Synopsis
We have gained enormous insight into the mechanisms underlying both activity-dependent and (to a lesser degree) -independent plasticity of excitatory synapses. Recently, cortical inhibition has been shown to play a vital role in the formation of critical periods for sensory plasticity. As such, sculpting of neuronal circuits by inhibition may be a common mechanism by which activity organizes or reorganizes brain circuits. Disturbances in the balance of excitation and inhibition in the neocortex provoke abnormal activities, such as epileptic seizures and abnormal cortical development. However, both the process of experience-dependent postnatal maturation of neocortical inhibitory networks and its underlying mechanisms remain elusive. Mechanisms that match excitation and inhibition are central to achieving balanced function at the level of individual circuits. The goal of this review is to reinforce our understanding of the mechanisms by which developing inhibitory networks are able to adapt to sensory inputs, and to maintain their balance with developing excitatory networks. Discussion will be centered on the following questions related to experience-dependent plasticity of neocortical inhibitory networks. 1) What are the roles of GABAergic inhibition in the postnatal maturation of neocortical circuits? 2) Does the maturation of neocortical inhibitory circuits proceed in an activity-dependent manner or do they develop independently of sensory inputs? 3) Does activity regulate inhibitory networks in the same way it regulates excitatory networks? 4) What are the molecular and cellular mechanisms that underlie the activity-dependent maturation of inhibitory networks? 5) What are the functional advantages of experience-dependent plasticity of inhibitory networks to network processing in sensory cortices?
Keywords: GABA, inhibition, cortex, microcircuits, synaptic plasticity, development
Sensory experience drives the refinement of sensory maps in developing and adult sensory cortices (Wiesel, 1982; Crair et al., 1998; Merzenich et al., 1984; Stryker et al., 1978; Feldman and Brecht, 2005). Sensory deprivation causes the cortical area representing the deprived sensory input to shrink, and neighboring spared representations to enlarg, in somatosensory (Feldman and Brecht, 2005a; Merzenich et al., 1984), auditory (Merzenich and Sameshima, 1993), visual (Crair et al., 1998; Hubel et al., 1977; Wiesel, 1982) and language cortex (Neville and Bavelier, 2002). Contributions from both genes and neural activity instruct the development of sensory maps. Tremendous progress has been made toward understanding both the process of maturation of excitatory networks, and the mechanisms underlying the activity-dependent modification of glutamatergic synapses in principal neurons (Sur and Leamey, 2001). Recently, cortical inhibition has been shown to play a vital role in the formation of critical periods for sensory plasticity (Hensch et al., 1998). However, both the process of experience-dependent postnatal maturation of neocortical inhibitory networks and the underlying mechanisms remain elusive (Alonso and Swadlow, 2005; Feldman, 2000; Micheva and Beaulieu, 1996). This review focuses on the mechanisms underlying activity-dependent regulation of neocortical inhibitory circuits and the roles of inhibition in postnatal sensory map plasticity. Focus will be placed on the following questions related to experience-dependent plasticity of neocortical inhibitory networks. 1) What are the roles of GABAergic inhibition in the postnatal maturation of neocortical circuits? 2) Does the maturation of neocortical inhibitory circuit proceed in an activity-dependent manner or do they develop independently of sensory inputs? 3) Does activity regulate inhibitory networks in the same way it regulates excitatory networks? 4) What are the molecular and cellular mechanisms that underlie the activity-dependent maturation of inhibitory networks? 5) What are the functional advantages of experience-dependent plasticity of inhibitory networks to network processing in sensory cortices?
1. Role of GABAergic synaptic inhibition in postnatal cortical development
In the immature hippocampus and neocortex, GABAergic interneurons form functional synapses earlier than glutamatergic neurons. These pioneering interneurons form functional “inhibitory networks” that generate excitatory depolarizing potentials thought to be very important for the early development of the neural networks. Several important functions of GABA have been elucidated, particularly its role as a trophic factor that influences cell proliferation, migration, and circuit maturation (reviewed by Owens et al., 1999). During postnatal brain development, the reversal potential for GABAA mediated responses highly dependent upon intracellular Cl− concentrations and is shifted from −46 mV (postnatal day 0) to −82 mV (>postnatal day 12; Owens et al., 1999). The increased expression of a K+-Cl− coupled co-transporter (KCC2) is primarily responsible for the developmental switch of the GABA mediated response (Rivera et al., 1999).
Apart from the trophic actions of depolarizing GABA in early development, inhibitory networks are also recognized for playing crucial a role in experience-dependent refinement of neural networks. Distinct genes encode two isoforms of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD65 & GAD67). GAD67 is the larger protein, and provides a constitutive concentration of GABA throughout the CNS. Mice lacking GAD67 show a significant reduction in brain GABA concentrations, and die at birth. GAD65 is found primarily in the synaptic terminals, and serves the rapid changes in synaptic demand following intense neuronal activity. Mice lacking GAD65 survive and develop normal gross cortical morphology, and normal adult GABA concentrations (Kash et al., 1999). However, the GAD65 knockout prevents ocular dominance plasticity (Hensch et al., 1998). In contrast, pharmacologically enhancing activity-dependent GABA transmission can prematurely enhance ocular dominance plasticity (Fagiolini et al., 1994; Iwai et al., 2003). In normal wild type animals, enhancing local existing GABA transmission did not perturb visual responsiveness but did widen ocular dominance column spacing. This suggests that local cortical inhibitory synapses might modulate incoming TC inputs (Fig. 1). Despite these clear demonstrations of the importance of intracortical inhibition in visual cortical plasticity, the underlying mechanisms involved are not clear. These experiments suggest the important roles of GABA in experience-dependent early cortical development. In the somatosensory cortex, the role of cortical inhibition in shaping barrel plasticity is even less clear. It is unknown whether genetic deletion of fast GABAergic transmission will affect cortical barrel formation. However, indirect evidence suggests that inhibition may be at least partially responsible for the activity-dependent barrel plasticity. For example, enhancing whisker activity increases the number of GABA synapses formed on dendritic spines (Knott et al., 2002). Recent evidence also suggests that regulation of NMDA receptor subtype composition has no effect on barrel critical period formation (Lu et al., 2001). In the barrel cortex, GAD65 expression appears late in the critical period for barrel formation (Kiser et al., 1998), indicating GABA’s role in the refinement of barrel structure. Additional experiments that thoroughly examine the roles of GABA in barrel plasticity, are necessary for understanding the roles of inhibition in somatosensory cortical development.
Figure 1. Role of basket cells in sensory-mediated feed-forward inhibition and modulation of receptive field properties.
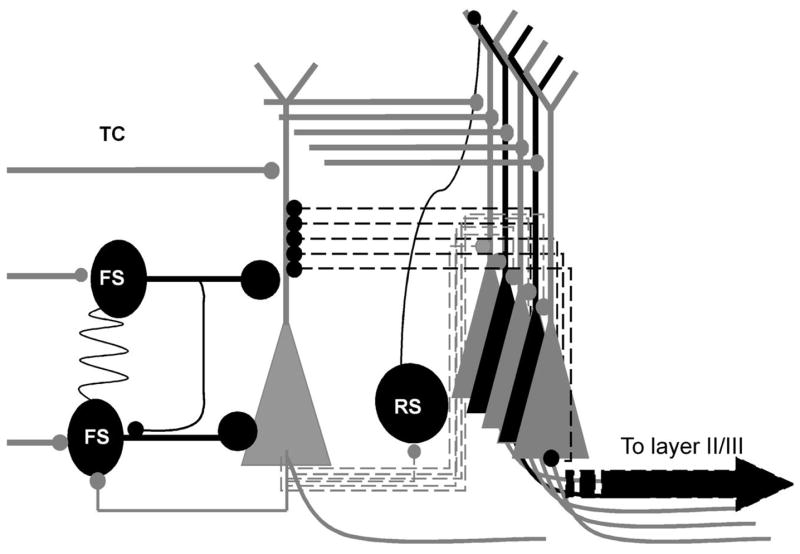
Thalamocortical excitatory synapses onto spiny stellate cells as well as fast-spiking basket cells, which form connections via gap junctions. Spiny stellate cells out-number basket cells and form elaborate recurrent connections with other spiny stellate cells. However, the strength of the FS-mediated perisomatic inhibition is on average 10 times larger than the intracortical excitatory synaptic excitation. Therefore the sensory feed-forward inhibition plays a very important role in modulating the receptive field properties by limiting the lateral propagation of intracortically mediated recurrent excitations and spike-timing of sensory mediated spikes in spiny stellate cells. Black cells: interneurons and inhibitory synapses; Gray cells spiny stellate cells and glutamatergic synapses.
2. Does the maturation of neocortical inhibitory networks proceed in an activity-dependent manner or do they develop independently of sensory inputs (or both)?
Cortical inhibition plays a vital role in the formation of critical periods for visual plasticity. How does inhibition contribute to the formation of neocortical critical periods? The current dogma regarding sensory map plasticity is centered on plasticity of excitatory connections, which follows a “use it or loose it” rule, i.e. connections with stimulated (or correlated) inputs grows stronger and connections with inactive (or uncorrelated) inputs grows weaker. This process was also known as the Hebbian rule (Hebb, 1955). A very compelling hypothesis about the role of inhibition in the formation of critical periods was that lateral cortical inhibition modulated the Hebbian-type plasticity by enhancing correlative activities of adjacent cortical neurons and producing anti-correlative activities in distal cells (Ferster, 2004). To serve this role, i.e. modulating the spike-timing and lateral spread of excitation, strength of inhibitory synapses must be developmentally regulated as well. Prior to the formation of neocortical critical periods, the strength of thalamocortical and intracortical glutamatergic synapses undergo drastic morphological, molecular and functional changes (Feldman et al., 1998; Feldman and Knudsen, 1998). Disturbances in the balance of excitation and inhibition in the neocortex induce cortical epileptic seizure. Therefore, a key requirement for maturation of sensory cortices, based on a “use it or loose it” rule, was that excitation and inhibition must be delicately balanced to achieve appropriate functioning at the level of cortical local circuits.
2.1.) Experience-dependent plasticity
In the barrel cortex of rodents, intra-barrel inhibition plays an important role in sensory mediated refinement of receptive fields (Shoykhet et al., 2005). Sensory deprivation has been shown to induce a dynamic adjustment in the balance of excitation and inhibition, which may allow networks within layer 4 to maintain stable levels of activity in the face of variable sensory inputs (Alonso and Swadlow, 2005). In an early study, sensory loss by selected whisker removal produces immediate disinhibition in the somatosensory cortex of behaving rats (Kelly et al., 1999). However, it is unclear how this process is regulated if the sensory loss persists throughout a critical period of postnatal development. There is considerable evidence suggesting that the amount of inhibitory neurotransmitter (GABA), its receptors, and the number of synapses, are correlated with levels of neuronal activity (Knott et al., 2002; Micheva and Beaulieu, 1996). Recent studies of visual and auditory systems provide further evidence that the reorganization of inhibitory connections occurs at the circuitry level (Hensch et al., 1998c; Kim and Kandler, 2003). In the barrel cortex, active whisking enhances the emergence of mature inhibition (Kiser et al., 1998). In contrast, whisker trimming during the second through fourth postnatal week induced very robust down-regulation of perisomatic inhibitory synapses from fast-spiking basket cells. This down-regulation is accompanied by changes in presynaptic calcium dynamics and GAD expression in the nerve terminals as well (Jiao et al., 2006). Visual cortical GABAergic synapses of basket cells also show clearly defined dependence on sensory experience (Jiang et al., 2005). Between the time at which the eyes first open and the end of the critical period for experience-dependent plasticity, the total GABAergic input converging onto layer II/III pyramidal cells of the visual cortex increases threefold. This increase reflects changes in the number of quanta released by presynaptic axons and is prevented by dark rearing (sensory deprivation). Thus, sensory experience appears to play a permissive role in the maturation of intracortical GABAergic circuits (Morales et al., 2002, Fig. 2). Recently, using microarray analysis combined with other molecular and immunohistochemical methods, a set of signaling genes, whose expression is regulated by visual deprivation, were identified (Majdan and Shatz, 2006; Tropea et al., 2006). Among these genes, genes for GABAA receptor subunits α2 & 3, β1 & 3 genes were found to be up-regulated by monocular derivation or dark rearing. Other genes, such as those encoding for GAD67 and parvalbumin, have been implicated in experience-dependent plasticity, and were all found to be affected by visual experiences (Tropea et al., 2006). Thus activity-dependent regulation of gene expression appears to be a major means of remodeling inhibitory networks through experiences.
Figure 2. Postnatal maturation and plasticity of perisomatic inhibitory synaptic boutons in sensory cortices.
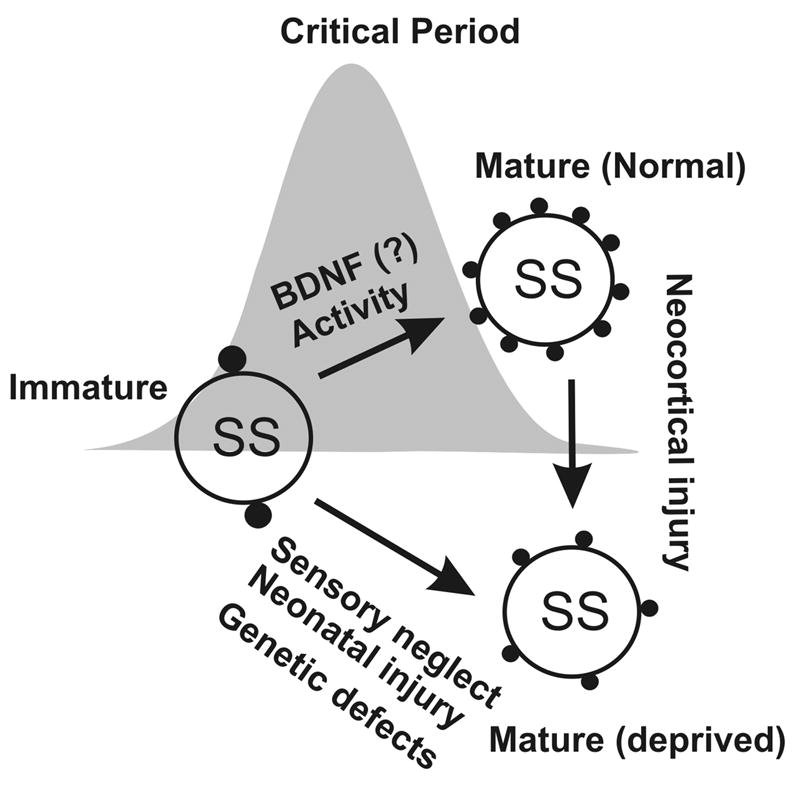
Perisomatic inhibitory synapses undergo a process of activity-dependent maturation during the first few postnatal weeks. This process is presumably regulated via activity-dependent processes and is delayed or diminished by the sensory neglect or neonatal injury. As implicated, the maturation of inhibitory network plays a role in activity-dependent formation of sensory critical periods. Mature inhibitory circuits can be changed into ‘immature network’, if the cortices are injured.
2.2.) Activity-independent plasticity
In addition to these clearly defined activity-dependent processes that underlie GABAergic maturation, activity-independent plasticity has been reported in the sensory cortices. It has been reported that both GAD67, which produces the basal pool of GABA, and GAD65, which is specialized to respond to short-term increases in demand in synaptic terminals, develop normal levels of expression and normal intracellular and laminar distributions in the absence of visual input (Mower and Guo, 2001). In another study, both changes in GABAA receptor expression and synaptic functioning were initiated well before eye opening. Moreover, dark rearing could not prevent the robust up-regulation of alpha1 or the change in sIPSC kinetics, indicating that these parameters are not dependent upon sensory (visual) input. Dark rearing experiments have shown that a lack of extrinsic input to the visual cortex does not affect the overall developmental regulation of synaptic functioning of GABAA receptors (Heinen et al., 2004). In the barrel cortex, the density of GABAA receptors is reduced in lamina IV following complete loss of peripheral afferent input. However, less severe tactile deprivation, which is known to affect cortical neuron responsiveness, produces little or no change in GABAA receptor distribution (Land et al., 1995). Taken together these results imply that certain components of the GABAergic network, particularly the presynaptic features of the GABAergic system (such as total number of synaptic boutons, functional active synapses, and property of presynaptic inhibitory boutons) are sensitive to regulation by sensory activities. On the other hand, postsynaptic properties of GABAergic system (such as postsynaptic GABAA receptor subunits), may be regulated by activity-independent processes. The results of the following studies will help to further our understanding of the dilemma between the activity-dependent and -independent components of GABAergic maturation. In this study, it was reported that over-expression of BDNF promotes the maturation of GABA transmission in the absence of activity (via dark rearing) in the visual cortex (Gianfranceschi et al., 2003; Kiser et al., 1998). Therefore, trophic factors such as BNDF appear to regulate the maturation of the GABAergic system, however, the release of BDNF is activity-dependent and developmentally regulated. A further understanding of these different components of GABAergic maturation and how they are regulated is of great importance to future studies.
2.3) Homeostatic synaptic plasticity
Homeostatic synaptic plasticity is a form of plasticity that is triggered by changes in the overall level of activity of a neural circuit and has a crucial role in stabilizing the activity of neurons and networks (Marder and Goaillard, 2006; Turrigiano and Nelson, 2004). Without this stabilizing mechanism, activity-dependent forms of plasticity could drive neural activity towards runaway excitation or quiescence. Homeostatic plasticity is mediated by mechanisms that include: global changes in synaptic strength, changes in neuronal excitability, and the regulation of synapse number. Synaptic scaling is a major form of homeostatic plasticity that scales synaptic strength up or down to compensate for prolonged changes in activity (Wierenga et al., 2005). Because homeostatic plasticity occurs in the absence of sensory activity, this type of regulation can be considered a form of activity-independent plasticity. Glutamatergic synapses and glutamatergic cells are known to exhibit very well characterized synaptic scaling and other forms of homeostatic plasticities in the visual cortex and other sensory regions (Marder and Goaillard, 2006;Turrigiano and Nelson, 2004). However, example of synaptic scaling of inhibitory synapses is very sparse. In the visual cortex, homeostatic potentiation of inhibitory feedback between interneurons and excitatory neurons may underlie the loss of visual responsiveness to the deprived eye (Maffei et al., 2006; Wierenga et al., 2005). The mechanisms of synaptic scaling are poorly understood. It may involve presynaptic or postsynaptic changes and the release of neural active substance from glial cells (Stellwagen and Malenka, 2006). It is important to test how GABAergic neurons undergo homeostatic changes and other activity-independent changes under various sensory deprivation paradigms and to examine the potential mechanisms underlying such homeostatic changes.
3. Comparison of activity-dependent regulation of inhibitory vs. excitatory networks
Sensory experience drives the plasticity of the body map in developing adult sensory cortices (Sur and Leamey, 2001; Feldman and Brecht, 2005). Early studies of visual receptive field plasticity during early postnatal life have established the role of activity in fine tuning the receptive field properties of a visual cortical neuron, where inputs from the two eyes can either ‘associate’ or ‘compete’, depending on how well they are correlated (Wiesel, 1982). Donald Hebb postulated that associative memories are formed in the brain by a process of synaptic modification that strengthens connections when presynaptic activity correlates with postsynaptic firings (Hebb, 1955). Later, Gunther Stent modified this proposal by including mechanisms of synaptic weakening, i.e. connections weaken when they are inactive at the same time that postsynaptic neurons is active (Stent, 1973). Long-term potentiation (LTP) and depression (LTD) of excitatory synaptic transmission has been demonstrated in almost all excitatory neurons (reviewed by Malenka and Bear, 2004). It is clear that cortical activity, mediated through glutamate receptors, contributes to the experience-dependent refinement of the sensory map (Crair et al., 1998). Although the cellular mechanisms underlying changes in cortical maps are not entirely clear, considerable evidence now confirms that LTP and LTD, or similar processes, are induced at specific cortical excitatory synapses onto principal neurons during map plasticity (Bear et al., 1992), where the LTP and LTD appears to induce either synaptic strengthening or elimination, respectively. The increase in synaptic strength that occurs during LTP is likely to involve structural changes in dendritic spines, either through the expansion of existing spines or through an increased connectivity mediated via the addition of new spines. LTP causes a rapid local increase in the extension of filopodia and the formation of new spines at the site of stimulation (Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999). This increase requires the activation of NMDA receptors and can be induced by a focal application of Ca2+. Induction of LTD is accompanied by a marked shrinkage of spines. The spine shrinkage requires activation of NMDA receptors and calcineurin, similar to that for LTD. This activity-induced spine shrinkage may contribute to the activity-dependent elimination of synaptic connections (Zhou et al., 2004).
There is little evidence available that would indicate a similar process occurs at specific cortical excitatory synapses onto neocortical interneurons (Alonso and Swadlow, 2005). In the hippocampus, where LTP of glutamatergic synapses onto pyramidal neurons is very robust; LTP of glutamatergic transmission has been reported in interneurons (Ouardouz and Lacaille, 1995; Laezza et al., 1999). However, a number of studies have documented the lack of long-term modifications in these synapses (McBain and Maccaferri, 1997; McBain et al., 1999). In the sensory cortices, input discrimination depends upon a delicate balance between inhibition and excitation (Alonso and Swadlow, 2005). Selective LTP of excitatory transmission to spiny neurons, without a corresponding potentiation of inhibitory transmission, would lead to a compromise of spatial and temporal precision and a degradation in the fidelity of signal processing (Alonso and Swadlow, 2005; Turrigiano et al., 1998).
In excitatory neurons, postsynaptic forms of LTP and LTD of glutamatergic synapses are dependent on Ca2+-signaling cascades, mostly on two key enzymes, calcineurin (CN) and Ca2+/calmodulin-dependent protein kinase II (CaMKII; Nayak et al., 1996; Leamey et al., 2003). Large increases in intracellular Ca2+ levels activate CaMKII and induce LTP (Lledo et al., 1995), whereas smaller increases activate CN preferentially and can result in LTD (Nayak et al., 1996; Cummings et al., 1994). These two enzymes provide a switch-like mechanism for regulating glutamate receptor-dependent Ca2+ signaling processes, such as AMPA receptor trafficking and neurite outgrowth in cortical pyramidal neurons (Beaumont et al., 2001; Isaac et al., 1997; Cooke and Bliss, 2003; Yang et al., 2005). However, both enzymes are lacking in most hippocampal interneurons (McBain et al., 1999; Yang et al., 2005). In developing sensory cortices, CaMKII is largely lacking in most neocortical interneurons (McDonald et al., 2002). Although heavily labeled calcineurin neurons appeared in layer IV of the barrel cortex between 3 and 5 weeks of age (Goto et al., 1993), it is unclear which neuronal subtypes express CN. Interneurons and excitatory neurons also differ in intracellular calcium dynamics induced by excitatory synaptic inputs. In excitatory neurons, Ca2+ influx is compartmentalized in dendritic spines, which cortical interneurons lack. Different subtypes of cortical interneurons also vary in the Ca2+ signals they generate. Using two photon confocal imaging techniques, Goldberg and Yuste, have shown that fast-spiking basket interneurons are coincidence detectors: AMPA receptors generate fast Ca2+ microdomains in speed-optimized circuits, whereas dendrite-targeting interneurons may serve as burst detectors: active dendrites amplify local synaptic inputs and generate global Ca2+ signals (Goldberg and Yuste, 2005). Because local spikes and spatially control the expression of specific conductance underlies Hebbian plasticity, the differences in calcium dynamics indicate different mechanisms underlying activity-dependent plasticity in interneurons vs. pyramidal neurons.
4. What are the molecular and cellular mechanisms that regulate the maturation of inhibitory networks?
Patterned sensory inputs provide pathway-specific and glutamate receptor-dependent increases in intracellular calcium (Castro-Alamancos and Connors, 1996; Castro-Alamancos, 2004;Sur and Rubenstein, 2005), which in turn activate downstream signaling cascades that are important for the formation and stabilization of synapses. Neuronal activity induces gene transcription by modulating transcriptional activators and repressors (Greenberg et al., 1986). This type of regulation has been shown to be crucial for many different types of long-term neural plasticity (Nestler, 2001; Spitzer et al., 2000; West et al., 2002). A vast body of literature reveals the importance of NMDARs and mGluRs in the development of excitatory neural networks and their plasticity. Information regarding involvement of both NMDARs and mGluRs in experience-dependent plasticity of intracortical glutamatergic synapses on inhibitory interneurons is sparse. In most examples mentioned below, activity induces calcium influx, which in turn regulates gene transcription in GABAergic neurons. This type of regulation occurs during synaptogenesis throughout synaptic plasticity.
4.1). Brain-derived neurotrophic factor (BDNF) and maturation of specific GABAergic inhibitory networks
Brain-derived neurotrophic factor (BDNF) is essential for the differentiation of multiple interneuron subtypes and the formation of their synaptic contacts. The expression and release of BDNF correlates with the amount of excitatory neuronal activity (Lu, 2003), suggesting that it might act in a feedback dependent manner to maintain a balance between excitation and inhibition during development. A role of BNDF in modulating the maturation of the neocortical inhibitory network was described in visual cortex, where maturation is influenced by visual experience during an early postnatal period. In transgenic mice in which the postnatal rise of BDNF was accelerated, the maturation of GABAergic innervation and inhibition was also accelerated. These transgenic mice also showed a precocious development of visual acuity and an earlier termination of the critical period for ocular dominance plasticity. This study indicates that BDNF promotes the maturation of cortical inhibition during early postnatal life, thereby regulating the critical period for visual cortical plasticity (Huang et al., 1999). Using organotypic cortical slice cultures from neonatal mice and biolistically transfected with green fluorescent protein (GFP) driven by the GAD67 promoter, Jin et. al., further showed that BDNF, released by neocortical pyramidal neurons in response to depolarization, enhances dendritic growth and branching in nearby inhibitory interneurons (Jin et al., 2003). In another study, it was found that postsynaptic BDNF-TrkB signaling contributes to the target-selective potentiation of inhibitory presynaptic machinery. Since BDNF is expressed in an activity-dependent manner in vivo, this selectivity may be one of the key mechanisms by which the independence of functional neuronal circuits is maintained (Ohba et al., 2005). In addition, the activity-dependent scaling of inhibitory synaptic strength can be modulated by BDNF/TrkB-mediated signaling (Swanwick et al., 2006). In purified fast-spiking interneuronal culture preparations, BDNF promoted FS cell differentiation by increasing the somatic diameter, dendritic branching and the frequency of action potential firing. In addition, BDNF treatment led to a significant up-regulation of synaptophysin and vesicular GABA transporter expression, components of the synaptic machinery critical for GABA release, which was paralleled by an increase in synaptic strength (Berghuis et al., 2004).
4.2.) Ionotropic and metabotropic glutamate receptors and experience-dependent plasticity of interneuronal networks
N-methyl-D-aspartate receptors (NMDARs)
Local GABA circuits contribute to sensory experience-dependent refinement of neuronal connections in the developing nervous system; a few recent studies showed that GABAergic synapses themselves can be rapidly modified by sensory stimuli. Like experience-dependent plasticity in excitatory networks, NMDARs appear to play an important role in the plasticity of GABAergic synapses. However, the cellular mechanisms by which NMDARs regulate GABAergic synapses appear to differ from those observed in excitatory synapses, in that their actions take place in presynaptic terminals (Fiszman et al., 2005). In developing cerebellar cultures, NMDARs alter GABAergic synapses by increasing the size of the terminal and the spontaneous GABA release. These findings support recent results which show parallel changes in inhibitory synaptic efficacy in vivo in the molecular layer of the cerebellum (Fiszman et al., 2005). In the developing Xenopus retinotectal system, repetitive light stimuli or theta burst stimulation of the optic nerve induces LTP of glutamatergic inputs, but LTD of GABAergic inputs to the same tectal neuron. The LTD is due to a reduction in presynaptic GABA release and requires activation of presynaptic NMDARs and coincident, high-level GABAergic activity. Thus, the presynaptic NMDAR may function as a coincidence detector for adjacent glutamatergic and GABAergic activities, leading to coordinated synaptic modification by sensory experience (Lien et al., 2006).
Metabotropic glutamate receptors (mGluRs)
In a recent study (Liu et al., 1998), mGluR1a, mGluR5, and mGluR2/3 were found to be concentrated in layer IV of somatosensory cortex from its early differentiation and were densely expressed in the barrel hollows, peaking between P4 and P9, a time when intense NMDAR1 immunoreactivity was present in layer IV (Rema and Ebner, 1996). This finding suggests the involvement of mGluRs in the developmental plasticity of TC synapses during the establishment of the somatotopic whisker representational maps in SI (DeFelipe, 1997). In addition, an interaction between mGluRs and NMDARs has been demonstrated (Liu et al., 1998; Kotecha et al., 2003). A key component of this interaction may due to synergistic changes of intracellular calcium signaling. For example, mGluRs, via the phospholipase C-b1 (PLC-b1) signaling pathway, regulate intracellular calcium signaling pathways. Indeed, in both PLC-b1 and mGluR5 knockout mice, barrel formation in somatosensory cortex was disrupted (Spires et al., 2005). Furthermore, mGluR1&5 receptors were found in dendrites of neocortical and hippocampal interneurons (Muly et al., 2003), indicating a potential role in regulating excitation-inhibition matching.
4.3.) Gamma-aminobutyric acid (GABA) mediated self-regulation
Spontaneous Ca2+ transients expressed prior to synaptogenesis regulate the developmental appearance of GABA. In cultured Xenopus spinal neurons, GAD, the enzyme responsible for GABA synthesis, is regulated by a Ca2+-dependent process and parallels the appearance of GABA. GAD67 transcripts first appear in the embryonic spinal cord during the period in which these Ca2+ spikes are generated, in a pattern that is temporally and spatially appropriate to account for differentiation of GABAergic interneurons (Watt et al., 2000). In mature circuits, a role for GABA in mediating activity-dependent plasticity has also been implicated. In hippocampal cultures and acute hippocampal slices, coincident pre- and postsynaptic activation of the GABAergic interneurons led to a persistent change in inhibitory synaptic strength (Woodin et al., 2003; Fiumelli et al., 2005). Is it possible that these mechanisms regulate excitation and inhibition matching in the sensory cortex in vivo? During sensory processing, FS interneurons, which are involved in sensory feed-forward inhibition, generate reliable and robust sensory-mediated action potentials and robust feed-forward inhibitory synaptic potentials that regulate firing of spiny neurons (Sun et al., 2006). Evidence linking spike timing of interneuron a to interneuron b is rare during sensory processing (except when a & b are connected via gap junctions, Fig. 1). In addition, early sensory deprivation and persistent sensory deprivation appears to have opposite effects on the strength of GABAergic transmission (Morales et al., 2002; Sun et al., 2006; Turrigiano and Nelson, 2004). Very interestingly, in a recent study, it was shown that GABAergic synaptic strength can be regulated bi-directionally. In the subthalamic nucleus (STN), rebound burst firing of STN neurons induces long-lasting bidirectional modifications of GABAergic synaptic transmission in STN neurons. The potentiation or depression of IPSPs was associated with a negative or positive shift in the reversal potential of IPSPs (Wang et al., 2006). In all these above mentioned examples, the modification required Ca2+ influx through postsynaptic L-type Ca2+ channels and was due to a local decrease in K+-Cl− co-transport activities (Woodin et al., 2003; Fiumelli et al., 2005; Wang et al., 2006). GABA also plays an essential role for synaptic integration of newly generated excitatory neurons in the adult brain, and for activity-dependent regulation of adult neurogenesis. In hippocampal dentate gyrus, newborn granule cells of the adult hippocampus are tonically activated by ambient GABA before being sequentially innervated by GABA - and glutamate-mediated synaptic inputs. This effect appears to be related to the excitatory action of GABA (Ge et al., 2006). In adult neocortex, neurogenesis occurs in GABAergic interneurons as well (Dayer et al., 2005), however, it is unclear whether GABA plays a similar role.
4.4.) Effects of sensory experience on activity-dependent gene regulation: gene profiling studies
Recently, using microarray analysis combined with other molecular and immunohistochemical methods, a pool of signaling genes, whose expression is regulated by visual deprivation (produced by monocular enucleation, ME) in visual cortex, were identified (Majdan and Shatz, 2006;Tropea et al., 2006). In one recent study, Majdan and Shatz showed that there are two pool of genes, a common sets of genes (~10 genes) and an age-related gene pools (about 50 genes), that are regulated differently by sensory deprivation (dark rearing). The common gene set defines a MAP kinase signaling pathway, and are regulated by vision at all ages studied. Dark rearing does not perturb the regulation of this common gene set, but instead profoundly changes the regulation of the age-specific gene sets. Thus, critical period formation and experience-dependent plasticity appear to be regulated by common genes as well as age-related genes (Majdan and Shatz, 2006). Among the common sets of target genes, MAP kinase signaling pathways have been implicated in experience-dependent, independent and homeostatic plasticity of inhibitory networks. The roles of other genes that are regulated only at specific ages in regulating inhibitory networks are largely unknown. Among these genes, connexin 43, annexin XI, regulator of G-protein signaling gene 4 (RGS4), Rho-associated, coiled-coil forming protein kinase p160 (Rock-2) are known to be involved in regulating gap junction coupling, dendritic morphology and the synaptic properties of inhibitory interneurons. In another related study (Tropea et al., 2006) GABAA receptor subunits α2 & 3, β1 & 3 genes were found to be up-regulated by monocular derivation or dark rearing. Other genes, such as GAD67 and parvalbumin, which have been implicated in experience-dependent plasticity, and all have been found to be affected by visual experiences (Tropea et al., 2006). Further experiments examining the physiological consequences of altered gene expression will certainly help to clarify mechanisms underlying the experience-dependent plasticity.
5. What are the functional consequences to a neural network?
There are different types of interneuerons whose laminar location, synaptic targets, firing properties and functions are extremely diverse (Kawaguchi and Kondo, 2002; Somogyi et al., 1998; Staiger et al., 2000;Thomson et al., 2002; Staiger et al. 1996). Neocortical local circuits can also be further divided into interlaminar and intralaminar connections (Thomson et al., 2002). Next the focus will center on inhibitory networks involved in mediating feed-forward inhibition in the sensory cortex.
5.1.) Feed-forward inhibition and receptive field
Sensory-mediated intracortical inhibition plays a role in the shaping of sensory cortical receptive fields (Nelson, 1991; Vidyasagar et al., 1996; Swadlow, 2002 & 2003). A powerful feed-forward inhibitory mechanism could serve to constrain the size of supra-threshold receptive fields and to modify the temporal response property of targeted cortical neurons (e.g. Fig 1). In the somatosensory cortex, putative inhibitory interneurons with sensitive and broadly tuned feed-forward inhibitory properties have been described in the rabbit and rat (Swadlow, 2002 & 2003; Simons, 1978).
Rodent whisker sensory input is represented somatopically in the barrel field of layer IV of S1 neocortex (Woolsey and Van der, 1970). A cohort of morphologically distinct excitatory neurons and inhibitory interneurons has been described in layer IV barrels (Keller and White, 1987; Woolsey and Van der, 1970). The vast majority (70–90%) of neurons within layer IV of a barrel forms a reciprocally connected excitatory network, and are the major targets for TC inputs (Egger et al., 1999; Feldmeyer et al., 1999; Feldmeyer et al., 2002; Petersen and Sakmann, 2000). By contrast, interneurons only represent about 10 % to 30% of the total number of neurons within the barrel cortex (Simons, 1978; Keller and White, 1987; Micheva and Beaulieu, 1995). In order for precise registration of sensory information to take place without runaway recurrent excitation in the population, excitation and inhibition must be delicately balanced (e.g. Chagnac-Amitai and Connors, 1989). How does the inhibition supplied by a limited number of interneurons provide the necessary inhibitory control of activities within an excitatory network? The ratio of excitatory and inhibitory synapses and their distribution are, at least as important as the numbers of involved cells, e.g., single chandelier cells likely control the firing of many pyramidal neurons. This dilemma can only be resolved through the quantitative analysis of the properties of unitary inhibitory and excitatory synaptic events in layer 4. In a recent study, we have shown that there is a striking contrast between the strength of unitary GABAergic inhibitory and glutamatergic excitatory synaptic events (Sun et al., 2006, Fig. 3). The average conductance of uIPSCs from individual FS interneurons is about 10 fold greater than that of uEPSCs in spiny neurons. This difference, together with rapid feed-forward inhibition, serves to counteract the convergent cortical excitation from spiny neurons (Sun et al., 2006, Fig. 2 & 3). Cross-correlation analysis of TC-evoked polysynaptic responses from pairs of unconnected spiny neurons located in the same barrel suggests that these cells receive inhibition from a common group of interneurons. FS interneurons are likely to be the major source of local feed-forward inhibition, since networks of electrically coupled FS cells produce highly synchronized activities (Amitai et al., 2002; Beierlein et al., 2003). This could have profound influences on the generation of thalamocortical mediated feed-forward inhibition. Essentially, the electrical coupling allows the firing FS neurons to be “phase locked” to their electrically coupled partners (Beierlein et al., 2000; Gibson et al., 1999), and thus contribute to the synchronization of FS spikes. In addition, the electrical coupling might increase the probability of firing in FS networks because a supra-threshold EPSP in one FS interneuron might increase the firing probability of its electrically coupled partners which have TC inputs slightly below the threshold. Feed-forward inhibition, provided by the FS interneurons, limits the TC-mediated excitation of spiny neurons and reduces the likelihood that disynaptic reciprocal excitation will occur, particularly when TC input is weak (Swadlow, 2002 & 2003; Sun et al., 2006, Fig. 3). Furthermore, FS neurons and spiny neurons could have different receptive field properties (Swadlow, 2002 & 2003). Our results show that FS cell activation can result in selective inhibition of spiking in spiny neurons located in the same barrel and in adjacent barrels (Sun et al., 2006). This conclusion supports the idea that FS neurons are involved in modifying receptive field properties in barrel cortices. In the auditory cortex, one of the roles of cortical inhibition in sound processing is to increase the temporal precision. This is achieved via feed-forward inhibition that occurs immediately following pyramidal neuron APs (Wehr and Zador, 2003). However, there are no differences in receptive fields between excitatory neurons and inhibitory neurons in auditory cortex. This finding is different from somatosensory cortex, where feed-forward inhibition controls both the temporal precision and, likely, the receptive field (Bruno and Simons, 2002). In the visual cortex, a sensitive and broadly tuned feed-forward inhibition could account for the contrast-invariant orientation tuning seen in feline visual cortex. However, the existence of such interneurons in cat visual cortex is uncertain (Hirsch and Martinez, 2006).
Figure 3. Reciprocal synaptic connection between spiny stellate cells and basket cells.
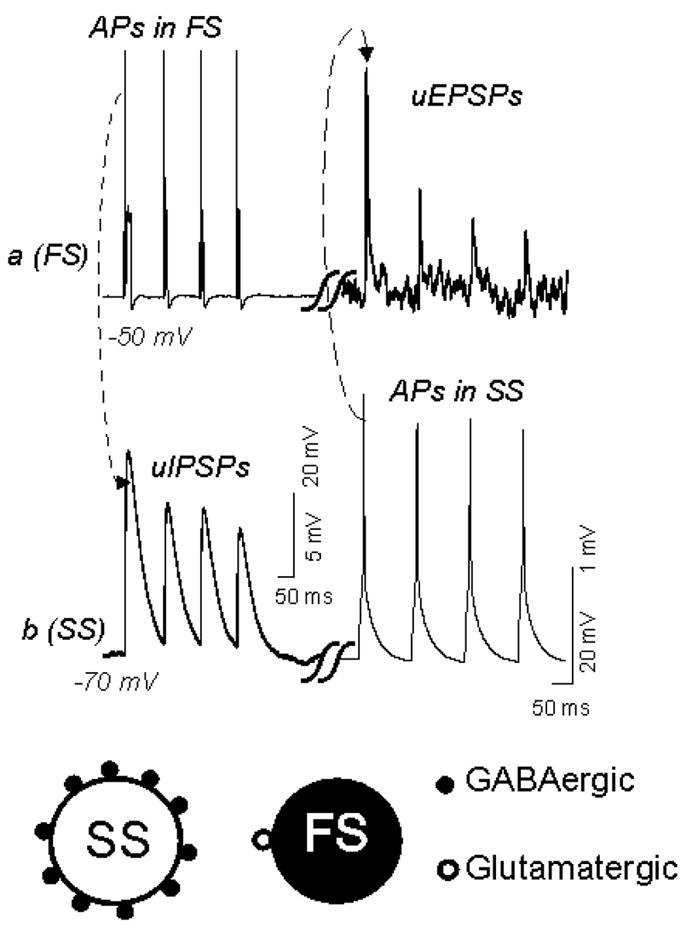
Top: Paired recordings from a reciprocally connected fast-spiking cell (a) and spiny stellate cell (b) show unitary synaptic potentials elicited by trains (B1) or single action potentials (B2) in the presynaptic cell. B1, Trains of action potentials (APs) elicited by depolarizing currents (250 pA, 100 ms) in cell a (top left) evoked unitary (u) IPSPs in cell b (bottom left, dotted line and arrow), while action potentials in cell b (bottom right, dashed line and arrow) elicited uEPSPs in cell a (top, left). Note that the amplitude of uIPSPs is >10 times larger than the uEPSPs. uIPSPs were recorded as outward currents due to high intracellular pipette Cl− content. Bottom: note that inhibitory synaptic boutons (from a single basket cell) in a spiny neuron out-numbers glutamatergic boutons from single spiny neurons in the basket cell. This figure was modified from Sun et al, 2006.
5.2.) Importance of inhibitory networks in experience-dependent refinement of sensory maps
Inhibition may play an important role in activity-dependent plasticity which underlies some of the most fundamental aspects of circuit maturation, such as sensory mediated refinement of receptive fields (Egger et al., 1999; Froemke and Dan, 2002; Feldman et al., 1999; Nelson et al., 2002). Experience-dependent synaptic plasticity in the sensory cortex requires precision in spike- timing of the postsynaptic excitatory cortical neurons. The role of inhibition in experience-dependent plasticity becomes clearer with two recent studies carried out in the auditory cortex and visual cortex. In the first series of studies, the influence of GABA-mediated inhibition on adaptive adjustment of the owl’s auditory space map during the initial phase of plasticity was studied. Zhang and Knudsen have found that the pattern of feed-forward inhibition is less dynamic than the pattern of feed-forward excitation at the site of map plasticity (Zheng and Knudsen, 1999; Zheng and Knudsen, 2001). In a second sets of experiments, the intracortical inhibitory influences upon developing visual afferents are further examined by altering intrinsic GABA-mediated inhibition with benzodiazepines in the visual cortex. Local enhancement by agonist (diazepam) infusion did not perturb visual responsiveness, but did widen column spacing. An inverse agonist (DMCM) produced the opposite effect. Thus, intracortical inhibitory circuits shape the geometry of incoming thalamic arbors, suggesting that cortical columnar architecture depends on neuronal activity (Hensch et al., 1998; Hensch and Stryker, 2004). Similar roles of inhibition in the somatosensory cortex have also been implicated (Foeller et al., 2005). These results suggest that intracortical inhibition, presumably via modulating spike patterns of spiny neurons, regulate the experience-dependent plasticity and columnar organization (cf. Fig. 1). Interestingly, in a rat model of neocortical dysplasia, LTP is impaired, presumably due to diminished GABAergic inhibitory connections in affected areas (Peters et al., 2004). In animal models of epilepsy and patients with developmental epilepsy (van Rijckevorsel, 2006), where the balance of inhibition and excitation was disturbed, the capacity for forming Hebbian-forms of plasticity (in animals) and learning (in humans) is severally undermined (von der et al., 2006). Mental retardation, and learning difficulties is also common problems in several neurological disorders (such as autism and fragile-X syndrome), and were accompanied by a perturbation of inhibition and excitation (Dong and Greenough, 2004).
Homeostatic synaptic plasticity also regulates excitation and inhibition separately within recurrent cortical networks to preserve balanced function during constant activity-dependent changes in synaptic drive (Marder and Goaillard, 2006; Turrigiano and Nelson, 2004). In sensory deprived cortices, lateral excitation is enhanced whereas feed-forward and feed-back inhibition are reduced, leading to enhanced Hebbian plasticity and a strengthening of synaptic connections between deprived and spared cortex. In cultured cortical and hippocampal networks, activity blockade reversibly decreases perisomatic inhibition and increases the quantal distribution of EPSCs in pyramidal neurons (Rutherford et al., 1997). Thus, it appears that inhibition and excitation onto pyramidal neurons are regulated in opposite directions by activity blockade.
6. Concluding remarks
Experience and the resulting changes in neuronal activity shape the nervous system and its function. Activity-dependent changes in neuronal function are essential for the survival of the animal and normal brain function. The impact of restricting neuronal activity through sensory neglect is evident in the human population. Each year in the United States alone, over 500,000 children suffer from “neglect” and these children have a much higher probability of emotional, behavioral, cognitive, and physical delays than normal children. As such, research aimed at identifying mechanisms underlying activity-induced plasticity of brain circuits and brain function has immediate health relevance. It is becoming clearer that GABAergic inhibitory circuit plays a very important role in sensory-dependent refinement of functional neocortical circuits. Future experiments aimed at unraveling the mechanisms underlying activity-dependent plasticity of inhibitory circuits will help to further develop the concept that early experiences shape the structure of neocortical excitatory networks, and they also fine tune the inhibitory networks to maintain a balance between excitation and inhibition. These types of experiments will also help to determine the correlation between patterns of synaptic maturation and the occurrence of critical periods for forming functional sensory inhibitory structure. In this vein, several priorities of future experiments should be addressed: 1) the mechanisms involved in the activity-dependent maturation of brain inhibitory circuits in vivo; 2) roles and mechanisms of GABAergic activity in regulating the presynaptic and postsynaptic properties of GABAergic synaptic connections; 3) roles of sensory experiences in regulating GABA-mediated self-regulation; 4) roles and mechanisms of glutamatergic synaptic transmission in regulating the presynaptic and postsynaptic properties of GABAergic synaptic connections and their modulation by sensory activity; 5) experience-dependent regulation of dendritic-targeting interneurons and interlaminar or intercolumar inhibitory connections; 6) Homeostatic synaptic plasticity of GABAergic synapses and its regulation by sensory experiences in different sensory structures. Thoroughly understanding inhibition-excitation matching will bridge the gap between the experience–dependent plasticity of synapses and the maturation of functionally relevant neocortical circuits. It will also help us to gain further insights into the mechanisms underlying several developmentally related neurological disorders such as cortical dysplasia, schizophrenia, epilepsy and dyslexia, in which early unfavorable endogenous and exogenous conditions create a long-lasting impact on the mature cortex.
Acknowledgments
Research conducted in Sun lab is supported by National Institutes of Health Grants P20 RR15640 from NCRR and 5R01NS057415-02 from NINDS. I thank Mr. Andrew Young for editing the manuscript.
References
- Alonso JM, Swadlow HA. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. J Neurophysiol. 2005;94:26–32. doi: 10.1152/jn.01281.2004. [DOI] [PubMed] [Google Scholar]
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Press WA, Connors BW. Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol. 1992;67:841–851. doi: 10.1152/jn.1992.67.4.841. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Sousa KM, Schulte G, Mager PP, Hartig W, Gorcs TJ, Zilberter Y, Ernfors P, Harkany T. Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking GABAergic interneurons. Eur J Neurosci. 2004;20:1290–1306. doi: 10.1111/j.1460-9568.2004.03561.x. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci U S A. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. The genetic enhancement of memory. Cell Mol Life Sci. 2003;60:1–5. doi: 10.1007/s000180300000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Nicola SM, Malenka RC. Induction in the rat hippocampus of long-term potentiation (LTP) and long-term depression (LTD) in the presence of a nitric oxide synthase inhibitor. Neurosci Lett. 1994;176:110–114. doi: 10.1016/0304-3940(94)90883-4. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Ment Retard Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Inhibition and plasticity. Nat Neurosci. 2000;3:303–304. doi: 10.1038/73849. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Knudsen EI. Experience-dependent plasticity and the maturation of glutamatergic synapses. Neuron. 1998;20:1067–1071. doi: 10.1016/s0896-6273(00)80488-2. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J Physiol. 1999;521 Pt 1:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Neuroscience. Blocking plasticity in the visual cortex. Science. 2004;303:1619–1621. doi: 10.1126/science.1096224. [DOI] [PubMed] [Google Scholar]
- Fiszman ML, Barberis A, Lu C, Fu Z, Erdelyi F, Szabo G, Vicini S. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumelli H, Cancedda L, Poo MM. Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron. 2005;48:773–786. doi: 10.1016/j.neuron.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Foeller E, Celikel T, Feldman DE. Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J Neurophysiol. 2005;94:4387–4400. doi: 10.1152/jn.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R. Space matters: local and global dendritic Ca2+ compartmentalization in cortical interneurons. Trends Neurosci. 2005;28:158–167. doi: 10.1016/j.tins.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Goto S, Nagahiro S, Korematsu K, Ushio Y, Fukunaga K, Miyamoto E, Hofer W. Cellular colocalization of calcium/calmodulin-dependent protein kinase II and calcineurin in the rat cerebral cortex and hippocampus. Neurosci Lett. 1993;149:189–192. doi: 10.1016/0304-3940(93)90768-g. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The mammal and his environment. Am J Psychiatry. 1955;111:826–831. doi: 10.1176/ajp.111.11.826. [DOI] [PubMed] [Google Scholar]
- Heinen K, Bosman LW, Spijker S, van Pelt J, Smit AB, Voorn P, Baker RE, Brussaard AB. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 2004;303:1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Martinez LM. Circuits that build visual cortical receptive fields. Trends Neurosci. 2006;29:30–39. doi: 10.1016/j.tins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Hu H, Mathers PH, Agmon A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Keller A, White EL. Synaptic organization of GABAergic neurons in the mouse SmI cortex. J Comp Neurol. 1987;262:1–12. doi: 10.1002/cne.902620102. [DOI] [PubMed] [Google Scholar]
- Kelly MK, Carvell GE, Kodger JM, Simons DJ. Sensory loss by selected whisker removal produces immediate disinhibition in the somatosensory cortex of behaving rats. J Neurosci. 1999;19:9117–9125. doi: 10.1523/JNEUROSCI.19-20-09117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Land PW, De Blas AL, Reddy N. Immunocytochemical localization of GABAA receptors in rat somatosensory cortex and effects of tactile deprivation. Somatosens Mot Res. 1995;12:127–141. doi: 10.3109/08990229509101504. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Ho-Pao CL, Sur M. Role of calcineurin in activity-dependent pattern formation in the dorsal lateral geniculate nucleus of the ferret. J Neurobiol. 2003;56:153–162. doi: 10.1002/neu.10226. [DOI] [PubMed] [Google Scholar]
- Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- Liu XB, Munoz A, Jones EG. Changes in subcellular localization of metabotropic glutamate receptor subtypes during postnatal development of mouse thalamus. J Comp Neurol. 1998;395:450–465. doi: 10.1002/(sici)1096-9861(19980615)395:4<450::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Maccaferri G. Synaptic plasticity in hippocampal interneurons? A commentary. Can J Physiol Pharmacol. 1997;75:488–494. [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Sameshima K. Cortical plasticity and memory. Curr Opin Neurobiol. 1993;3:187–196. doi: 10.1016/0959-4388(93)90209-h. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Postnatal development of GABA neurons in the rat somatosensory barrel cortex: a quantitative study. Eur J Neurosci. 1995;7:419–430. doi: 10.1111/j.1460-9568.1995.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD, Guo Y. Comparison of the expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) in the visual cortex of normal and dark-reared cats. Brain Res Dev Brain Res. 2001;126:65–74. doi: 10.1016/s0165-3806(00)00139-5. [DOI] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Nayak AS, Moore CI, Browning MD. Ca2+/calmodulin-dependent protein kinase II phosphorylation of the presynaptic protein synapsin I is persistently increased during long-term potentiation. Proc Natl Acad Sci U S A. 1996;93:15451–15456. doi: 10.1073/pnas.93.26.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB. Temporal Interactions in the Cat Visual-System.3. Pharmacological Studies of Cortical Suppression Suggest A Presynaptic Mechanism. Journal of Neuroscience. 1991;11:369–380. doi: 10.1523/JNEUROSCI.11-02-00369.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Sjostrom PJ, Turrigiano GG. Rate and timing in cortical synaptic plasticity. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2002;357:1851–1857. doi: 10.1098/rstb.2002.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Neville H, Bavelier D. Human brain plasticity: evidence from sensory deprivation and altered language experience. Prog Brain Res. 2002;138:177–188. doi: 10.1016/S0079-6123(02)38078-6. [DOI] [PubMed] [Google Scholar]
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex. 2005;15:291–298. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Lacaille JC. Mechanisms of selective long-term potentiation of excitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. J Neurophysiol. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu XL, Kriegstein AR. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Peters O, Redecker C, Hagemann G, Bruehl C, Luhmann HJ, Witte OW. Impaired synaptic plasticity in the surround of perinatally acquired [correction of aquired] dysplasia in rat cerebral cortex. Cereb Cortex. 2004;14:1081–1087. doi: 10.1093/cercor/bhh067. [DOI] [PubMed] [Google Scholar]
- Petersen CCH, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. Journal of Neuroscience. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Postnatal changes in NMDAR1 subunit expression in the rat trigeminal pathway to barrel field cortex. J Comp Neurol. 1996;368:165–184. doi: 10.1002/(SICI)1096-9861(19960429)368:2<165::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoykhet M, Land PW, Simons DJ. Whisker trimming begun at birth or on postnatal day 12 affects excitatory and inhibitory receptive fields of layer IV barrel neurons. J Neurophysiol. 2005;94:3987–3995. doi: 10.1152/jn.00569.2005. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978a;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Spires TL, Molnar Z, Kind PC, Cordery PM, Upton AL, Blakemore C, Hannan AJ. Activity-dependent regulation of synapse and dendritic spine morphology in developing barrel cortex requires phospholipase C-beta1 signalling. Cereb Cortex. 2005;15:385–393. doi: 10.1093/cercor/bhh141. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. Coding of neuronal differentiation by calcium transients. Bioessays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Kotter R, Zilles K, Luhmann HJ. Laminar characteristics of functional connectivity in rat barrel cortex revealed by stimulation with caged-glutamate. Neurosci Res. 2000;37:49–58. doi: 10.1016/s0168-0102(00)00094-8. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci U S A. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Sherk H, Leventhal AG, Hirsch HV. Physiological consequences for the cat’s visual cortex of effectively restricting early visual experience with oriented contours. J Neurophysiol. 1978;41:896–909. doi: 10.1152/jn.1978.41.4.896. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1717–1727. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol Cell Neurosci. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Rijckevorsel K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure. 2006;15:227–234. doi: 10.1016/j.seizure.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Pei X, Volgushev M. Multiple mechanisms underlying the orientation selectivity of visual cortical neurones. Trends in Neurosciences. 1996;19:272–277. doi: 10.1016/S0166-2236(96)20027-X. [DOI] [PubMed] [Google Scholar]
- von der BC, Waltereit R, Zhang L, Beck H, Kirschstein T. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. doi: 10.1111/j.1460-9568.2006.04594.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Kitai ST, Xiang Z. Activity-dependent bidirectional modification of inhibitory synaptic transmission in rat subthalamic neurons. J Neurosci. 2006;26:7321–7327. doi: 10.1523/JNEUROSCI.4656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SD, Gu X, Smith RD, Spitzer NC. Specific frequencies of spontaneous Ca2+ transients upregulate GAD 67 transcripts in embryonic spinal neurons. Mol Cell Neurosci. 2000;16:376–387. doi: 10.1006/mcne.2000.0871. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl-transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der LH. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fischer QS, Zhang Y, Baumgartel K, Mansuy IM, Daw NW. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nat Neurosci. 2005;8:791–796. doi: 10.1038/nn1464. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Gabaergic inhibition antagonizes adaptive adjustment of the owl’s auditory space map during the initial phase of plasticity. J Neurosci. 2001;21:4356–4365. doi: 10.1523/JNEUROSCI.21-12-04356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]


