect parasitoids in population cycles of the spruce neddleminer in Denmark. In: Berryman A.A, editor. Population cycles: the case for trophic interactions. Oxford University Press; Oxford, UK: 2002. pp. 29–43. [Google Scholar]
- Münster-Swendsen M, Berryman A. Detecting the causes of population cycles by analysis of R-functions: the spruce needle-miner, Epinotia tedella, and its parasitoids in Danish spruce plantations. Oikos. 2005;108:495–502. doi: 10.1111/j.0030-1299.2005.13747.x. [DOI] [Google Scholar]
- Murray J.D. Springer; New York, NY: 2002. Mathematical biology I: an introduction. [Google Scholar]
- Nilssen A.C, Tenow O, Bylund H. Waves and synchrony in Epirrita autumnata/Operophtera brumata outbreaks. II. Sunspot activity cannot explain cyclic outbreaks. J. Anim. Ecol. 2007;76:269–275. doi: 10.1111/j.1365-2656.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- Okland B, Liebhold A.M, Bjørnstad O.N, Erbilgin N, Krokene P. Are bark beetle outbreaks less synchronous than forest Lepidoptera outbreaks? Oecologia. 2005;146:365–372. doi: 10.1007/s00442-005-0221-2. [DOI] [PubMed] [Google Scholar]
- Oli M.K. Population cycles of small rodents are caused by specialist predators: or are they? Trends Ecol. Evol. 2003;18:105–107. doi: 10.1016/S0169-5347(03)00005-3. [DOI] [Google Scholar]
- Ostfeld R.S. The fence effect reconsidered. Oikos. 1994;70:340–348. doi: 10.2307/3545771. [DOI] [Google Scholar]
- Pascual M. Diffusion-induced chaos in a spatial predator–prey system. Proc. R. Soc. B. 1993;25:1–7. doi: 10.1098/rspb.1993.0001. [DOI] [Google Scholar]
- Pascual M, Caswell H. Environmental heterogeneity and biological pattern in a chaotic predator–prey system. J. Theor. Biol. 1997;185:1–13. doi: 10.1006/jtbi.1996.0272. [DOI] [Google Scholar]
- Petrovskii S.V, Malchow H. A minimal model of pattern formation in a prey–predator system. Math. Comput. Model. 1999;29:49–63. doi: 10.1016/S0895-7177(99)00070-9. [DOI] [Google Scholar]
- Petrovskii S.V, Malchow H. Critical phenomena in plankton communities: Kiss model revisited. Nonlin. Anal. Real World Appl. 2000;1:37–51. doi: 10.1016/S0362-546X(99)00392-2. [DOI] [Google Scholar]
- Petrovskii S.V, Malchow H. Wave of chaos: new mechanism of pattern formation in spatio-temporal population dynamics. Theor. Popul. Biol. 2001;59:157–174. doi: 10.1006/tpbi.2000.1509. [DOI] [PubMed] [Google Scholar]
- Petrovskii S.V, Vinogradov M.E, Morozov A.Y. Spatial–temporal dynamics of a localized populational burst in a distributed prey–predator system. Okeanologiya. 1998;38:881–890. [Google Scholar]
- Petrovskii S.V, Morozov A.Y, Venturino E. Allee effect makes possible patchy invasion in a predator–prey system. Ecol. Lett. 2002;5:345–352. doi: 10.1046/j.1461-0248.2002.00324.x. [DOI] [Google Scholar]
- Petrovskii S.V, Malchow H, Hilker F.M, Venturino E. Patterns of patchy spread in deterministic and stochastic models of biological invasion and biological control. Biol. Invasions. 2005;7:771–793. doi: 10.1007/s10530-005-5217-7. [DOI] [Google Scholar]
- Piertney S.B, MacColl A.D.C, Bacon P.J, Dallas J.F. Local genetic structure in red grouse (Lagopus lagopus scoticus): evidence from microsatellite DNA markers. Mol. Ecol. 1998;7:1645–1654. doi: 10.1046/j.1365-294x.1998.00493.x. [DOI] [PubMed] [Google Scholar]
- Pitelka F.A, Batzli G.O. Population cycles of lemmings near Barrow, Alaska: a historical review. Acta Theriol. 2007;52:323–336. [Google Scholar]
- Rademacher J.D.M, Scheel A. Instabilities of wave trains and Turing patterns in large domains. Int. J. Bifurcat. Chaos. 2007a;17:2679–2691. doi: 10.1142/S0218127407018683. [DOI] [Google Scholar]
- Rademacher J.D.M, Scheel A. The saddle-node of nearly homogeneous wave trains in reaction–diffusion systems. J. Dyn. Differ. Eqns. 2007b;19:479–496. doi: 10.1007/s10884-006-9059-5. [DOI] [Google Scholar]
- Rademacher J.D.M, Sandstede B, Scheel A. Computing absolute and essential spectra using continuation. Physica D. 2007;229:166–183. doi: 10.1016/j.physd.2007.03.016. [DOI] [Google Scholar]
- Ranta E, Kaitala V. Travelling waves in vole population dynamics. Nature. 1997;390:456. doi: 10.1038/37261. [DOI] [Google Scholar]
- Ranta E, Kaitala V, Lindstrom J. Dynamics of Canadian lynx populations in space and time. Ecography. 1997;20:454–460. doi: 10.1111/j.1600-0587.1997.tb00412.x. [DOI] [Google Scholar]
- Redpath S.M, Mougeot F, Leckie F.M, Elston D.A, Hudson P.J. Testing the role of parasites in driving the cyclic population dynamics of a gamebird. Ecol. Lett. 2006;9:410–418. doi: 10.1111/j.1461-0248.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Risler E. Generic instability of spatial unfoldings of almost homoclinic periodic orbits. Commun. Math. Phys. 2001;216:325–356. doi: 10.1007/s002200000330. [DOI] [Google Scholar]
- Rosenzweig M.L, MacArthur R.H. Graphical representation and stability conditions of predator–prey interactions. Am. Nat. 1963;97:209–223. doi: 10.1086/282272. [DOI] [Google Scholar]
- Royama T. Moran effect on nonlinear population processes. Ecol. Monogr. 2005;75:277–293. doi: 10.1890/04-0770. [DOI] [Google Scholar]
- Rueness E.K, Stenseth N.C, O'Donoghue M, Boutin S, Ellegren H, Jakobsen K.S. Ecological and genetic spatial structuring in the Canadian lynx. Nature. 2003;425:69–72. doi: 10.1038/nature01942. [DOI] [PubMed] [Google Scholar]
- Sandstede B. Stability of travelling waves. In: Fiedler B, editor. Handbook of dynamical systems II. North-Holland; Amsterdam, The Netherlands: 2002. pp. 983–1055. [Google Scholar]
- Sandstede B, Scheel A. Absolute versus convective instability of spiral waves. Phys. Rev. E. 2000;62:7708–7714. doi: 10.1103/PhysRevE.62.7708. [DOI] [PubMed] [Google Scholar]
- Sandstede B, Scheel A. Defects in oscillatory media: toward a classification. SIAM J. Appl. Dyn. Syst. 2004;3:1–68. doi: 10.1137/030600192. [DOI] [Google Scholar]
- Scheel A. Radially symmetric patterns of reaction–diffusion systems. Memoir. Am. Math. Soc. 2003;165:A7. [Google Scholar]
- Schwartz M.K, Mills L.S, McKelvey K.S, Ruggiero L.F, Allendorf F.W. DNA reveals high dispersal synchronizing the population dynamics of Canada lynx. Nature. 2002;415:520–522. doi: 10.1038/415520a. [DOI] [PubMed] [Google Scholar]
- Scott S.K. Oxford University Press; Oxford, UK: 1994. Oscillations, waves and chaos in chemical kinetics. [Google Scholar]
- Scott S.K, Johnson B.R, Taylor A.F, Tinsley M.R. Complex chemical reactions. Chem. Eng. Sci. 2000;55:209–215. doi: 10.1016/S0009-2509(99)00314-0. [DOI] [Google Scholar]
- Selås V. Cyclic population fluctuations of herbivores as an effect of cyclic seed cropping of plants: the mast depression hypothesis. Oikos. 1997;80:257–268. doi: 10.2307/3546594. [DOI] [Google Scholar]
- Selås V. UV-B-induced plant stress as a possible cause of ten-year hare cycles. Popul. Ecol. 2006a;48:71–77. doi: 10.1007/s10144-005-0235-y. [DOI] [Google Scholar]
- Selås V. Explaining bank vole cycles in southern Norway 1980–2004 from bilberry reports 1932–1977 and climate. Oecologia. 2006b;147:625–631. doi: 10.1007/s00442-005-0326-7. [DOI] [PubMed] [Google Scholar]
- Selås V, Hogstad O, Andersson G, von Proschwitz T. Population cycles of autumnal moth, Epirrita autumnata, in relation to birch mast seeding. Oecologia. 2001;129:213–219. doi: 10.1007/s004420100711. [DOI] [PubMed] [Google Scholar]
- Selås V, Hogstad A, Kobro S, Rafoss T. Can sunspot activity and ultraviolet-B radiation explain cyclic outbreaks of forest moth pest species? Proc. R. Soc. B. 2004;271:1897–1901. doi: 10.1098/rspb.2004.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D.J, Haydon D.T, Cattadori I.M, Hudson P.J, Thirgood S.J. The shape of red grouse cycles. J. Anim. Ecol. 2004;73:767–776. doi: 10.1111/j.0021-8790.2004.00853.x. [DOI] [Google Scholar]
- Sherratt J.A. On the evolution of periodic plane waves in reaction–diffusion equations of λ–ω type. SIAM J. Appl. Math. 1994;54:1374–1385. doi: 10.1137/S0036139993243746. [DOI] [Google Scholar]
- Sherratt J.A. Unstable wavetrains and chaotic wakes in reaction–diffusion systems of λ–ω type. Physica D. 1995;82:165–179. doi: 10.1016/0167-2789(94)00224-E. [DOI] [Google Scholar]
- Sherratt J.A. Oscillatory and chaotic wakes behind moving boundaries in reaction–diffusion systems. Dyn. Stab. Syst. 1996a;11:303–324. [Google Scholar]
- Sherratt J.A. Periodic travelling waves in a family of deterministic cellular automata. Physica D. 1996b;95:319–335. doi: 10.1016/0167-2789(96)00070-X. [DOI] [Google Scholar]
- Sherratt J.A. Invasive wave fronts and their oscillatory wakes are linked by a modulated travelling phase resetting wave. Physica D. 1998;117:145–166. doi: 10.1016/S0167-2789(97)00317-5. [DOI] [Google Scholar]
- Sherratt J.A. Periodic travelling waves in cyclic predator–prey systems. Ecol. Lett. 2001;4:30–37. doi: 10.1046/j.1461-0248.2001.00193.x. [DOI] [Google Scholar]
- Sherratt J.A. Periodic travelling wave selection by Dirichlet boundary conditions in oscillatory reaction–diffusion systems. SIAM J. Appl. Math. 2003;63:1520–1538. doi: 10.1137/S0036139902392483. [DOI] [Google Scholar]
- Sherratt, J. A. Submitted. A comparison of periodic travelling wave generation by Robin and Dirichlet boundary conditions in oscillatory reaction–diffusion equations.
- Sherratt J.A, Lewis M.A, Fowler A.C. Ecological chaos in the wake of invasion. Proc. Natl Acad. Sci. USA. 1995;92:2524–2528. doi: 10.1073/pnas.92.7.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt J.A, Eagan B.T, Lewis M.A. Oscillations and chaos behind predator–prey invasion: mathematical artifact or ecological reality? Phil. Trans. R. Soc. B. 1997;352:21–38. doi: 10.1098/rstb.1997.0003. [DOI] [Google Scholar]
- Sherratt J.A, Lambin X, Thomas C.J, Sherratt T.N. Generation of periodic waves by landscape features in cyclic predator–prey systems. Proc. R. Soc. B. 2002;269:327–334. doi: 10.1098/rspb.2001.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt J.A, Lambin X, Sherratt T.N. The effects of the size and shape of landscape features on the formation of traveling waves in cyclic populations. Am. Nat. 2003;162:503–513. doi: 10.1086/377186. [DOI] [PubMed] [Google Scholar]
- Sherratt T.N, Lambin X, Petty S.J, MacKinnon J.L, Coles C.F, Thomas C.J. Application of coupled oscillator models to understand extensive synchrony domains and travelling waves in populations of the field vole in Kielder forest, UK. J. Appl. Ecol. 2000;37(Suppl. 1):148–158. doi: 10.1046/j.1365-2664.2000.00472.x. [DOI] [Google Scholar]
- Smith C.H. Spatial trends in Canadian snowshoe hare, Lepus Americanus, population cycles. Can. Field Nat. 1983;97:151–160. [Google Scholar]
- Smith M.J, Sherratt J.A. The effects of unequal diffusion coefficients on periodic travelling waves in oscillatory reaction–diffusion systems. Physica D. 2007;236:90–103. doi: 10.1016/j.physd.2007.07.013. [DOI] [Google Scholar]
- Smith M.J, Sherratt J.A, Armstrong N.J. The effects of obstacle size on periodic travelling waves in oscillatory reaction–diffusion equations. Proc. R. Soc. A. 2008;464:365–390. doi: 10.1098/rspa.2007.0198. [DOI] [Google Scholar]
- Stenseth N.C, Bjørnstad O.N, Saitoh T. A gradient from stable to cyclic populations of Clethrionomys rufocanus in Hokkaido, Japan. Proc. R. Soc. B. 1996;263:1117–1126. doi: 10.1098/rspb.1996.0164. [DOI] [PubMed] [Google Scholar]
- Stenseth N.C, et al. Common dynamic structure of Canada lynx populations within three climatic regions. Science. 1999;285:1071–1073. doi: 10.1126/science.285.5430.1071. [DOI] [PubMed] [Google Scholar]
- Stenseth N.C, Kittilsen M.O, Hjermann D.O, Viljugrein H, Saitoh T. Interaction between seasonal density-dependence structures and length of the seasons explain the geographical structure of the dynamics of voles in Hokkaido: an example of seasonal forcing. Proc. R. Soc. B. 2002;269:1853–1863. doi: 10.1098/rspb.2002.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, et al. The effect of climatic forcing on population synchrony and genetic structuring of the Canadian lynx. Proc. Natl Acad. Sci. USA. 2004;101:6056–6061. doi: 10.1073/pnas.0307123101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L, He D. Chaotic oscillations and cycles in multi-trophic ecological systems. J. Theor. Biol. 2007;248:382–390. doi: 10.1016/j.jtbi.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Tanhuanpää M, Ruohomäki K, Turchin P, Ayres M.P, Bylund H, Kaitaniemi P, Tammaru T, Haukioja E. Population cycles of the autumnal moth in Fennoscandia. In: Berryman A.A, editor. Population cycles: the case for trophic interactions. Oxford University Press; Oxford, UK: 2002. pp. 142–154. [Google Scholar]
- Taylor C.M, Hastings A. Allee effects in biological invasions. Ecol. Lett. 2005;8:895–908. doi: 10.1111/j.1461-0248.2005.00787.x. [DOI] [Google Scholar]
- Tenow O, Nilssen A.C, Bylund H, Hogstad O. Waves and synchrony in Epirrita autumnata/Operophtera brumata outbreaks. I. Lagged synchrony: regionally, locally and among species. J. Anim. Ecol. 2007;76:258–268. doi: 10.1111/j.1365-2656.2006.01204.x. [DOI] [PubMed] [Google Scholar]
- Truscott J.E, Brindley J. Ocean plankton populations as excitable media. Bull. Math. Biol. 1994;56:981–998. [Google Scholar]
- Tsyganov M.A, Brindley J, Holden A.V, Biktashev V.N. Soliton-like phenomena in one-dimensional cross-diffusion systems: a predator–prey pursuit and evasion example. Physica D. 2004;197:18–33. doi: 10.1016/j.physd.2004.06.004. [DOI] [Google Scholar]
- Turchin P. Princeton University Press; Princeton, NJ: 2003. Complex population dynamics: a theoretical/empirical synthesis. [Google Scholar]
- Turchin P, Reeve J.D, Cronin J.T, Wilkens R.T. Spatial pattern formation in ecological systems: bridging thoretical and empirical approaches. In: Bascompte J, Sole R.V, editors. Modeling spatiotemporal dynamics in ecology. Springer; Berlin, Germany: 1998. pp. 199–213. [Google Scholar]
- Turchin P, Taylor A.D, Reeve J.D. Dynamical role of predators in population cycles of a forest insect: an experimental test. Science. 1999;285:1068–1071. doi: 10.1126/science.285.5430.1068. [DOI] [PubMed] [Google Scholar]
- Turchin P, Briggs C.J, Ellner S.P, Fischlin A, Kendall B.E, McCauley E, Murdoch W.W, Wood S.N. Population cycles of the larch budmoth in Switzerland. In: Berryman A.A, editor. Population cycles: the case for trophic interactions. Oxford University Press; Oxford, UK: 2002. pp. 130–141. [Google Scholar]
- Webb S.D, Sherratt J.A. Oscillatory reaction–diffusion equations with temporally varying parameters. Math. Comput. Model. 2004;39:45–60. doi: 10.1016/S0895-7177(04)90505-5. [DOI] [Google Scholar]
- Wilder J.W, Vasquez D.A, Christie I, Colbert J.J. Wave trains in a model of gypsy moth population dynamics. Chaos. 1995;5:700–706. doi: 10.1063/1.166102. [DOI] [PubMed] [Google Scholar]
- Xin J. Front propagation in heterogeneous media. SIAM Rev. 2000;42:161–230. doi: 10.1137/S0036144599364296. [DOI] [Google Scholar]
- Yang S, Ruuhola T, Rantala M.J. Impact of starvation on immune defense and other life-history traits of an outbreaking geometrid, Epirrita autumnata: a possible causal trigger for the crash phase of population cycle. Ann. Zool. Fenn. 2007;44:89–96. [Google Scholar]