Abstract
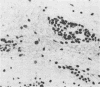
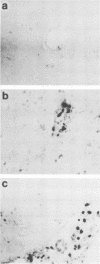
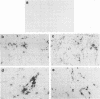
Street rabies virus (SRV)-infected T-lymphocyte-deficient (nude) mice, in contrast to euthymic mice, did not develop hindlimb paralysis prior to death. To document the role of T lymphocytes in rabies virus-associated paralysis, 10(8) spleen cells from normal immunocompetent euthymic mice were transferred to nude mice and the recipient mice were challenged with SRV. One hundred percent of the reconstituted mice developed paralysis and died. Depletion of T cells from the donor spleen suspension prior to transfer abrogated the development of paralysis but did not prevent the deaths of the recipient animals. Mice receiving 10(8) rabies virus-immune spleen cells did not become paralyzed and did not die. Nude mice inoculated with either rabies virus-immune or normal mouse serum prior to and following SRV inoculation did not develop paralysis. Immune serum protected the mice, whereas animals inoculated with normal serum died. Central nervous system inflammatory responses in nude mice immunologically reconstituted with normal spleen cells were characterized by diffuse cellular infiltrates in the parenchyma and extensive perivascular cuffing. Perivascular infiltrates included CD8+ and CD4+ T lymphocytes and Mac-1+ macrophage-microglial cells. Inflammatory cells in the parenchyma were limited to CD8+ lymphocytes and Mac-1+ cells. These observations indicate that paralysis of SRV-infected mice is dependent on T lymphocytes. Whether injury leading to paralysis is mediated by T lymphocytes or by an influence of T lymphocytes on macrophage-microglial cells or other cells remains to be determined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J., Hengartner H., Zinkernagel R. M., Cole G. A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986 Apr;16(4):387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Cleary W. F. A model in mice for the pathogenesis and treatment of rabies. J Infect Dis. 1972 May;125(5):520–527. doi: 10.1093/infdis/125.5.520. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Cleary W. F., Díaz A. M., Perl D. F. Characteristics of 11 rabies virus isolates in mice: titers and relative invasiveness of virus, incubation period of infection, and survival of mice with sequelae. J Infect Dis. 1977 Sep;136(3):336–345. doi: 10.1093/infdis/136.3.336. [DOI] [PubMed] [Google Scholar]
- Blancou J., Andral B., Andral L. A model in mice for the study of the early death phenomenon after vaccination and challenge with rabies virus. J Gen Virol. 1980 Oct;50(2):433–435. doi: 10.1099/0022-1317-50-2-433. [DOI] [PubMed] [Google Scholar]
- Chopra J. S., Banerjee A. K., Murthy J. M., Pal S. R. Paralytic rabies: a clinico-pathological study. Brain. 1980 Dec;103(4):789–802. doi: 10.1093/brain/103.4.789. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C. T cells and viral infections. Br Med Bull. 1985 Jan;41(1):7–14. doi: 10.1093/oxfordjournals.bmb.a072028. [DOI] [PubMed] [Google Scholar]
- Fekadu M., Chandler F. W., Harrison A. K. Pathogenesis of rabies in dogs inoculated with an Ethiopian rabies virus strain. Immunofluorescence, histologic and ultrastructural studies of the central nervous system. Arch Virol. 1982;71(2):109–126. doi: 10.1007/BF01314881. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Gerhard W., Clark H. F. Role of host immune response in the development of either encephalitic or paralytic disease after experimental rabies infection in mice. Infect Immun. 1977 Oct;18(1):220–225. doi: 10.1128/iai.18.1.220-225.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Tishon A., Oldstone M. B. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in vivo. J Immunol. 1989 Sep 15;143(6):2013–2016. [PubMed] [Google Scholar]
- Lodmell D. L., Bell J. F., Moore G. J., Raymond G. H. Comparative study of abortive and nonabortive rabies in mice. J Infect Dis. 1969 Jun;119(6):569–580. doi: 10.1093/infdis/119.6.569. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C. Pathogenesis of street rabies virus infections in resistant and susceptible strains of mice. J Virol. 1985 Sep;55(3):788–795. doi: 10.1128/jvi.55.3.788-795.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L. Genetic control of resistance to street rabies virus in mice. J Exp Med. 1983 Feb 1;157(2):451–460. doi: 10.1084/jem.157.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Wiedbrauk D. L., Ewalt L. C. Interferon induced within the central nervous system during infection is inconsequential as a mechanism responsible for murine resistance to street rabies virus. J Gen Virol. 1989 Feb;70(Pt 2):473–478. doi: 10.1099/0022-1317-70-2-473. [DOI] [PubMed] [Google Scholar]
- Maehlen J., Olsson T., Löve A., Klareskog L., Norrby E., Kristensson K. Persistence of measles virus in rat brain neurons is promoted by depletion of CD8+ T cells. J Neuroimmunol. 1989 Feb;21(2-3):149–155. doi: 10.1016/0165-5728(89)90170-7. [DOI] [PubMed] [Google Scholar]
- Marcovistz R., Germano P. M., Rivière Y., Tsiang H., Hovanessian A. G. The effect of interferon treatment in rabies prophylaxis in immunocompetent, immunosuppressed, and immunodeficient mice. J Interferon Res. 1987 Feb;7(1):17–27. doi: 10.1089/jir.1987.7.17. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., ter Meulen V. Viral particles induce Ia antigen expression on astrocytes. Nature. 1986 Apr 10;320(6062):543–546. doi: 10.1038/320543a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mori S., Sawai T., Teshima T., Kyogoku M. A new decalcifying technique for immunohistochemical studies of calcified tissue, especially applicable to cell surface marker demonstration. J Histochem Cytochem. 1988 Jan;36(1):111–114. doi: 10.1177/36.1.3275709. [DOI] [PubMed] [Google Scholar]
- Morris A., Tomkins P. T., Maudsley D. J., Blackman M. Infection of cultured murine brain cells by Semliki Forest virus: effects of interferon-alpha beta on viral replication, viral antigen display, major histocompatibility complex antigen display and lysis by cytotoxic T lymphocytes. J Gen Virol. 1987 Jan;68(Pt 1):99–106. doi: 10.1099/0022-1317-68-1-99. [DOI] [PubMed] [Google Scholar]
- Olsson T., Maehlen J., Löve A., Klareskog L., Norrby E., Kristensson K. Induction of class I and class II transplantation antigens in rat brain during fatal and non-fatal measles virus infection. J Neuroimmunol. 1987 Oct;16(2):215–224. doi: 10.1016/0165-5728(87)90076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. L., Hotchkiss J. D., Lodmell D. L. Murine susceptibility to street rabies virus is unrelated to induction of host lymphoid depletion. J Immunol. 1990 May 1;144(9):3552–3557. [PubMed] [Google Scholar]
- Prabhakar B. S., Fischman H. R., Nathanson N. Recovery from experimental rabies by adoptive transfer of immune cells. J Gen Virol. 1981 Sep;56(Pt 1):25–31. doi: 10.1099/0022-1317-56-1-25. [DOI] [PubMed] [Google Scholar]
- Prabhakar B. S., Nathanson N. Acute rabies death mediated by antibody. Nature. 1981 Apr 16;290(5807):590–591. doi: 10.1038/290590a0. [DOI] [PubMed] [Google Scholar]
- Richt J., Stitz L., Deschl U., Frese K., Rott R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J Gen Virol. 1990 Nov;71(Pt 11):2565–2573. doi: 10.1099/0022-1317-71-11-2565. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Pierce M. L., Howie E. A. Immune response gene products (Ia antigens) on glial and endothelial cells in virus-induced demyelination. J Immunol. 1987 May 15;138(10):3438–3442. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sikes R. K., Cleary W. F., Koprowski H., Wiktor T. J., Kaplan M. M. Effective protection of monkeys against death from street virus by post-exposure administration of tissue-culture rabies vaccine. Bull World Health Organ. 1971;45(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., McCelland C. L., Reid F. L., Baer G. M. Dual role of the immune response in street rabiesvirus infection of mice. Infect Immun. 1982 Jan;35(1):213–221. doi: 10.1128/iai.35.1.213-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S. Mouse model for abortive rabies infection of the central nervous system. Infect Immun. 1981 Jan;31(1):297–308. doi: 10.1128/iai.31.1.297-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973 May;48(5):535–541. [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Sugamata M., Ewalt L. C., Perry L. L., Lodmell D. L. Detection of anti-rabies virus cytotoxic T lymphocytes in mice of four distinct H-2 haplotypes using target cells persistently infected with ERA rabies virus. J Virol Methods. 1990 Jul;29(1):1–11. doi: 10.1016/0166-0934(90)90002-w. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Weiss S. R., Silberberg D. H. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986 May 23;232(4753):991–993. doi: 10.1126/science.3010460. [DOI] [PubMed] [Google Scholar]
- Tignor G. H., Shope R. E., Gershon R. K., Waksman B. H. Immunopathologic aspects of infection with Lagos bat virus of the rabies serogroup. J Immunol. 1974 Jan;112(1):260–265. [PubMed] [Google Scholar]
- Tirawatnpong S., Hemachudha T., Manutsathit S., Shuangshoti S., Phanthumchinda K., Phanuphak P. Regional distribution of rabies viral antigen in central nervous system of human encephalitic and paralytic rabies. J Neurol Sci. 1989 Aug;92(1):91–99. doi: 10.1016/0022-510x(89)90178-0. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Hart D. N., Fabre J. W., Morris P. J. Distribution and quantitation of HLA-ABC and DR (Ia) antigens on human kidney and other tissues. Transplantation. 1980 Apr;29(4):274–279. doi: 10.1097/00007890-198004000-00002. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., McKimm-Breschkin J. L., Schrader J. W. Interferon-gamma induces the expression of H-2 and Ia antigens on brain cells. J Neuroimmunol. 1985 Feb-Mar;7(5-6):255–278. doi: 10.1016/s0165-5728(84)80026-0. [DOI] [PubMed] [Google Scholar]