Abstract
Macrophages are important mediators of injury in most types of human kidney diseases; however, the pathogenic importance of both macrophage number and activation status is unknown. To examine this question, severe-combined immunodeficient mice with adriamycin nephrosis, an experimental model of human focal segmental glomerulosclerosis, were treated intravenously with either resting (1 × 106 to 5 × 106) or activated (1 × 103 to 1 × 106) macrophages on day 6 postadriamycin administration, and the effects on kidney injury were examined. On day 28, renal injury was worse in the group that received activated macrophages at doses as low as 1 × 104 macrophages per mouse compared with control adriamycin nephrotic mice. However, treatment with resting macrophages at doses as high as 5 × 106 macrophages per mouse had no significant effect on either renal histology or function. The transferred activated macrophages homed to inflamed kidneys during the middle-to-late stages of the disease, but such homing was not observed for resting macrophages. This study of in vivo cell adoptive transfer supports the importance of macrophage activation status over macrophage number in causing renal injury. These data suggest that therapeutic strategies for treating progressive kidney diseases should target activated macrophages.
Macrophages have been proposed as important mediators of injury in most types of primary and secondary human kidney diseases.1,2 The density of macrophage accumulation correlates with the degree of renal dysfunction and is predictive of disease progression.3 A variety of strategies have been used to examine the role of macrophages in experimental renal injury, including adoptive transfer of macrophages, systemic depletion of macrophages, blockade of molecules involved in monocyte recruitment into the inflamed kidney, and gene modification of macrophages.4,5,6,7,8 In both immune and nonimmune models of kidney disease, a clear association has been shown between macrophage accumulation and the severity of renal injury.9,10
Adriamycin nephrosis (AN) is a robust experimental model of human focal segmental glomerulosclerosis, characterized by changes in both tubulointerstitial and glomerular compartments.11 It can be induced in immunocompetent mice, and also in severe-combined immunodeficient (SCID) mice that are homozygous for an autosomal recessive mutation that leads to absence of lymphocytes and hypogammaglobulinaemia.12 Previous studies in our laboratory have demonstrated macrophage accumulation in both immunocompetent and SCID mice with AN, and significant reduction of injury following macrophage depletion or reduced macrophage recruitment with chemokine C ligand 2 (CCL2) vaccination.6,13,14
Adoptive transfer of macrophages has been shown to worsen inflammation in animal models of acute renal injury.15,16,17 In contrast, macrophages with an anti-inflammatory phenotype induced by inhibition of nuclear factor kappa-B-ameliorated renal injury in an animal model of glomerulonephritis.18 In addition, macrophages transfected or pretreated with anti-inflammatory cytokines have been shown to reduce renal injury.19,20,21 These studies support the importance of activation status of macrophages in mediating renal injury.22,23 The influence of activation status on the potency of macrophages in their effect on kidney diseases has not been explored fully.
The aim of present study was to determine the importance of macrophage activation status on renal injury. To examine this, the effect of dose and disposition of resting and activated transfused macrophages was evaluated in SCID mice with AN.
Materials and Methods
Mice and AN Model
Six- to 8-week-old, male SCID and BALB/c mice obtained from the Animal Resources Centre (Perth, Australia) were used in this study. The Animal Ethics Committee of Westmead Hospital approved all procedures. Dose-finding studies defined an optimal dose of 5.2 mg/kg body weight of adriamycin (ADR; doxorubicin hydrochloride; Pharmacia & Upjohn Pty Ltd., Australia). ADR was injected once via the tail vein of each nonanesthetized SCID mouse.11
Macrophage Separation from Spleens and in Vitro Culture Conditions
BALB/c mice splenocytes were harvested and washed in ice-cold RPMI 1640 medium (Invitrogen, Australia). Tissue was triturated with the sterile syringes, and the resulting cell suspension was filtered through 40 μm nylon mesh (BD Biosciences, Australia) and then incubated at 37°C for 30 minutes. The adherent cells were harvested and purified by magnetic activated cell sorting CD11b+ MicroBeads (Miltenyi Biotec, Germany). These spleen-derived macrophages were rinsed three times with RPMI 1640 medium. Cells were cultured at 37°C in humidified 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum in the presence or in the absence of CpG DNA (10 μg/ml for 24 hours). CPG DNA was purchased from Sigma-Genosys (Woodlands, TX). RPMI 1640 was purchased from Invitrogen (Australia). Cells were washed three times (300g, 10 minutes) and counted, and viability was defined by lack of trypan blue staining.
Experimental Design
Macrophages were separated from normal BALB/c mice. Activated macrophages were prepared by stimulation with CPG DNA (Sigma, Australia). Different dosages of resting macrophages (1 × 106, 2 × 106, 5 × 106) and activated macrophages (1 × 103, 1 × 104, 1 × 105, 1 × 106) were transfused into mice by a single i.v. injection at day 5 after ADR. Mice (n = 6 per group/at each time point) were sacrificed at days 6, 14, and 28. At day 28, functional (serum creatinine and urine protein) and histological measures of renal injury (histology and immunohistochemistry) were assessed.
Isolation of Infiltrated Macrophages from Mouse Kidney
Warm saline (37°C) was perfused to the right heart atrium under anesthesia to remove peripheral blood within mouse kidney. Kidney tissue was then triturated in RPMI 1640 medium with the sterile syringes, and the resulting cell suspension was filtered through 40 μm nylon mesh. Mononuclear cells were separated using a step-gradient sucrose separation procedure (Lymphoprep, Pharmacia, Uppsala, Sweden). Purification of macrophages was performed by positive selection using magnetic activated cell sorting CD11b+ MicroBeads.
Macrophage Purity and Characteristics
For flow cytometry detection, macrophages were incubated at 4°C for 20 minutes with Fc Block (PharMingen, San Diego, California) and were used for double-staining of cell surface and intracellular activation markers as described previously. Briefly, the cells were stained for 30 minutes at 4°C in staining buffer (PBS, 1% bovine serum albumen) with fluorescence-conjugated mAbs for cell surface activation molecules major histocompatibility complex class II and CD86 together for 30 minutes or CD11b, CD11c (dendritic cell marker), CD19 (B cell marker), and CD49b (natural killer cell marker). After Ab staining, cells were analyzed by flow cytometry on a FACScan with CellQuest software (Becton Dickinson, Mountain View, CA) gated to exclude nonviable cells. At least 10,000 viable cells were analyzed per sample. Cells stained with isotype-matched antibodies were used as isotype controls for fluorescence activated cell sorting analysis. For detection of CCL-2, CCL-3, and inducible nitric oxide synthase (iNOS) expression in kidney macrophages, cells were homogenized and the total RNA was isolated using RNeasy Mini Kit (Qiagen). cDNA was synthesized with a reverse transcriptase reaction using a standard technique (Superscript First Strand Synthesis System for RT-PCR, Invitrogen). The PCR primer sequences used in this study were designed using Oligoperfect software (Invitrogen) and are presented in Table 1. Primers were designed to span intron-exon boundaries. For RT-PCR, a 0.2 μg portion of cDNA was used in a 25 μl PCR mixture with 2 ng/μl of each primer and 2U of TaqDNA polymerase (Roche). Housekeeping gene 18S was used as the endogenous control. PCR products after amplification were analyzed by 1.5% agarose gel electrophoresis. For real-time PCR, mRNA levels were quantified using the Rotogene-3000 Real-Time Thermo cycler (Corbett Research, Australia). PCR mixtures containing 0.3 μmol/L primers in 25 μl final SYBR mastermix (Invitrogen) were cycled for 2 minutes at 50°C, 2 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C. The normalized value for mRNA expression in each sample was calculated as the relative quantity of relevant primers divided by the relative quantity of the housekeeping gene 18s.
Table 1.
Real-Time PCR Primers and Products
| Gene | Primer sequence (5′… 3′) | Product |
|---|---|---|
| CCL3 (F) | tgcccttgctgttcttctct | 124 |
| CCL3 (R) | gatgaattggcgtggaatct | |
| iNOS (F) | caccttggagttcacccagt | 170 |
| iNOS (R) | accactcgtacttgggatgc | |
| TNF-α (F) | gctgagctcaaaccctggta | 118 |
| TNF-α (R) | cggactccgcaaagtctaag | |
| CCL2 (F) | cccaatgagtaggctggaga | 125 |
| CCL2 (R) | tctggacccattccttcttg |
F, forward; R, reverse.
Serum and Urine Analysis for Renal Function
Serum creatinine concentration and 16 hour urinary protein excretion at the end of experiment were analyzed by the Institute of Clinical Pathology and Medical Research, Westmead Hospital, using a BM/Hitachi 747 analyzer (Tokyo, Japan).
Histological and Microscopic Preparation of Tissues
Kidneys were rapidly removed on day 7, day 14, and day 28. Coronal sections of renal tissue were immersion-fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections 5 μm thick were stained with trichrome. The remaining cortex of the same kidney was frozen in liquid nitrogen and used for RNA analysis and immunohistochemistry. To evaluate the degree of renal injury, 20 fields of view/section at a magnification of 200× were assessed and the degree of renal injury was estimated by evaluating the percentage of renal injury per field and was graded on a scale of 0 to 4: 0, normal glomeruli, tubules and interstitial volume; 0.5, small focus of glomerular and tubular injury and interstitial infiltration; 1, involvement of <10% of the cortex; 2, involvement of up to 25% of the cortex; 3, 26%−75% of the cortex; and 4, extensive damage involving >75% of the cortex.24
For immunohistochemical staining of macrophages, rat anti-mouse F4/80 (PharMingen) and CCL2 (Santa Cruz) were used as the primary antibody and biotinylated rabbit anti-rat Ig (Dako Corporation, USA) was used as the secondary antibody. Kidney sections were placed in ornithine carbamoyltransferase (Sakura Fintek Inc, USA). Five-μm sections were cut, dried overnight, and fixed in cold acetone for 8 minutes. Endogenous peroxidase activity was blocked by 0.3% (v/v) H2O2 solution for 15 minutes when incubating the slides. Biotin Blocking System (Dako) was used to block endogenous avidin binding activity. Normal rat immunoglobulin was used for control sections. Sections were incubated with secondary antibodies, ABC, and 3, 3-diaminobenzidine substrate-chromogen solution (Dako) were applied and then washed. Slides were counterstained with hematoxylin (Sigma). For assessment of interstitial infiltration, positively stained cells located in the tubulointerstitial area only were counted from more than 20 random cortical fields (magnification 400×) in each section, and the numbers averaged for each section, as previously described.11
For immunohistochemical staining of α-SMA, tissue sections were dewaxed and dehydrated according to standard protocols. For antigen retrieval, the sections were placed in 10 mmol/L citric acid buffer (pH 6.0) and incubated in a preheated water-bath maintained at 95°C for 15 minutes. Endogenous peroxidase was blocked with hydrogen peroxide in methanol (3% [v/v]) followed by the blocking with Biotin Blocking System from Dako and Blockbuster. A rabbit anti α-SMA first antibody (Lab vision) was applied for one hour at room temperature at a concentration of 1:200. After incubation with biotinylated anti-rabbit second antibody (1:400), the sections were developed using the same protocols as the above.
For detection of tubular chemokine expression in AN, the level of chemokine mRNA expressed in tubules of AN mice was analyzed by Laser Capture MicroDissection (Microlaser Technologies AG, Bernried, Germany) of ornithine carbamoyltransferase (Tissue-Tek, Sakura Rinetek, Torrance, CA) frozen tissue sections and real-time PCR. Ornithine carbamoyltransferase-embedded frozen kidney cortex was cut in 10-μm sections and mounted on diethyl pyrocarbonate-pretreated glass slides, fixed in 70% ethanol and briefly stained in Mayers Hematoxylin, then laser captured immediately into lysis buffer (Absolutely RNA Nanoprep Kit; Stratagene, La Jolla, CA) under x200 magnification. mRNA from microdissected tubular samples was amplified by real-time PCR to detect the expression of chemokines and prepared as above.
In Vivo Transferred Macrophages Tracking
For macrophage in vivo tracking, macrophages were labeled with red fluorescent membrane label, DiI (Invitrogen) according to the manufacturer’s instructions, and harvested into serum-free medium immediately before injection. In brief, 50 μg DiI was dissolved into 50 μl pure ethanol. To a one-million cell suspension in 1 ml RPMI 1640, 4 μl DiI was added for 10 minutes incubation at 37°C, followed by 15 minutes incubation at 4°C. Cells were transfused into SCID mice by a single tail vein injection at day 6 after ADR. Kidney tissue slides were visualized under fluorescence microscopy and the red fluorescent cells were considered to be transfused macrophages. The viability and phenotype of macrophages were determined by Trypan blue and real time-PCR.
Macrophage Chemotaxis Assay
The assay of chemotaxic cell migration was performed using enhanced chemiluminescence Cell Invasion Assay (Millipore, Australia) (whose insert contains a 5 μm pore size membrane). Resting or activated macrophages were loaded into an upper chamber coated with ECM at the density of 2 × 105 cells/100 μl of 0.1 ml serum-free RPMI 1640. The lower chamber was loaded with graded concentrations of monoclonal antibody CCL-2 (BD Australia) (0, 50, 100, and 300 μg/ml RPMI 1640 medium). The plates with cells were incubated for 8 hours at 37°C in a CO2 incubator. Migrating cells on the bottom of the insert were incubated with 150 μl of prewarmed Cell Detachment Buffer for 30 minutes, dissociated from the membrane, and detected by CyQuant GR dye. Absorbances (OD480 and OD530) were read using a fluorescence plate reader and protein concentrations calculated using a standard curve generated with known concentrations of CCL-2 antibody.
Statistical Analysis
Renal functional data (serum creatinine and urine protein) were log transformed before analysis to stabilize the variance. The statistical package SPSS for windows version 14 was used to analyze the data. Analysis of variance was used to test for an association between the log transformed outcome and treatment (one way analysis of variance). The pairwise comparisons between treatments were adjusted for multiple comparisons using the Bonferroni technique. Other data analysis was performed directly by one-way analysis of variance (analysis of variance) for multiple comparisons of parametric data, and the Kruskal-Wallis test for nonparametric data. Results are expressed as the group mean ± SEM. A P value of less than 0.05 was considered statistically significant.
Results
Macrophage Characteristics in Vitro and in Vivo
Macrophages were obtained from spleen of BALB/c mice. Following purification, the fraction of CD11b positive cells was 96%, while CD11c, CD19, and CD49b positive cells accounted for less than 1%. The expression of MHC class II and CD86 by activated macrophages was stronger in comparison to that of resting macrophages. INOS expression by activated macrophages was significantly greater than by resting macrophages (Table 2).
Table 2.
Properties of Resting Macrophages and Activated Macrophages
| Properties | Assay procedures | RM | AM |
|---|---|---|---|
| CCL3 mRNA expression | RT-PCR | ± | + |
| iNOS mRNA expression | RT-PCR | − | + |
| TNF-α protein expression | Western Blot | ± | + |
| CCL2 expression | Western Blot | + | ++ |
| MHC class II expression | FACS | 1.5% | 33.6% |
| CD86 expression | FACS | 42.8% | 92.6% |
RM, resting macrophages; AM, activated macrophages.
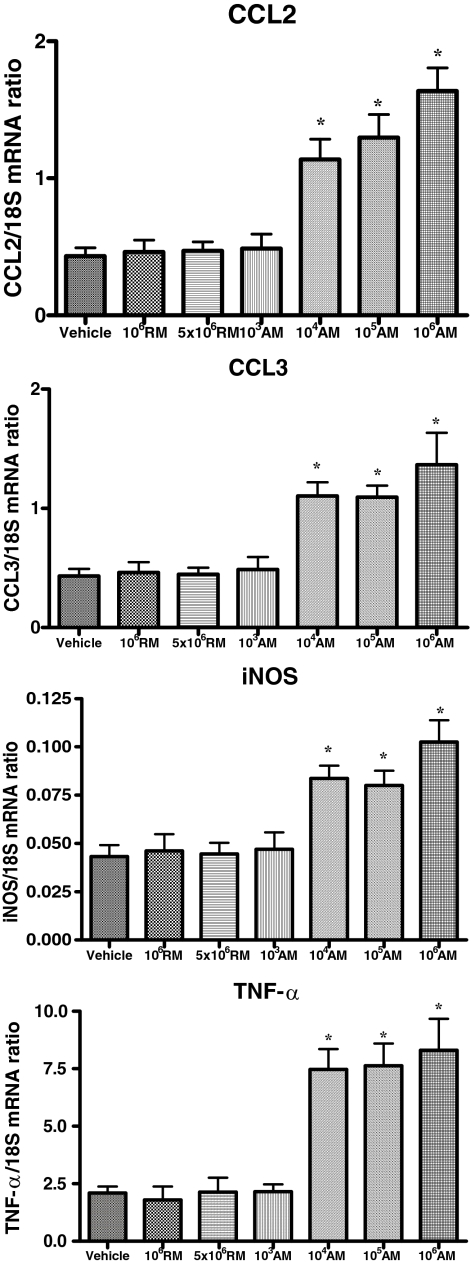
Macrophages were also isolated from kidney of AN mice at day 28. INOS and CCL3 gene expression of renal macrophages was significantly up-regulated in all AN mice transfused with activated macrophages (except at the dose of 1 × 103 macrophages) in comparison to AN mice transfused with resting macrophages (Figure 1).
Figure 1.
Cytokine expression of macrophages isolated from AN kidney at day 28 of mice transfused with resting (RM) or activated (AM) macrophages. The expression of CCL2, CCL3, iNOS, and TNF-α mRNA was assessed by real-time PCR. *P < 0.05 vs. non-asterisked groups.
In Vivo Tracking of Transfused Macrophages
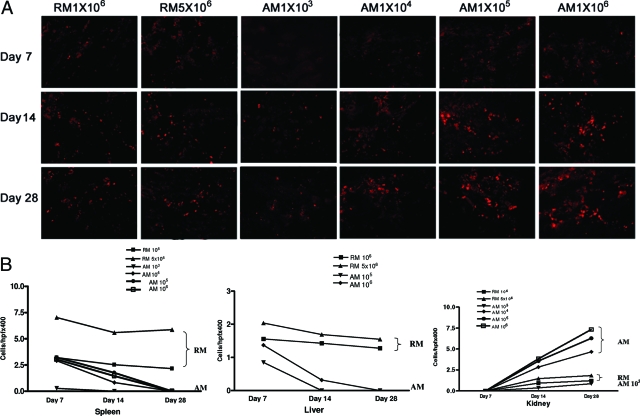
One to five million fluorescently labeled resting, or 1 × 103 to 1 × 106 activated macrophages were injected via tail vein at day 6 post ADR or into control normal mice. Fluorescently labeled cells were distributed to kidney, spleen, and liver of AN mice 24 hours after transfer. Interestingly there was no trace of fluorescently labeled cells in lung and heart 24 hours after transfusion. Few of these cells were seen in spleen and liver at day 14 (8 days post transfusion) and none was detectable at day 28 in AN mice transfused with activated macrophages, whereas in AN mice transfused with resting macrophages, their number in liver and spleen remained constant. In contrast, transfused activated macrophages from a dose of 1 × 104 accumulated progressively in kidneys up to day 28 after ADR. This phenomenon of macrophage migration in kidneys was less marked with resting macrophages, even with transfusion with as many as 5 × 106 cells per mouse (Figure 2, A and B, and Table 3).
Figure 2.
A: In vivo tracking of transfused macrophages in AN kidney. Representative fluorescence photomicrographs showing transfused resting (RM) and activated (AM) macrophages in kidney at day 7, 14, and 28 after ADR, following macrophage transfusion at day 6. B: Quantitation of transfused resting and activated macrophages in spleen, liver, and kidney at day 7, 14, and 28 after ADR, following macrophage transfusion at day 6. There were no transfused AM seen in liver following transfusion at a dose of 103 and 104. Error bars are omitted for clarity (also see Table 3). Original magnification ×200.
Table 3.
Quantitative Analysis of Tissue Distribution of Transfused Resting or Activated Macrophages after Transfusion on Day 6 after ADR
| RM
|
AM
|
|||||
|---|---|---|---|---|---|---|
| 106 | 5 × 106 | 103 | 104 | 105 | 106 | |
| Liver | ||||||
| D7 | 1.56 ± 0.51 | 2.04 ± 0.79 | ± | ± | 0.85 ± 0.27 | 1.37 ± 0.48 |
| D14 | 1.43 ± 0.42 | 1.69 ± 0.51 | ± | ± | ± | 0.32 ± 0.14 |
| D28 | 1.28 ± 0.43 | 1.55 ± 0.36 | ± | ± | ± | ± |
| Spleen | ||||||
| D7 | 3.21 ± 1.18 | 7.03 ± 1.94* | 0.28 ± 0.11 | 2.91 ± 0.98 | 3.22 ± 1.38 | 3.04 ± 0.92 |
| D14 | 2.53 ± 0.74 | 5.59 ± 1.33* | ± | 0.83 ± 0.24 | 1.76 ± 0.39 | 1.45 ± 0.58 |
| D28 | 2.16 ± 0.75 | 5.87 ± 1.39* | ± | ± | ± | ± |
| Kidney | ||||||
| D7 | ± | ± | ± | ± | ± | ± |
| D14 | 0.93 ± 0.36 | 1.46 ± 0.42 | 0.36 ± 0.11 | 2.85 ± 0.88* | 3.57 ± 0.96* | 3.84 ± 0.87* |
| D28 | 1.22 ± 0.48 | 1.83 ± 0.59 | 0.86 ± 0.27 | 4.67 ± 1.12* | 6.29 ± 1.74* | 7.34 ± 1.93* |
The numbers represent counted DiI-labeled cells per hpf at 400× magnification.
±, occasional cells, too few to quantitate; D, day after ADR; RM, resting macrophages; AM, activated macrophages.
P < 0.05 vs. non-asterisked groups at each time point.
Macrophage Infiltration and Chemotactic Motility
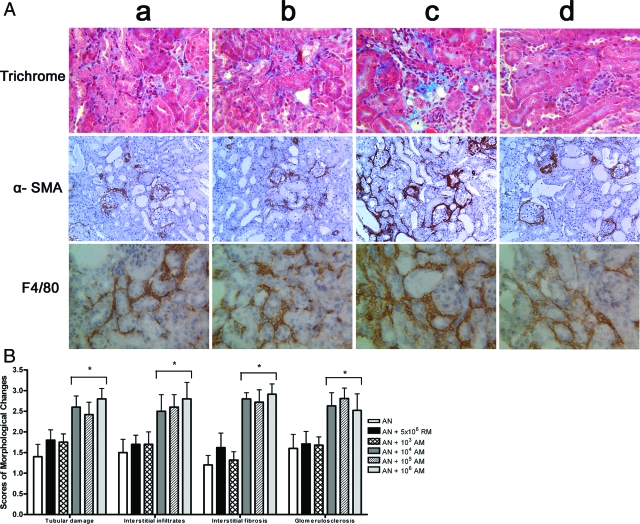
In AN mice, transfusion of 1 × 104 activated macrophages induced a 30% rise in numbers of F4/80-positive macrophages within the renal interstitium. This increase in F4/80-positive cells was not seen in AN mice transfused with 103 activated macrophages, nor in mice transfused with resting macrophages in doses up to 5 × 106 (Figure 3 and Table 4).
Figure 3.
A: Effect of macrophage transfusion on histological injury, macrophage accumulation, and fibrosis of AN. Representative Gomori trichrome, α-SMA, and F4/80 stained sections of renal cortices at day 28 (magnification ×200) from AN mice transfused with vehicle (a), 5 × 106 resting macrophages (b), 104 activated macrophages (c), 103 activated macrophages (d) (n = 6 per group). The injury caused by 105 and 106 AM was no different to that caused by 104AM. B: Semiquantitative assessment of kidney injury. Each evaluation represents the mean ± SEM. *P < 0.05 vs. other three groups (n = 6 per group).
Table 4.
Quantitative Analysis of the Proportion of Trichrome-Positive Area, α-SMA-Positive Area, and Number of F4/80-Positive Cells within Interstitium of AN Mice at Day 28 after ADR
| AN + Vehicle | AN + 106 RM | AN + 5 × 106 RM | AN + 103 AM | AN + 104 AM | AN + 105 AM | AN + 106 AM | |
|---|---|---|---|---|---|---|---|
| Trichrome-positive area (%) | 9.44 ± 3.11 | 9.86 ± 3.05 | 10.82 ± 3.49 | 11.41 ± 4.56 | 19.09 ± 5.27* | 20.77 ± 5.57* | 21.09 ± 7.40* |
| α-SMA-positive area (%) | 5.07 ± 1.49 | 5.29 ± 1.81 | 5.64 ± 1.77 | 6.46 ± 2.24 | 14.77 ± 4.31* | 15.15 ± 5.51* | 15.69 ± 5.36* |
| F4/80-positive cells (numbers) | 38.45 ± 11.28 | 40.23 ± 10.52 | 42.45 ± 11.26 | 44.80 ± 10.83 | 69.45 ± 15.85* | 71.45 ± 12.67* | 73.25 ± 17.04* |
P < 0.05 compared to non-asterisked groups.
RM, resting macrophages; AM, activated macrophages.
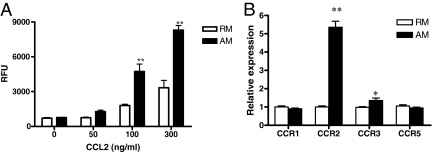
Migration of resting and activated macrophages with or without addition of CCL2 was examined using the ECM migration chamber assay. In response to CCL2, activated macrophages demonstrated much greater motility than resting macrophages (Figure 4A).
Figure 4.
CCL-2-mediated macrophage chemotaxis and expression of chemokine receptors. A: Macrophage chemotaxis toward CCL-2 was tested for 8 hours. 20,000 cells were used in each assay and fluorescence was determined as described in methods. (**P < 0.01 compared to RM group). B: mRNA expression of chemokine receptors in RM and AM was examined by microdissection and real-time PCR (*P < 0.01 and **P < 0.001 compared to RM group).
The expression of chemokine receptors by RM and AM was also examined. CCR2 mRNA expression was greatly increased, while CCR3 mRNA expression was mildly increased with AM compared to RM (Figure 4B).
The Expression of Chemokines and Cytokines in AN
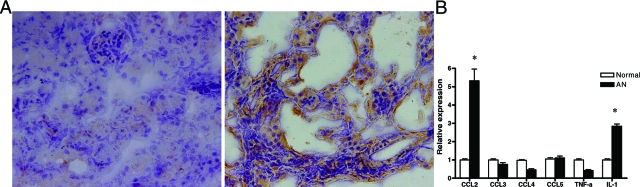
CCL2 expression was examined in the cortex of AN mice by immunohistochemistry. CCL2 was strongly expressed in injured tubule cells, and moderately expressed in glomerular and interstitium (Figure 5A).
Figure 5.
Expression of cytokines and chemokines in AN. A: CCL2 staining, left: normal kidney; right: AN kidney. B: mRNA expression of chemokines and cytokines in tubules of AN (*P < 0.001 compared with normal controls).
CCL2 and other chemokines in tubule cells were examined quantitatively by laser capture microdissection and real-time PCR. Significantly higher levels of CCL2 and interleukin-1β gene expression were detected in tubules 4 weeks after ADR as compared with normal control (Figure 5B).
Effects of Transfused Macrophages on Renal Injury
Transfusion of as few as 1 × 104 activated macrophages per mouse induced progressive fibrosis, whereas transfusion of up to 5 × 106 resting macrophages per mouse had no effect on interstitial fibrosis in comparison with AN controls. Similarly, there was much stronger α-SMA expression in mice transfused with 1 × 104 activated macrophages, but not those transfused with resting macrophages in doses up to 5 × 106 in comparison with control AN mice (Table 3 and Figure 3).
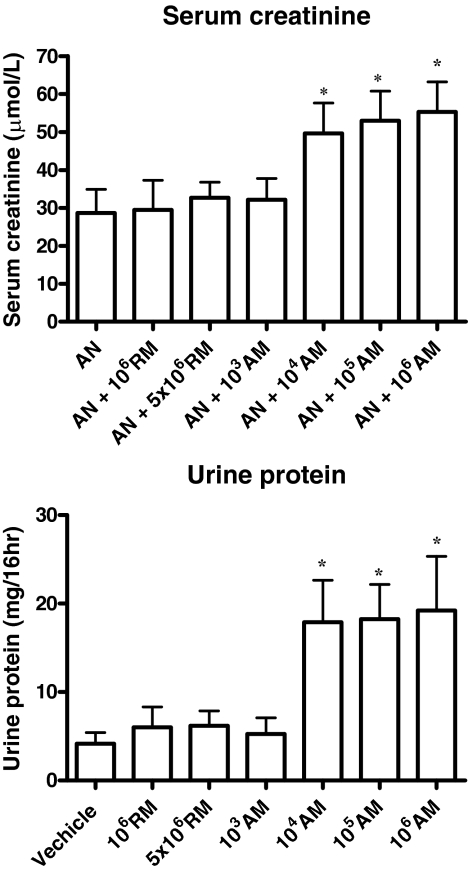
Both serum creatinine and urinary protein excretion at day 28 were significantly worse following transfusion with activated macrophages in doses as low as 1 × 104 (but not 1 × 103) per mouse, but were unaffected by resting macrophages in doses up to 5 × 106 per mouse (Figure 6). Transfusion of culture medium from final stages of macrophage preparation (before their transfusion) had no effect on kidney function or structure in normal or AN mice (data not shown).
Figure 6.
Serum creatinine and 16-hour urine protein at day 28. The values represent the mean ± S.E.M. of evaluations from each group (n = 6 per group). *P < 0.05 for activated macrophages at doses 104, 105, or 106 versus other five groups.
Discussion
In this study we provide evidence that renal injury mediated by macrophages in chronic kidney disease depends more on macrophage activation status than on dose. Transfusion of activated macrophages but not resting macrophages worsened renal glomerulosclerosis, interstitial inflammation and fibrosis, and functional impairment in AN SCID mice.
Perhaps the most striking finding in this study is that with a dose as low as only 1 × 104 activated macrophages per mouse, kidney injury was significantly worse than in untransfused AN mice, yet a dose 500 times higher (5 × 106) of resting macrophages had no effect. This finding emphasizes the importance of macrophage activation status in causing or exacerbating kidney injury. Activation status of macrophages has been examined in several studies. One such study examined the properties and responsiveness to cytokines of macrophages purified from normal and nephritic glomeruli and found that macrophages from normal glomeruli did not generate nitric oxide spontaneously but only after treatment with interferon-γ and tumor necrosis factor (TNF)-α.25 Interestingly, another study using the unilateral ureteric obstruction model found that wild-type mice reconstituted with marrow lacking the Agtr1 gene (Agtr1 (−/−)) developed more severe interstitial fibrosis with fewer interstitial macrophages than those in mice reconstituted with Agtr1 (+/+) marrow. In vivo assays revealed a significantly impaired phagocytic capability of Agtr1(−/−) macrophages.26
The mechanisms underlying the exacerbation of renal injury by activated macrophages were examined in present study. First, activated macrophages expressed high levels of MHC II and CD86, which are important molecules in the initiation of adaptive immune responses. Secondly, activated macrophages expressed inflammatory mediators including nitric oxide and TNF-α, which are known to cause renal injury directly. It is noteworthy that macrophages separated from AN kidney more than 3 weeks after transfusion still exhibited up-regulated inflammatory cytokine expression that was similar to that of the macrophages immediately after initial activation. Studies from Rees’ group demonstrated that macrophages separated from a focus of immunologically mediated inflammation in nephrotoxic nephritis were programmed in vivo, and were unresponsive to activating cytokines such as interleukin-4, transforming growth factor-β, and TNF-α.25 Their studies raised the question of whether macrophage function alters with time during the resolution of inflammation. In the current study transfused macrophages appeared to maintain their activation status within the inflammatory focus during the progression of renal injury.
Macrophage recruitment to the kidney is an important step in initiation of inflammation and tissue destruction.27 As expected, transfused resting macrophages were distributed mostly to spleen and liver at different time points after transfusion. The number of transfused macrophages in spleen and liver did not change significantly with time for transfused resting macrophages but fell for activated macrophages. In contrast, transfused activated macrophages in doses as low as 1 × 104 per mouse accumulated progressively at the site of injury (the kidney), but not in other organs up until day 28. Interestingly, transfusion of resting macrophages in doses up to 500 times greater than those of activated macrophages did not result in significant macrophage accumulation in injured kidney. Moreover there was no significant difference in the number of transfused activated macrophages, as well as total macrophages at the site of injury (kidney) across a 100-fold dose range of transfused macrophages (1 × 104 to 1 × 106). This is the likely explanation for why the effect of activated macrophages on structural and functional injury was not dose-dependent across this range. It is unknown whether a greater chemotactic stimulus could increase renal macrophage accumulation nor whether this could exacerbate injury to a greater extent.
To clarify why activated macrophages more effectively accumulate in sites of injury, macrophage motility in response to CCL2 was examined. This study showed that activated macrophages demonstrated greater motility in response to CCL2 than resting macrophages. These data suggest that enhanced responsiveness of activated macrophages to chemotactic stimuli could be a key factor determining their accumulation at sites of injury. In this study, activated macrophages also expressed higher CCR2 than resting macrophages. Izikson et al have reported that CCR2 expression is consistent with the ability of monocytes to traffic to inflammatory sites.28 In this study, activated macrophages showed higher levels of CCR2 compared to resting macrophages. Furthermore Xu et al also showed that inflammatory monocyte recruitment to inflamed tissue is consistent with reduction of CCR2+ cells in circulation after adoptive transfer of bone marrow derived macrophages.29
An unexpected finding in this study was that transfusion of resting macrophages did not exacerbate kidney injury in comparison to that of untransfused AN mice, even at doses 500 times higher than that at which activated macrophage exacerbated injury. This result contrasts with that of Nikolic-Paterson’s group who reported that transfusion of resting macrophages increased proteinuria and mesangial cell proliferation in anti-GBM nephritis.15 The explanation for these contrasting results may relate to differences in the models, with AN being primarily a model of innate immunity and anti-GBM nephritis of adaptive immunity. Acuteness of disease may be another determinant of whether resting macrophages can exacerbate injury.30 Moreover, under appropriate conditions, resting macrophages serve as a reservoir for activated macrophages. This has been demonstrated in a two-shot model of Thy 1.1 nephritis, in which resting macrophages become activated by interferon only after a second dose of anti Thy 1.1 antibody.31
Another notable observation in the present study is that of the large number of renal cells that were positive for α-SMA after transfusion of activated macrophages. This finding suggests that activated macrophages may directly or indirectly increase the number of α-SMA-expressing myofibroblasts. In unilateral ureteric obstruction and diabetic nephropathy, macrophage accumulation is accompanied by the development of fibrotic lesions following the rapid accumulation of interstitial myofibroblasts. Support for a direct role for macrophages in increasing myofibroblast numbers comes from an in vitro study of hepatoma cells where conditioned medium from activated macrophages induced epithelial-mesenchymal transition and down-regulated the E-cadherin/β-catenin complex.32
In conclusion, this study demonstrates the importance of activation status in determining whether and to what extent macrophages cause or exacerbate renal injury. Activated macrophages, but not resting macrophages increased renal injury in murine AN. Under situations in which activated macrophages are shown to be pathogenic, therapeutic targeting of activated macrophages rather than all macrophages is likely to be a more effective approach to treating progressive renal disease.
Acknowledgments
We acknowledge Dr. Karen Byth for the data statistical analysis and Ms Aysen Yuksel for histological assistance from Westmead Millennium Institute.
Footnotes
Address reprint requests to Dr. Yiping Wang, Centre for Transplantation and Renal Research, The University of Sydney at Westmead Millennium Institute, Westmead, Sydney 2145, Australia. E-mail: Yiping_Wang@wsahs.nsw.gov.au.
Supported by the National Health & Medical Research Council of Australia (NHMRC, grant 457345 to Dr. Yiping Wang), Johnson &Johnson (Focused Funding to Professor David Harris), and Kidney Health Australia (Biomedical scholarship to Ying Wang).
Ying Wang and Yiping Wang contributed equally to this work.
References
- Ferrario F, Castiglione A, Colasanti G, Barbiano di Belgioioso G, Bertoli S, D'Amico G. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28:513–519. doi: 10.1038/ki.1985.158. [DOI] [PubMed] [Google Scholar]
- Schreiner GF. Macrophages and cellular immunity in experimental nephrosis and glomerulonephritis. Contrib Nephrol. 1985;45:115–122. doi: 10.1159/000410454. [DOI] [PubMed] [Google Scholar]
- Atkins RC. Macrophages in renal injury. Am J Kidney Dis. 1998;31:xlv–xlvii. doi: 10.1016/s0272-6386(14)70003-4. [DOI] [PubMed] [Google Scholar]
- Holdsworth SR, Neale TJ, Wilson CB. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981;68:686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol. 2003;170:3254–3262. doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mahajan D, Tay YC, Bao S, Spicer T, Kairaitis L, Rangan GK, Harris D. Partial depletion of macrophages by ED7 reduces renal injury in adriamycin nephropathy. Nephrology (Carlton) 2005;10:470–477. doi: 10.1111/j.1440-1797.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M. Adoptive transfer of genetically modified macrophages elucidated TGF-beta-mediated ‘self-defense’ of the glomerulus against local action of macrophages. Nephrol Dial Transplant. 1999;14 Suppl 1:35–38. doi: 10.1093/ndt/14.suppl_1.35. [DOI] [PubMed] [Google Scholar]
- Diamond JR. Macrophages and progressive renal disease in experimental hydronephrosis. Am J Kidney Dis. 1995;26:133–140. doi: 10.1016/0272-6386(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- Lee VW, Wang Y, Qin X, Zheng G, Mahajan D, Coombes J, Rangan G, Alexander SI, Harris DC. Adriamycin nephropathy in severe combined immunodeficient (SCID) mice. Nephrol Dial Transplant. 2006;21:3293–3298. doi: 10.1093/ndt/gfl413. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC. DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int. 2005;67:2178–2186. doi: 10.1111/j.1523-1755.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- Zheng G, Wang Y, Xiang SH, Tay YC, Wu H, Watson D, Coombes J, Rangan GK, Alexander SI, Harris DC. DNA vaccination with CCL2 DNA modified by the addition of an adjuvant epitope protects against “nonimmune” toxic renal injury. J Am Soc Nephrol. 2006;17:465–474. doi: 10.1681/ASN.2005020164. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Atkins RC, Nikolic-Paterson DJ. Interferon-gamma augments acute macrophage-mediated renal injury via a glucocorticoid-sensitive mechanism. J Am Soc Nephrol. 2003;14:888–898. doi: 10.1097/01.asn.0000056604.13964.62. [DOI] [PubMed] [Google Scholar]
- Holdsworth SR, Neale TJ. Macrophage-induced glomerular injury. Cell transfer studies in passive autologous antiglomerular basement membrane antibody-initiated experimental glomerulonephritis. Lab Invest. 1984;51:172–180. [PubMed] [Google Scholar]
- Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol. 2005;167:27–37. doi: 10.1016/s0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth DC, Ainslie CV, Pearce WP, Finlay S, Clarke D, Anegon I, Rees AJ. Macrophages transfected with adenovirus to express IL-4 reduce inflammation in experimental glomerulonephritis. J Immunol. 2001;166:4728–4736. doi: 10.4049/jimmunol.166.7.4728. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Stewart KN, Brown PA, Anegon I, Chettibi S, Rees AJ, Kluth DC. Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol Ther. 2002;6:710–717. doi: 10.1006/mthe.2002.0802. [DOI] [PubMed] [Google Scholar]
- Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997–1004. doi: 10.1681/ASN.2004050367. [DOI] [PubMed] [Google Scholar]
- Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Stout RD. T cell-mediated cognate signaling of nitric oxide production by macrophages. Requirements for macrophage activation by plasma membranes isolated from T cells, Eur J Immunol. 1993;23:2916–2921. doi: 10.1002/eji.1830231128. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Kurosawa T, Matsumoto K, Mizuno-Horikawa Y, Okamoto M, Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J Clin Invest. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig LP, Stewart K, Rees AJ. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am J Pathol. 2000;156:295–301. doi: 10.1016/S0002-9440(10)64730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo AB, Davidson JM, Linton MF, Fazio S, Homma T, Yoshida H, Ichikawa I. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin Immunol. 2002;103:125–131. doi: 10.1006/clim.2001.5167. [DOI] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezumi Y, Hurst L, Atkins RC, Nikolic-Paterson DJ. Macrophage-mediated renal injury is dependent on signaling via the JNK pathway. J Am Soc Nephrol. 2004;15:1775–1784. doi: 10.1097/01.asn.0000131272.06958.de. [DOI] [PubMed] [Google Scholar]
- Minto AW, Erwig LP, Rees AJ. Heterogeneity of macrophage activation in anti-Thy-1.1 nephritis. Am J Pathol. 2003;163:2033–2041. doi: 10.1016/S0002-9440(10)63561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin CJ, Chen KH, Wu JC, Huang SH, Wang SM. Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett. 2006;580:3042–3050. doi: 10.1016/j.febslet.2006.04.049. [DOI] [PubMed] [Google Scholar]