Abstract
Heterotopic cardiac xenotransplantation from α1,3-galactosyltransferase gene-knockout (GalT-KO) swine to baboons was performed to characterize immunological reaction to the xenograft in the absence of anti-Gal antibody-mediated rejection. Eight baboons received heterotopic cardiac xenografts from GalT-KO porcine donors. All baboons were treated with chronic immunosuppressive therapy. Both histological and immunohistochemical studies were performed on biopsy and graftectomy samples. No hyperacute rejection was observed. Three baboons were euthanized or died 16 to 56 days after transplantation. The other five grafts ceased beating between days 59 and 179 (median, 78 days). All failing grafts exhibited thrombotic microangiopathy (TM) with platelet-rich fibrin thrombi in the microvasculature, myocardial ischemia and necrosis, and focal interstitial hemorrhage. TM developed in parallel with increases in immunoglobulin (IgM and IgG) and complement (C3, C4d, and C5b-9) deposition, as well as with subsequent increases in both TUNEL+ endothelial cell death and procoagulant activation (increased expression of both tissue factor and von Willebrand factor and decreased expression of CD39). CD3+ T-cell infiltration occurred in all grafts and weakly correlated with the development of TM. In conclusion, although the use of GalT-KO swine donors prevented hyperacute rejection and prolonged graft survival, slowly progressive humoral rejection—probably associated with non-Gal antibodies to the xenograft—and disordered thromboregulation represent major immunological barriers to long-term xenograft survival.
Xenotransplantation with pig donor organs offers a potential solution to the critical shortage of organs available for clinical transplantation.1,2,3,4 Vascularized cardiac grafts transplanted into primates undergo hyperacute rejection (HAR) and subsequently acute humoral xenograft rejection (AHXR), also known as acute vascular rejection or delayed xenograft rejection.1,2,3,4,5 Both HAR and AHXR are triggered by the binding of xenoreactive natural antibodies to a specific epitope (galactose α1-3 galactose: Gal) on porcine vascular endothelium.6,7 To date, considerable effort has been applied to the depletion of anti-Gal antibodies and the inhibition of complement in pig-to-nonhuman primate models.7,8,9,10,11,12 These approaches include the depletion of antibody using extracorporeal or intravascular immunoadsorption of anti-Gal antibodies, and the inhibition of complement either systemically or through the use of transgenic pig organs that express one or more human complement-regulatory proteins, such as decay-accelerating factor (hDAF). However, despite the successful prevention of HAR by these methods, with the return or continuing presence of anti-Gal antibodies, all swine xenografts undergo AHXR, resulting in graft failure within a few weeks.9,10,11,12
To overcome this problem, α1,3-galactosyltransferase gene-knockout (GalT-KO) pigs have recently been produced that do not express the Gal epitope.13,14 We have reported elsewhere our initial trial of cardiac transplantation from GalT-KO miniature swine to baboons with chronic immunosuppression.15,16 None of the grafts succumbed to HAR and graft survival was consistently improved over previous results that were obtained using miniature swine or hDAF transgenic pig donors.10,17,18 However, all GalT-KO heart grafts underwent graft failure within 6 months of transplantation. This study is the first detailed pathology report of the GalT-KO cardiac xenografts transplanted into baboons; it characterizes the immunological and vascular reaction in the grafts in the predicted absence of anti-Gal natural antibody-mediated rejection.
Materials and Methods
Animals
Baboons (Papio hamadryas, n = 8; Mannheimer Foundation, Homestead, FL) of body weight 9 to 22 kg were used as recipients. GalT-KO miniature swine (n = 8) of body weight 9 to 27 kg served as sources of hearts. All pigs were bred by nuclear transfer from modified fibroblasts from Massachusetts General Hospital major histocompatibility complex (MHC)-inbred miniature swine.14 All animal care procedures were performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (publication no. 86-23, revised 1996). Protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Transplantation and Immunosuppression
The surgical procedures associated with heterotopic heart transplantation in baboons, and the immunosuppressive treatment, supportive therapy, and monitoring of recipient baboons have been previously described in detail.15,16 The chronic immunosuppressive regimen for these baboons included an induction treatment of horse anti-human thymocyte globulin (ATGAM; Upjohn, Kalamazoo, MI) 50 mg/kg/day i.v. on days −3, −2, and −1. Thymic irradiation (700 cGy) was given on day −1 except in one baboon (B228). Complement was depleted in five of eight baboons by cobra venom factor for either 4 days (B226, B228, B229) or 14 days (B214, B216). Maintenance therapy consisted of a human anti-human CD154 monoclonal antibody (mAb) (ABI793; Novartis Pharma AG, Basel, Switzerland) administered intravenously at 25 mg/kg on days −1, 0, 1, 4, 7, 10, and 14, followed by 20 or 25 mg/kg every 5 days thereafter, mycophenolate mofetil that was administered by continuous intravenous infusion from day −2 to maintain a whole blood level of 3 to 5 μg/ml, and methylprednisolone that was given from day 0 (2 mg/kg × 2 i.v. daily for 7 days, followed by tapering to 0.5 mg/kg i.v. daily throughout the next 35 days). Heparin (3 to 60 U/kg/hour), recombinant human antithrombin (750 U/kg/day, generously provided by GTC Biotherapeutics, Framingham, MA), and/or aspirin (40 mg p.o. on alternate days) were administered as anticoagulant therapy. Graft function was monitored by measuring graft palpation scores (grade 3 representing excellent graft beat and grade 0 representing cessation of contractions) and serum troponin T levels.15,16
Histological and Immunohistochemical Examination
Heart graft samples were taken from open needle biopsies on various days after transplantation and from graftectomies. For light microscopic examination, tissue was fixed in 10% buffered formalin and embedded in paraffin. Sections were examined after hematoxylin and eosin (H&E) and elastica-Masson Goldner staining. Tissues for electron microscopy were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde, postfixed with 1% osmium tetroxide, and embedded in Epon 812. Ultrathin sections were stained with lead citrate. Frozen tissue sections were stained by the direct immunofluorescent technique, using fluorescein isothiocyanate (FITC)-conjugated rabbit polyclonal antibody to human IgG, IgM, C3, and fibrinogen (all from DAKO, Carpinteria, CA); and the indirect immunofluorescent technique, using anti-human C4d mAb (Quidel, San Diego, CA), polyclonal rabbit anti-human C4d antibody (American Research Products, Inc., Belmont, MA) and anti-human C5b-9 mAb (DAKO). The following primary antibodies were stained by the standard avidin-biotin-peroxidase complex (ABC) technique19: 1) anti-swine CD31 (PCAM1) mAb (Serotec, Raleigh, NC) and anti-MHC class II mAb (ISCR3)20 that detect capillary endothelium in swine grafts; 2) polyclonal rabbit anti-tissue factor (TF) antibody (the cross-reactive anti-porcine TF antibody was kindly provided by Prof. Yale Nemerson, Mount Sinai School of Medicine, New York, NY),21,22 which detects TF on porcine activated endothelial cells; 3) anti-pig CD39 mAb,23 which detects NTP diphosphohydrolase on porcine endothelial cells; 4) anti-human CD41 mAb (5B12, DAKO), which detects baboon platelets; 5) polyclonal rabbit anti-human von Willebrand factor (vWF, DAKO), which detects vWF in endothelial cells and thrombi; and 6) anti-proliferating cell nuclear antigen (PCNA) mAb (PC10, DAKO), which detects proliferating cells. To detect platelet-fibrin thrombi in xenografts, two-color immunohistochemistry for CD41 (Texas Red) and fibrinogen (FITC) was performed. The relationship between CD41+ platelet aggregation and the deposition of immunoglobulin or complement was assessed using two-color immunohistochemistry for CD41 (Texas Red) and IgM (FITC) or C4d (FITC). In histological sections, fragmented nuclear DNA associated with apoptosis was labeled by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) method.24
Quantification of Histological Findings
In each graft sample, randomly selected fields were assessed without prior knowledge of the clinical or histological findings. The number of CD3+ cells and TUNEL+ cells in capillaries was counted in 40 randomly selected fields (at ×400, using an optical grid area of 0.0625 mm2), and the mean number of cells per single field was calculated. The quantitative evaluation of CD41, IgM, IgG, C3, C4d, C5b-9, TF, or CD39 was performed using a computer-assisted image analysis system based on an Olympus (Tokyo, Japan) BX50 microscope connected via video camera to a PC. Data were analyzed using the WinROOF image processing software (Mitani Corp., Tokyo, Japan). At least 20 digitized images of cardiac parenchyma at ×200 magnification (0.569 mm2) were evaluated for each sample, and the percentage area of positive staining per field was evaluated. CD41+ platelet-rich thrombi formation in small and large pericardial arteries was also examined in all arterial cross-sections in all fields of the grafts and the percentage of arteries affected was evaluated. Correlations were computed and analyzed using Pearson’s test.
Results
Clinical Course and Graft Survival
Eight heterotopic heart transplantations were performed with a chronic immunosuppressive regimen using baboon recipients and GalT-KO pigs as donors.15,16 No HAR occurred. Three baboons were euthanized or died between postoperative days (PODs) 16 and 56 with beating heart grafts (Table 1). The remaining five grafts ceased beating between PODs 59 and 179. The median graft survival for these five baboons was 78 days.
Table 1.
Graft Survival and the Day of Biopsy and Graftectomy
| Baboon (ID) | Graft survival (POD)* | Cause of graft loss and last palpation score† | POD of biopsy or graftectomy, stage 1 to 3 (S1 to S3),‡ and troponin T levels (ng/ml)§ | |||||
|---|---|---|---|---|---|---|---|---|
| B226 | >16 | Euthanized¶ | (PS 3.0) | 0 (S1) | 16 (S2) (0.19) | |||
| B225 | >23 | Euthanized∥ | (PS 3.0) | 13 (S2) (0.21) | 23 (S2) (1.88) | |||
| B216 | >56 | Died** | (PS 2.0) | 27 (S1) (0.01) | 56 (S2) (0.14) | |||
| B214 | 59 | Graft failure | (PS 1.0) | 59 (S3) (0.76) | ||||
| B218 | 67 | Graft failure | (PS 0) | 67 (S3) (1.08) | ||||
| B229 | 78 | Graft failure | (PS 0) | 27 (S1) (0.01) | 50 (S2) (0.66) | 78 (S3) (3.34) | ||
| B223 | 110 | Graft failure | (PS 1.0) | 0 (S1) | 7 (S2) (0.13) | 69 (S2) (0.27) | 110 (S3) (0.23) | |
| B228 | 179 | Graft failure | (PS 1.5) | 27 (S1) (0.01) | 62 (S1) (0.01) | 95 (S1) (0.01) | 127 (S1) (0.01) | 179 (S3) (0.02) |
POD, postoperative day.
Palpation score (PS, 0 to 3): contraction of grafts were monitored as score 0 to 3.
Stage 1 to 3 (S1 to S3): the results were divided into three stages (see Results).
Troponin T levels (ng/ml): serum troponin T levels at the time of biopsy or graftectomy.
B226 was euthanized because of development of anemia (hematocrit, <15%).
B225 was euthanized because of development of an ischemic leg.
B216 died from a spontaneous abdominal bleeding.
Thrombotic Microangiopathy (TM) in Development of Graft Failure in GalT-KO Heart Grafts
For analysis, histology samples were divided into three stages according to the serum troponin T levels and the palpation score (Table 1); stage 1 (n = 8), grafts were stable and troponin T levels were normal; stage 2 (n = 7), grafts were beginning to fail and troponin T levels were rising; stage 3 (n = 5), grafts had failed completely and beating had either substantially weakened or stopped.
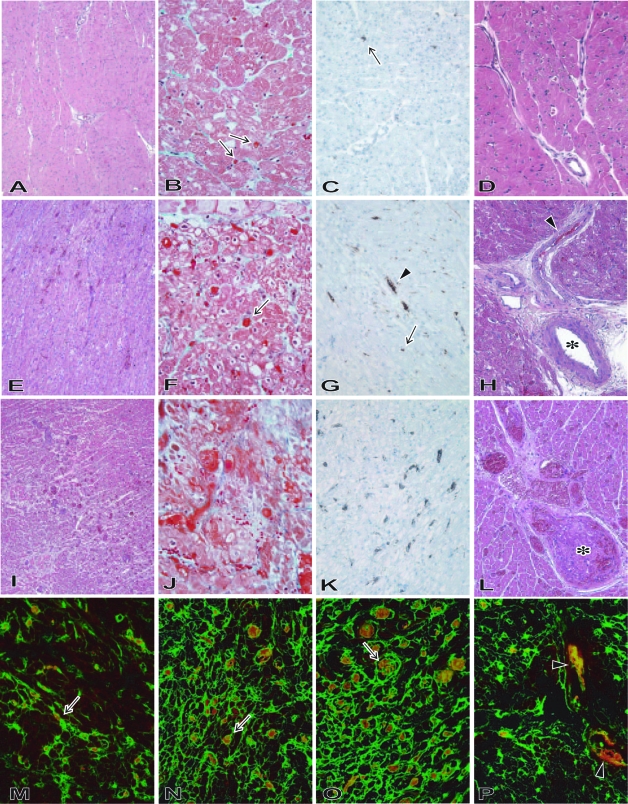
During the progression of graft failure from stage 1 to stage 3, TM developed in the grafts (Figure 1, A–L). Multiple microthrombi in vessels were characterized by platelet-fibrin thrombi (Figure 1, M–P). In addition, multiple thrombi involved various sized vessels (capillaries, small arteries, and pericardial large arteries) (Figure 2, A–C). First signs of TM were apparent in capillaries, and larger vessels were involved in lesions that had progressed. In stage 1 samples, a few microthrombi were evident in capillaries without myocardial ischemia or interstitial hemorrhage. In stage 2 samples, thrombi were more prominent in capillaries. Small arteries also displayed thrombotic changes; however, large arteries were intact. Myocardial degeneration was evident, as judged by vacuolization around nuclei. In stage 3 samples, microthrombi developed diffusely in capillaries, and thrombotic changes usually involved arteries of all sizes, with myocardial ischemia, necrosis, and focal hemorrhage. One graft in stage 3 (B228), however, showed thrombosis in only a small percentage of small and large arteries.
Figure 1.
The development of TM in the grafts (A, E, and I: H&E stain; B, F, and J: Masson stain; C, G, and K: CD41 stain; D, H, and L: H&E stain; M–P: two-color immunohistochemistry for CD41+ platelets (red color) and fibrin (green color). In the grafts of stage 1 (A–D: B228, POD 27), only a small number of capillaries contained thrombi (arrow). In the grafts of stage 2 (E–H: B223, POD 69), thrombi were noted in capillaries (arrow) as well as in small arteries (arrowhead), but were not seen in large arteries (*). In the grafts of stage 3 (I–L: B223, POD 110), all sizes of arteries were involved in thrombotic change with focal interstitial hemorrhage. In capillaries (arrow) in the grafts of stage 1 (M: B229, POD 27), stage 2 (N: B229, POD 50), and stage 3 (O: B229, POD 78), and arteries (arrowhead) in stage 3 (P: B229, POD 78), platelet accumulation was detected together with fibrin deposition. Original magnifications: ×100 (A, E, H, I, L); ×200 (C, D, G, K, P); ×400 (B, F, J, M–O).
Figure 2.
The deposition of immunoglobulin and complement and the correlation with the development of TM. CD41+ thrombi formation in various sizes of vessels (A–C), the deposition of immunoglobulin and complement components (D–H) in the grafts, and the correlation between immunoglobulin or complement deposition and CD41+ thrombi formation (I–L) are shown. During the development of graft failure from stage 1 to stage 3, CD41+ thrombi seemed to develop first in graft capillaries and then gradually spread to small and large arteries. Immunoglobulin and complement deposition increased in the grafts from stage 1 to stage 3. The frequency of CD41+ thrombi strongly correlated with the degree of IgM, IgG, C4d, and C5b-9 deposition.
Pathogenesis and Mechanism of TM in GalT-KO Heart Grafts
To clarify the pathogenesis involved in multiple microthrombi formation, evidence for antibody-mediated rejection was sought (Figure 2, D–H; and Figure 3, A–L). One hour after transplantation biopsies were performed in two baboons (B223 and B226). In two biopsy samples, IgM was clearly positive, and IgG was weakly positive. During the development of graft damage from stage 1 to stage 3, deposition of IgM, IgG, C3, C4d, and C5b-9 increased in the capillary walls. The frequency of CD41+ thrombi was strongly correlated with the degree of immunoglobulin and complement deposition (Figure 2, I–L). Two-color immunohistochemistry for CD41 and IgM, C3, or C4d showed that almost all of the CD41+ thrombi were detected within vessels with depositions of immunoglobulin and complement (Figure 3, M–P). These findings strongly suggested that immunoglobulin deposition and complement activation are associated with multiple microthrombi formation during the development of TM.
Figure 3.
Immunoglobulin and complement deposition in the grafts. The biopsy taken 1 hour after transplantation in B223 (stage 1) showed minimal but clearly present IgM (A), IgG (B), and C4d (C) deposition. The focal deposition of IgM (D) and C4d (E) was seen in the graft of B223 on day 69 (stage 2). The graft with stage 3 in B223 on day 110 showed diffuse depositions of IgM (F), IgG (G), C4d (H), C3 (I), and C5b-9 (J). The deposition of IgM (K) and C4d (L) was also detected on large vessel. Two-color immunostaining for CD41 (red color) and IgM (M, O), or C4d (N, P) (green color) showed that almost all of the CD41+ thrombi were detected within vessels that contained deposits of immunoglobulin and complement.
We next assessed the mechanism of TM, focusing on cell death and activation of endothelial cells. Apoptotic cells with condensed nuclei and TUNEL+ dead cells were evident in damaged capillaries (Figure 4, A and C) and arteries (Figure 4, B and D). The number of TUNEL+ cells gradually increased in the grafts during the progression of graft dysfunction (Figure 4, E–G). Lack of CD31 and MHC class II staining in vessels indicated the loss of endothelial cells in capillaries and arteries (Figure 4, H–J). In electron microscopy, necrosis, apoptosis, complete loss of endothelial cells, and the presence of large intercellular gaps were seen in damaged capillaries with fibrin thrombi (Figure 5, A–D). Activated endothelial cells with enlarged nuclei and pronounced nucleoli were also evident in the grafts (Figure 5, E and F). Endothelial cells were PCNA+, suggesting that they were proliferating (Figure 6, A and B). vWF was prominent in endothelial cells and thrombi as well as in the interstitium of the grafts experiencing failure (Figure 6, C and D). The expression of TF gradually increased in the grafts during the development of graft failure (Figure 6, E–H). In naïve GalT-KO hearts, the expression of CD39 was detected on endothelial cells in capillaries and arteries (Figure 1). However, CD39 expression decreased markedly in capillaries during the progression of graft failure (Figure 6, J–L). During the development of graft damage, the number of TUNEL+ capillary cells and the expression of TF increased, whereas the expression of CD39 decreased (Figure 7, A–C): each of these three variables strongly correlated with IgM and/or C4d deposition (Figure 7, E–H). In addition, the severity of CD41+ microthrombi strongly correlated with the number of TUNEL+ capillary cells and the expression of TF and CD39 (Figure 7, I–K).
Figure 4.
Dead endothelial cells in damaged vessels. Apoptotic cells (arrow) with pyknotic nuclei and TUNEL+ dead cells (arrow) were detected in the damaged capillaries (A, C) and small arteries (B, D) (A and B: H&E stain; C and D: TUNEL stain). TUNEL+ dead cells (arrow) were more prominent in the grafts with graft failure (E: stage 1, F: stage 2, G: stage 3; E–G: TUNEL stain). Partial loss of CD31+ or MHC class II+ staining (arrowhead) indicated endothelial cell loss in the damaged capillaries and arteries (H and J: CD31 stain; I: MHC class II stain). Arrow indicates capillaries without thrombi. Original magnifications: ×400 (A–D, H, I); ×200 (E–G, J).
Figure 5.
Electron microscopy of injured and activated endothelial cells in damaged capillaries with fibrin thrombi (*), fibrin exudation (arrow), or platelet accumulation (arrowhead). Necrosis (double arrow) (A), apoptosis (double arrow) (B), complete loss (C) of endothelial cells, and the presence of large intercellular gaps (between double arrows) (D) were associated with fibrin thrombi formation in damaged capillaries. In the grafts with graft failure, capillaries also showed activated endothelial cells with fibrin deposition (arrow) (E) and platelet accumulation (F). Original magnifications: ×9100 (A, D); ×7100 (B, C); ×5500 (E); ×11,000 (F).
Figure 6.
Activation of endothelial cells in the grafts. A and B: Endothelial cells in rejecting grafts are indicated by enlarged nuclei and PCNA+ nuclei (arrow) (A: H&E stain; B: PCNA stain). Von Willebrand factor (vWF) is expressed on endothelial cells in naïve GalT-KO pig hearts (C), and is more prominent in the grafts of stage 3 (D) (C and D: vWF stain). The increase of TF and the decrease of CD39 expression are evident in the grafts during the development of graft failure (E and I: naïve GalT-KO pig heart, F and J: stage 1, G and K: stage 2, H and L: stage 3, E–H: TF stain; I–L: CD39 stain). CD39 expression remains in small arteries (arrowhead). Original magnifications: ×400 (A, B); ×200 (C, D, E–H).
Figure 7.
Cell death and activation of endothelial cells and cellular infiltration contributed to the development of TM. The number of TUNEL+ dead capillary cells (A), the expression of TF (B), and CD39 (C), and the number of CD3+ cells (D) in the grafts are shown. The number of TUNEL+ cells (E, F), and the expression of TF (G) and CD39 (H) correlated strongly with IgM and C4d deposition. CD41+ thrombi formation was strongly correlated with TUNEL+ cells (I), TF (J), and CD39 (K) expression, and was weakly correlated with CD3+ cell infiltration (L).
Lastly, we examined evidence of cellular infiltration in the grafts (Figure 8). The biopsy/graftectomy samples were grade 0R in stages 1 and 2, and grade 2R in stage 3, according to the International Society for Heart and Lung Transplantation recent criteria.25 Soon after transplantation, polymorphonuclear leukocytes and mononuclear cells infiltrated the capillaries (Figure 8A). In addition, a small number of CD3+ cells were present in all grafts, and were especially prominent in the grafts with graft failure (stage 3) (Figure 7D; and Figure 8, B and C). Thrombi were occasionally present with CD3+ T-cell infiltration. TUNEL+ dead cells were sometimes in contact with infiltrating mononuclear cells (Figure 8D). The frequency of CD41+ thrombi correlated weakly with the number of CD3+ cells (Figure 7L).
Figure 8.
A–D: Acute cellular rejection. Focal mononuclear cells infiltrating the grafts (A: H&E stain) included CD3+ T cells (arrowhead) (B and C: CD3 stain). Some thrombi (arrow in C) were present with CD3+ cell infiltration (arrowhead). Some TUNEL+ dead cells (arrow) were in contact with infiltrating mononuclear cells (D: TUNEL stain). Original magnifications: ×400.
Discussion
In the present study on GalT-KO cardiac xenografts transplanted into baboons, we demonstrated prevention of HAR and prolonged graft survival when compared to previous reports.10,17,18 However, in this series AHXR could still not be overcome, despite intensive immunosuppression. During the progression of graft failure, TM developed with extensive microvascular thrombi and myocardial ischemia. Here, both cell death and activation of endothelial cells, probably related with immunoglobulin deposition and complement activation, contributed to the formation of platelet-fibrin thrombi. AHXR, most likely associated with non-Gal antibodies, thus likely represents a major remaining immunological barrier to the success of xenotransplantation, even when using GalT-KO donors.
In our previous studies in pig-to-nonhuman primate models, cardiac xenografts from Massachusetts General Hospital miniature swine and hDAF transgenic pigs underwent AHXR despite immunosuppressive therapy with the depletion of anti-Gal antibodies and the inhibition of complement. The development of AHXR was correlated with immunoglobulin (mainly anti-Gal antibody) deposition and resulted in graft failure within a few weeks (Massachusetts General Hospital miniature swine) or within 4.5 months (hDAF pigs) of transplantation.10,17,18 In the present study, the GalT-KO heart grafts survived up to 6 months after transplantation without the continuous inhibition of complement and without any anti-Gal antibody depletion. However, all of the grafts underwent AHXR, which was characterized morphologically by the development of TM. These morphological findings were different from the usual pattern of acute humoral rejection,26,27,28 in that TM predominated with little cellular infiltrate and no obvious interstitial hemorrhage. Remarkably, in B228, graft palpation decreased by POD 179 with no significant increase in troponin level (Table 1). This graft was characterized morphologically by the development of TM mainly in capillaries, and not prominent in small and large arteries, with no obvious cellular infiltrate and interstitial hemorrhage.
Anti-CD154 mAbs have been reported to be associated with complications involving coagulation,29 but it is unlikely that this agent was a causative factor in the present study because TM was not seen in other organs of recipient (data not illustrated), recipients received anti-coagulation therapy from day 0 to prevent thromboembolic complications, and TM was also seen in the xenografts treated without anti-CD154 mAbs.10,24 Furthermore, in the present study, multiple microthrombi in the GalT-KO cardiac xenografts were accompanied by the deposition of IgM, IgG, and complement. The severity of TM was strongly correlated with the degree of immunoglobulin and complement deposition. We therefore concluded that TM in GalT-KO heart grafts was associated with antibody-mediated rejection, although anti-Gal antibodies could not play a role in graft injury. Immunoglobulin may react against porcine antigens other than the Gal epitope (non-Gal antibodies). Other recent reports also suggest that non-Gal antibodies could be involved in AHXR in xenotransplantation.30,31,32
Non-Gal antibodies in the present study were primarily directed against endothelial cells, because immunoglobulin was deposited in the capillary walls and initial graft injuries were detected in microvascular endothelial cells. Recently, several reports have suggested that the possible epitopes on pig grafts recognized by human non-Gal antibodies include swine leukocyte antigen, N-glycolylneuraminic acid (NeuGc) epitopes (Hanganutziu-Deicher antigen, although this would not be a target in baboons because these animals also express this antigen), and Forssman antigen.30,33,34 Further experiments are necessary to clarify the novel target epitope of non-Gal antibodies to consider other strategies to prevent non-Gal antibody-mediated rejection, including the deletion or modification of non-Gal epitopes by genetic engineering of the pig.
Several studies attribute graft injury in AHXR to both cell death and to activation of vascular endothelial cells in the grafts.19,22,28,35,36,37,38 Endothelial cell death can cause the loss of vascular integrity, exposure of thrombogenic matrix, and loss of endothelial function, such as the inhibition of coagulation and inflammation.28,35 Endothelial cell activation, with an increase in vWF and TF expression, and the linked decrease in CD39 expression, can also contribute to graft injury, because this is associated with a shift from an anticoagulant into a procoagulant state. TF, the major initiator of coagulation in vivo, acts as a cell surface receptor for factor VII and is in direct contact with coagulation factors in the blood.21,22,36,37,38 CD39, the dominant vascular ectonucleotidase is a NTP diphosphohydrolase that is expressed by quiescent endothelial cells with the capacity to degrade the extracellular inflammatory mediators ATP and ADP to AMP, thereby inhibiting platelet activation and modulating vascular thrombosis.23,38 In the present study, in addition to an increase in TUNEL+ dead capillary cells, the increase of TF and the decrease of CD39 expression were strongly correlated with platelet-fibrin thrombi formation during the development of graft failure.
In the present study, biopsies taken 1 hour after transplantation exhibited evidence for preformed non-Gal antibodies in the baboons. However, these preformed antibodies did not mediate HAR. During the development of TM in the grafts, extensive deposition of IgM and IgG developed with complement activation. In addition, T cells infiltrated during the period soon after transplantation. These findings suggest that the increase of IgM and IgG deposition during the development of TM was possibly associated with a T-cell-dependent and induced non-Gal IgM and IgG antibody response. Although in vitro assays showed general unresponsiveness of recipients to pig and baboon stimulators in MLR and circulating anti-pig IgM and IgG were undetectable by fluorescence-activated cell sorting,15,16 probably because of continuous immunosuppression, the T-cell infiltrates and IgG deposition seen in the grafts suggest that T cells were involved in the rejection. To overcome these problems, our group is developing approaches to induce xenogeneic T-cell tolerance,39 to prevent T-cell-dependent non-Gal antibody production and T-cell-mediated graft rejection.
Acknowledgments
We thank Ms. Emma Samelson-Jones for her special support in editing the manuscript; Novartis Pharma AG (Basel, Switzerland) for generously providing anti-CD154 mAb; and Immerge BioTherapeutics Inc. (Cambridge, MA) for providing LoCD2b mAb and cobra venom factor.
Footnotes
Address reprint requests to Akira Shimizu, M.D., Ph.D., Transplantation Biology Research Center, Massachusetts General Hospital, Building 149-9019, 13th St., Boston, MA 02129. E-mail: akira.shimizu@tbrc.mgh.harvard.edu.
Supported in part by the National Institutes of Health (program project 1PO1 A145897 and 1UO1 AI066331); a sponsored research agreement between the Massachusetts General Hospital and Immerge BioTherapeutics, Inc.; the Ter Meulen Fund from the Royal Netherlands Academy of Arts and Sciences (to F.J.M.F.D); the Prof. Michael van Vloten Fund (to F.J.M.F.D); the Netherland-America Foundation (to F.J.M.F.D); and the Japan Society for the Promotion of Science (grant-in-aid for scientific research C 18591787 to A.S.).
References
- Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- Samstein B, Platt JL. Physiologic and immunologic hurdles to xenotransplantation. J Am Soc Nephrol. 2001;12:182–193. doi: 10.1681/ASN.V121182. [DOI] [PubMed] [Google Scholar]
- Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- Cooper DK. Clinical xenotransplantation—how close are we? Lancet. 2003;362:557–559. doi: 10.1016/S0140-6736(03)14118-9. [DOI] [PubMed] [Google Scholar]
- Bach FH, Robson SC, Winkler H, Ferran C, Stuhlmeier KM, Wrighton CJ, Hancock WW. Barriers to xenotransplantation. Nat Med. 1995;1:869–873. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- Alwayn IP, Basker M, Buhler L, Cooper DK. The problem of anti-pig antibodies in pig-to-primate xenografting: current and novel methods of depletion and/or suppression of production of anti-pig antibodies. Xenotransplantation. 1999;6:157–168. doi: 10.1034/j.1399-3089.1999.00030.x. [DOI] [PubMed] [Google Scholar]
- Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, Monroy R, Xu Y, Awwad M, Colvin RB, Cosimi AB, Robson SC, Fishman J, Spitzer TR, Cooper DK, Sachs DH. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- Zhong R, Luo Y, Yang H, Garcia B, Ghanekar A, Luke P, Chakrabarti S, Lajoie G, Phillips MJ, Katopodis AG, Duthaler RO, Cattral M, Wall W, Jevnikar A, Bailey M, Levy GA, Grant DR. Improvement in human decay accelerating factor transgenic porcine kidney xenograft rejection with intravenous administration of gas914, a polymeric form of alphaGAL. Transplantation. 2003;75:10–19. doi: 10.1097/00007890-200301150-00003. [DOI] [PubMed] [Google Scholar]
- McGregor CG, Teotia SS, Byrne GW, Michaels MG, Risdahl JM, Schirmer JM, Tazelaar HD, Walker RC, Logan JS. Cardiac xenotransplantation: progress toward the clinic. Transplantation. 2004;78:1569–1575. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, Yamada K, Robson SC, Awwad M, Schuurman HJ, Sachs DH, Cooper DK. Alpha1,3-galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, Mueller N, Prabharasuth D, Alt A, Moran K, Cheng J, Behdad A, Sachs DH, Fishman JA, Schuurman HJ, Awwad M, Cooper DK. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, Shimizu A, Schuurman HJ, Cooper DK. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, Sachs DH, Colvin RB. Thrombotic microangiopathic glomerulopathy in hDAF-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- Choo JK, Seebach JD, Nickeleit V, Shimizu A, Lei H, Sachs DH, Madsen JC. Species differences in the expression of major histocompatibility complex class II antigens on coronary artery endothelium: implications for cell-mediated xenoreactivity. Transplantation. 1997;64:1315–1322. doi: 10.1097/00007890-199711150-00014. [DOI] [PubMed] [Google Scholar]
- Thiruvikraman SV, Guha A, Roboz J, Taubman MB, Nemerson Y, Fallon JT. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab Invest. 1996;75:451–461. [PubMed] [Google Scholar]
- Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, Kaczmarek E, Awwad M, Cooper DK, Robson SC. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, Meehan SM, Kozlowski T, Sablinski T, Ierino FL, Cooper DK, Sachs DH, Colvin RB. Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts. Lab Invest. 2000;80:815–830. doi: 10.1038/labinvest.3780086. [DOI] [PubMed] [Google Scholar]
- Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Rose AG, Cooper DK. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J Heart Lung Transplant. 1996;15:804–817. [PubMed] [Google Scholar]
- Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation. 2003;10:293–233. doi: 10.1034/j.1399-3089.2003.02092.x. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Colvin RB. Pathological features of antibody-meditated rejection. Curr Drug Targets–Cardiovasc Haemat Dis. 2005;5:199–214. doi: 10.2174/1568006054064744. [DOI] [PubMed] [Google Scholar]
- Knosalla C, Gollackner B, Cooper DKC. Anti-CD154 monoclonal antibody and thromboembolism revisited. Transplantation. 2002;74:416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- Buhler L, Xu Y, Li W, Zhu A, Cooper DK. An investigation of the specificity of induced anti-pig antibodies in baboons. Xenotransplantation. 2003;10:88–93. doi: 10.1034/j.1399-3089.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11:531–535. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, Liu W, Jevnikar A, Wall W, Cooper DK, Murase N, Dai Y, Wang W, Xiong Y, White DJ, Zhong R. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Ezzelarab M, Ayares D, Cooper DK. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- Holzknecht ZE, Kuypers KL, Plummer TB, Williams J, Bustos M, Gores GJ, Brunn GJ, Platt JL. Apoptosis and cellular activation in the pathogenesis of acute vascular rejection. Circ Res. 2002;91:1135–1141. doi: 10.1161/01.res.0000046236.20251.fa. [DOI] [PubMed] [Google Scholar]
- Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JL. The immunological barriers to xenotransplantation. Crit Rev Immunol. 1996;16:331–358. [PubMed] [Google Scholar]
- Robson SC, Schulte am Esch J, II, Bach FH. Factors in xenograft rejection. Ann NY Acad Sci. 1999;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]