Abstract
Atherosclerotic lesions contain T lymphocytes, which orchestrate adaptive immunity and regulate many innate immune pathways. This study examined the influence of Th1 and Th2 helper cell subsets on atherogenesis in two ApoE−/− mouse strains, C57BL/6 and BALB/c, which display opposite T-cell subset polarizations. ApoE−/− BL/6 mice showed predominant Th1-like immune responses on polyclonal stimulation of splenic CD4+ T cells and had IgG2a antibodies to oxidized low-density lipoprotein (a disease-related antigen) whereas ApoE−/− BALB/c mice displayed predominant Th2 responses by CD4+ T cells and an IgG1 isotype response toward oxidized low-density lipoprotein. ApoE−/− BL/6 and BALB/c mice consumed a high-cholesterol diet for 10, 16, and 24 weeks with equivalent cholesterolemic responses. The Th1-slanted BL/6 mice developed significantly more atherosclerosis in the aortic root and abdominal aorta at all time points compared with BALB/c mice, supporting a proatherogenic role for Th1 response. Progression of atherosclerosis was associated with increased levels of interleukin (IL)-6 in mouse serum and CD4+ T-cell culture supernatants and increased levels of the acute-phase protein, serum amyloid A (SAA). Both IL-6 and SAA levels rose significantly in ApoE−/− BL/6 mice compared with BALB/c mice. The circulating cytokine milieu (IL-6) and acute phase reactants such as SAA may reflect alterations in the Th1/Th2 balance. The results presented here illustrate how genetically determined modifiers of both immune and inflammatory responses can modulate atherogenesis independently of lipid levels.
Inflammation contributes critically to all phases of atherosclerosis. Atherosclerotic lesions contain T lymphocytes, notably CD4+ T cells, which recognize protein antigens presented to them in a MHC class II-dependent manner. A variety of disease-related antigens such as oxidized low-density lipoprotein (oxLDL), heat-shock protein 60, and Chlamydial proteins may stimulate CD4+ T cells1,2,3 in the context of atherogenesis.
In response to the local milieu of cytokines, CD4+ T lymphocytes differentiate into a T helper (Th) 1 or Th2 lineage. Interleukin (IL)-12 fosters development of Th1 cells, whereas IL-10 promotes a Th2 lymphocyte response. The cytokines they produce functionally define activated T-helper cell subsets, eg, Th1 cells secrete IL-2, interferon (IFN)-γ, and lymphotoxin, whereas Th2 cells produce IL-4, IL-5, IL-10, and IL-13.4 Among the prototypical Th1 cytokines, IFN-γ has attracted particular interest, because it activates macrophages and enhances inflammatory cell recruitment by augmenting cytokine, chemokine, and adhesion molecule expression. This Th1 cytokine also amplifies immune responses in several ways, including increasing levels of MHC I and II molecules on antigen-presenting cells and endothelium.5,6,7 In contrast, IL-4, the prototypical Th2 cytokine, promotes synthesis of IgE and allergic responses. IL-4 can inhibit Th1 responses and reduce macrophage activation and IFN-γ expression.8
Either Th1 or Th2 cells can promote humoral immune responses to protein antigens, but they stimulate production of different immunoglobulin subtypes. Production of IgG1 antibodies depends on Th2-type CD4+ T cells, whereas induction of IgG2a requires Th1 cells.4 Previous studies demonstrated the predominance of Th1 lymphocytes and their mediators within atherosclerotic lesions.9,10,11,12 Th1-slanted immune responses characterize atherogenesis in mice lacking either IFN-γ receptors13 or the cytokine itself.14,15 Although atherosclerosis involves primarily a Th1 immune response, severe hypercholesterolemia induces a switch from Th1 to Th2 in ApoE−/− mice.16
We sought to examine the impact of a Th1 versus Th2 immune response on atherogenesis in the context of two ApoE−/− mouse strains, C57BL/6 and BALB/c. C57BL/6 mice produce predominantly Th1 helper cells, whereas BALB/c mice lean toward Th2-dominated immune responses.17,18 This study tested the hypothesis that genetically determined differences in T-helper cell subsets influence the extent and quality of diet-induced advanced atherosclerosis, as well as cell-mediated and humoral immune responses to antigens associated with plaques. The results support the importance of immune and inflammatory modulators of atherogenesis independent of lipid levels, and further indicate that Th1/Th2 balance can influence the levels of circulating biomarkers of disease activity.
Materials and Methods
Mice
Male ApoE−/− mice, backcrossed 10 times onto a C57BL/6 background, were generated from an in-house breeding stock that was originally obtained from the Jackson Laboratory (Bar Harbor, ME). Dr. Jan Breslow (Rockefeller University, New York, NY) kindly provided the ApoE−/− BALB/c mice. ApoE−/− mice were maintained on an inbred 129/SV-ter background and subsequently bred onto the Balb/cByJ background using a speed congenic method.19 All mice were housed and bred in accordance with the institutional guidelines of Brigham and Women’s Hospital and Harvard Medical School.
Study Protocol
Beginning at 6 to 8 weeks of age, male ApoE−/− BL/6 and ApoE−/− BALB/c mice consumed a semipurified diet (product D12108; Research Diets, New Brunswick, NJ) containing 40% kcal lipid, 1.25% cholesterol, 0% cholate. After 10 (n = 12 per group), 16 (n = 16 per group), and 24 weeks (n = 15 ApoE−/− BALB/c, n = 9 ApoE−/− C57BL/6) mice were euthanized. Blood was collected by puncturing the right ventricle, and the arterial tree was perfused with phosphate-buffered saline (PBS). Perfused aortas were dissected from the aortic valve to the iliac bifurcation. The aortic roots were frozen in OCT (OCT compound; Tissue Tek, Torrance, CA), and the thoracic and abdominal aortas were fixed in 10% buffered formalin, as described previously.20,21
Serum Cholesterol Analysis
Serum total cholesterol concentrations were assayed by enzymatic methods using reagents from Wako Chemicals (Richmond, VA), adapted for microtiter plate assay.
In Vitro Assay of CD4+ Cytokine Secretion
The spleens were removed from ApoE−/− BL/6 and ApoE−/− BALB/c mice after 10 (n = 9 per group), 16 (n = 11 ApoE−/− BALB/c and n = 10 ApoE−/− C57BL/6), and 24 weeks on an atherogenic diet (n = 9 per group). CD4+ T cells were isolated by use of anti-CD4 magnetic beads on magnetic columns (Dynal, Great Neck, NY). The cells were stimulated in 96-well plates (5 × 105/well) with PMA (10 ng/ml; Sigma Aldrich, St. Louis, MO) and ionomycin (500 ng/ml, Sigma Aldrich). Culture supernatants were removed at 72 hours and analyzed using a Th1/Th2 cytokine cytometric bead array kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Splenic T-cell isolations that did not result in sufficient cell numbers were excluded from the analysis.
Serum Ig Analysis
Serum immunoglobulin subtypes and specific antibodies of different subclasses were quantified by enzyme-linked immunosorbent assay (ELISA). Total IgG2a levels in mouse serum were measured using reagents from BD Pharmingen (San Diego, CA). Total serum IgG1 concentrations were analyzed using a kit supplied by Alpha Diagnostic International (San Antonio, TX).
Serum IgG isotypes of oxidized LDL antibodies were determined by coating polystyrene microtiter plates overnight at 4°C with 50 μl of 10 μg/ml of human native LDL or copper oxidized LDL (Biomedical Technologies, Stoughton, MA) in PBS. Plates were blocked with 2% bovine serum albumin/PBS for 2 hours. Mouse serum (50 μl) at a dilution of 1:8 was added for 2 hours at room temperature. Plates were then incubated with biotinylated anti-mouse IgG1 or IgG2a (1:250, BD Pharmingen) for 1 hour at room temperature, followed by streptavidin-horseradish peroxidase (1:250) and the substrate TMB (BD Pharmingen). Plates were read at 650 nm. Specific anti-oxLDL antibody levels were determined by subtracting the optical density of the wells containing native LDL from those coated with oxLDL.
Immunohistochemistry
Serial cryostat sections (6 μm) of the aortic root were fixed in acetone, air-dried, and stained by the avidin-biotin-peroxidase method with the respective molecule-specific antibodies, as previously described.20,21 Antibodies used included rat anti-mouse Mac-3 (1:1000), rat anti-mouse CD4 (1:110), rat anti-mouse I-A/I-E antibody (murine MHC class II, 1:200; all Pharmingen, San Diego, CA), and monoclonal anti-human smooth muscle α-actin (1:100; DAKO, Carpinteria, CA).
Picrosirius Red Staining for Type I Collagen
Formalin-fixed frozen sections were incubated (4 hours) in a freshly prepared 0.1% solution of picrosirius red F3BA (Picrosirius Inc., Warrington, PA) in saturated aqueous picric acid. After rinsing twice in 0.01 N of HCl and distilled water, sections were briefly dehydrated in 70% ethanol and mounted in Permount (Vector Laboratories, Burlingame, CA). Picrosirius red staining was analyzed by polarization microscopy.
Oil Red O Staining for Lipids
Deposition of lipids in en face preparations of abdominal aortas and frozen sections of the aortic arch (fixed with 10% formalin) was determined by oil red O staining as previously described.20,21
Tissue Analysis
To quantify the extent and composition of the aortic lesions, cross-sections of the aortic root were analyzed microscopically in all mice, as described previously.20,21 To evaluate intimal lesion size within the aortic root, frozen sections were incubated with oil red O (0.5% in glycerol). Lesion areas were analyzed in cross-sections obtained at the level of all three leaflets of the aortic valve, immediately proximal to the right coronary artery ostium. The percentage of the total lesion area in the root stained for macrophages, T cells, smooth muscle cells, and lipids was determined via computer-assisted image analysis (ImagePro Plus Software; Media Cybernetics, Silver Spring, MD). The percentage surface area occupied by oil red O-stained lesions in the thoracic and abdominal aorta viewed en face was also measured using computer-assisted image analysis.
Analysis of IL-6 and Serum Amyloid A (SAA) Levels
Mouse serum and T-cell culture supernatants were analyzed for IL-6 by sandwich ELISA using reagents from R&D Systems (Minneapolis, MN). SAA levels were determined by ELISA using a kit supplied by Biosource (Camarillo, CA).
Statistical Analysis
Results are expressed as mean ± SEM. Significance of differences between two means was assessed by Student’s t-test. Significant relationships were analyzed by multiple linear regression analyses. Analyses were considered significant if P ≤ 0.05.
Results
Serum Cholesterol Analysis
This study used two mouse strains: C57BL/6, considered to exhibit a Th1 bias, and BALB/c mice, which tend to develop a Th2-predominant immune response. Each strain was crossbred with atherosclerosis-susceptible ApoE−/− mice. To investigate the role of Th1/Th2 balance at different stages of atherogenesis, ApoE−/− BL/6 and BALB/c mice consumed a cholesterol-rich diet for three different periods of time: 10, 16, and 24 weeks. Total cholesterol levels did not differ significantly between the two mouse strains (Table 1).
Table 1.
Plasma Cholesterol Levels
| Total cholesterol at | ApoE−/− BALB/c | ApoE−/− C57BL/6 |
|---|---|---|
| 10 weeks of atherogenic diet | 1208.1 ± 45.2 | 1221.0 ± 43.7 |
| 16 weeks of atherogenic diet | 1264.0 ± 46.2 | 1226.0 ± 50.5 |
| 24 weeks of atherogenic diet | 972.7 ± 44.2 | 986.1 ± 82.3 |
Values are total cholesterol (mg/dl) and represent mean ± SEM.
In Vitro CD4+ Cytokine Production
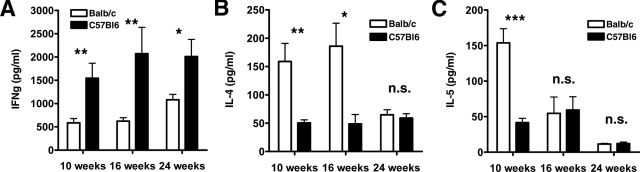
To explore the pattern of cytokine production in the two different mouse strains, we isolated CD4+ T cells from spleen and cultured them with PMA and ionomycin (72 hours). On stimulation, T cells from ApoE−/− BALB/c mice produced substantial IL-4, a Th2-type cytokine, but only low levels of IFN-γ, a Th1-type cytokine. In contrast, T cells from ApoE−/− BL/6 mice secreted a Th1 profile of cytokines with high IFN-γ and low IL-4 levels (Figure 1, A and B). CD4+ lymphocytes from ApoE−/− BALB/c mice produced significantly more IL-5 at the 10-week time point (153.80 ± 19.90 pg/ml versus 41.66 ± 6.02 pg/ml, P < 0.001), but not at the 16- and 24-week time points (Figure 1C). Production of TNF-α and IL-2 did not differ significantly between the two mouse strains (data not shown).
Figure 1.
Differences in cytokine production by CD4+ T cells from ApoE−/− BALB/c and ApoE−/− C57BL/6 mice. CD4+ T cells were isolated from mouse spleen after 10, 16, and 24 weeks on a high-cholesterol diet as described in the Materials and Methods. The cells were stimulated in culture with PMA and ionomycin. Culture supernatants were removed at 72 hours and analyzed by a cytometric bead array kit for IFN-γ (A), IL-4 (B), and IL-5 (C). Data represent mean ± SEM. P values ≤0.05 were considered significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; n.s., nonsignificant.
Serum Ig Analysis
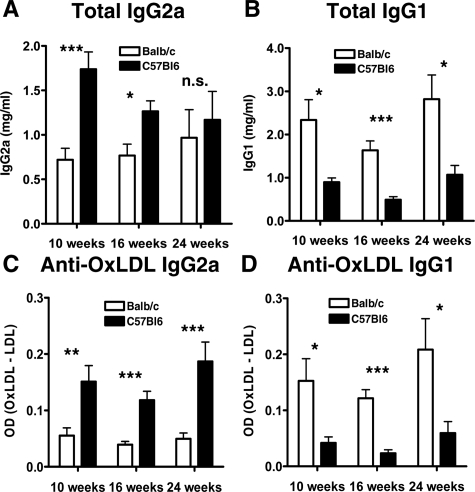
Th1 and Th2 cell subsets each stimulate production of different immunoglobulin isotypes. IgG2a is a Th1-dependent isotype and IgG1 a Th2-dependent isotype. We determined total IgG1 and IgG2a levels in serum of ApoE−/− BALB/c and BL/6 mice after 10, 16, and 24 weeks on an atherogenic diet. Indeed, we found significantly reduced total serum IgG2a in ApoE−/− BALB/c mice at 10 and 16 weeks (0.72 ± 0.13 mg/ml versus 1.74 ± 0.20 mg/ml at 10 weeks, P < 0.001; 0.77 ± 0.13 mg/ml versus 1.27 ± 0.12 mg/ml, P < 0.05 at 16 weeks; 0.97 ± 0.32 mg/ml versus 1.17 ± 0.32 mg/ml, nonsignificant, at 24 weeks; Figure 2A), indicating a suppressed Th1 immune response. Total IgG1 levels increased significantly in these mice (2.34 ± 0.47 mg/ml versus 0.90 ± 0.10 mg/ml, P < 0.05 at 10 weeks; 1.63 ± 0.22 mg/ml versus 0.49 ± 0.07 mg/ml, P < 0.001 at 16 weeks; 2.82 ± 0.56 mg/ml versus 1.07 ± 0.22 mg/ml, P < 0.05 at 24 weeks; Figure 2B).
Figure 2.
Total serum IgG2a (A) and IgG1 (B) antibodies and serum IgG2a (C) and IgG1 (D) antibodies to oxidized LDL in ApoE−/− BALB/c and ApoE−/− C57BL/6 mice at 10, 16, and 24 weeks of a high-cholesterol diet, measured by ELISA. Data in C and D were obtained by subtracting the optical density of the wells coated with native LDL from those coated with oxLDL. Data represent mean ± SEM. P values ≤0.05 were considered significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; n.s., nonsignificant.
We measured anti-oxidized LDL IgG subclasses to determine whether a significant Th1 or Th2 bias of the humoral immune response to this putative atherogenic antigen occurred in ApoE−/− BL/6 and ApoE−/− BALB/c mice. ApoE−/− BALB/c mice produced significantly lower levels of anti-oxLDL IgG2a antibodies at all three time points (Figure 2C), but had significantly elevated anti-oxLDL IgG1 levels compared to ApoE−/− BL/6 mice (Figure 2D), illustrating a diminished Th1 immune response toward a disease-related antigen in ApoE−/− BALB/c mice.
Atherosclerotic Lesion Formation in ApoE−/− C57BL/6 and ApoE−/− BALB/c Mice
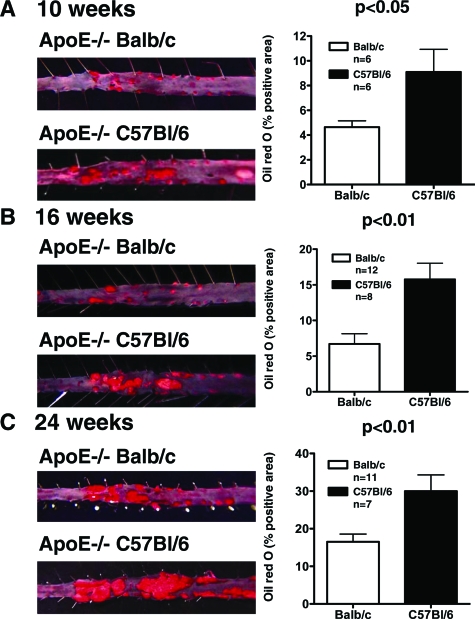
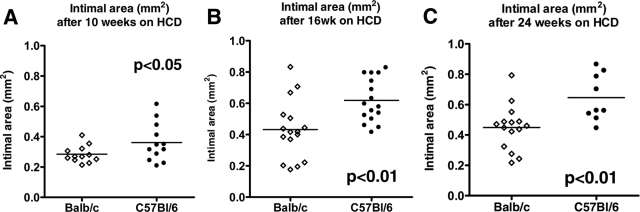
Analysis of lesion area in en face preparations of the descending aorta stained with oil red O showed significantly smaller lesions in ApoE−/− BALB/c mice at all three time points (4.64 ± 0.51 mm2 versus 9.10 ± 1.83 mm2, P < 0.05 at 10 weeks; 6.70 ± 1.44 mm2 versus 15.76 ± 2.26 mm2, P < 0.01 at 16 weeks; 16.51 ± 2.07 mm2 versus 29.97 ± 4.33 mm2, P < 0.01 at 24 weeks; Figure 3, A–C). Consistent with the observations on the descending aorta, the aortic roots from ApoE−/− BALB/c mice also showed a statistically significant reduction in plaque size after 10, 16, and 24 weeks on high-cholesterol diet compared to ApoE−/− BL/6 mice (0.28 ± 0.02 mm2 versus 0.36 ± 0.04 mm2, P < 0.05 at 10 weeks, n = 12; 0.43 ± 0.05 mm2 versus 0.62 ± 0.03 mm2, P < 0.01 at 16 weeks, n = 16; 0.45 ± 0.04 mm2 versus 0.65 ± 0.05 mm2, P < 0.01 at 24 weeks, n = 15 BALB/c, n = 9 BL/6; Figure 4, A–C). Multiple linear regression analysis showed a positive correlation between the amount of IFN-γ secreted by CD4+ T cells and intimal lesion size in the aortic roots of the respective mice (r = 0.43, P < 0.01). IL-4 concentrations correlated negatively with atherosclerotic plaque size (r = −0.31, P < 0.05).
Figure 3.
Atherosclerotic lesion area in the abdominal aorta of ApoE−/− BALB/c compared to ApoE−/− C57BL/6 mice after 10 (A), 16 (B), and 24 weeks (C) on atherogenic diet. Mouse abdominal aortas were isolated and stained for lipid deposition with oil red O. The left panels show representative specimens used to calculate the percentages of oil red O-positive areas shown in the bar graphs on the right.
Figure 4.
Reduced aortic atherosclerosis in ApoE−/− BALB/c mice compared to ApoE−/− C57BL/6 mice after 10 (A), 16 (B), and 24 (C) weeks on the atherogenic diet. Mouse aortic root cross-sections were stained with oil red O as described in the Materials and Methods. The intimal area was measured at the level of all three leaflets of the aortic valve using computer-assisted image analysis. P values ≤0.05 were considered significant.
Plaque Morphology in ApoE−/− C57BL/6 and ApoE−/− BALB/c Mice
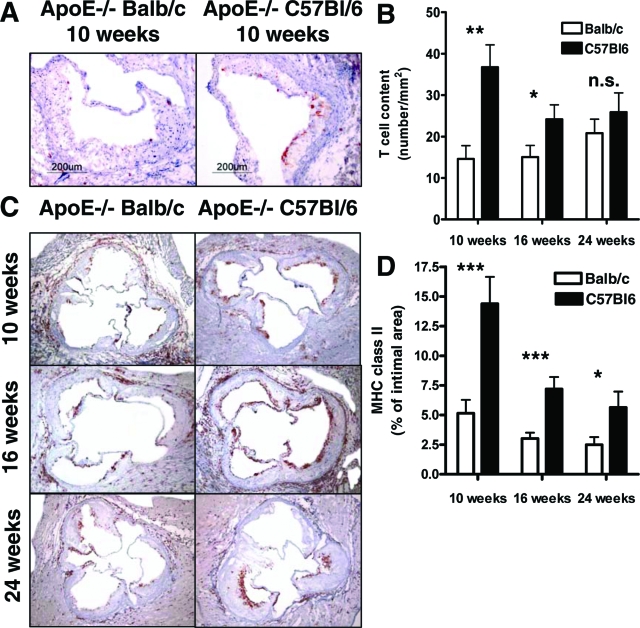
ApoE−/− BL/6 and ApoE−/− BALB/c mice developed atheroma-type atherosclerotic lesions with extracellular lipid cores in the aortic roots after 10 weeks on a high-cholesterol diet. The plaques at the 16- and 24-week time points showed greater extracellular matrix accumulation and cholesterol deposits. We analyzed the plaque composition in ApoE−/− BALB/c and BL/6 mice by measuring the relative content of macrophages, lipid deposition, smooth muscle cells, collagen, and CD4+ T cells in lesions of the aortic root. Data were normalized to lesion area in light of the significant differences in plaque size between the two mouse strains. The intimal area decreased significantly in cross-sections of the aortic root in ApoE−/− BALB/c mice compared to ApoE−/− BL/6 mice. CD4+ T-cell accumulation decreased markedly in the plaques of ApoE−/− BALB/c mice at the 10- and 16-week time points (14.61 ± 3.20 versus 36.73 ± 5.39, P < 0.01 at 10 weeks; 15.05 ± 2.80 versus 24.70 ± 3.40, P < 0.05 at 16 weeks, measured as number per mm2 intimal area; Figure 5, A and B). The CD4+ T-cell content tended to decrease in ApoE−/− BALB/c mice at the 24-week time point, but did not achieve statistical significance (20.81 ± 3.38 versus 25.89 ± 4.65). The percentage of plaque area composed of Mac-3-positive macrophages did not differ between ApoE−/− BL/6 and ApoE−/− BALB/c mice at all three time points (data not shown). CD4+ T-cell responses are, to a great degree, regulated by the class II histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells such as macrophages.22 MHC class II immunostaining served as an indicator of immune activation in sections of aortic roots. Expression of MHC class II relative to intimal area declined significantly in ApoE−/− BALB/c compared to ApoE−/− BL/6 mice at 10 (5.15 ± 1.13% versus 14.39 ± 2.27%, P = 0.001), 16 (3.02 ± 0.50% versus 7.19 ± 1.01%, P < 0.001), and 24 weeks (2.48 ± 0.66% versus 5.64 ± 1.34%, P < 0.05; Figure 5, C and D). Oil red O staining for lipid accumulation in the aortic root showed no differences between the two mouse strains. Additionally, we observed no significant differences in smooth muscle cells or collagen content (data not shown).
Figure 5.
Representative sections of mouse aortic roots immunostained for CD4 (T lymphocytes) after 10 weeks (A), and MHC class II after 10, 16, and 24 weeks on atherogenic diet (C). B: ApoE−/− BALB/c mice showed significantly fewer T lymphocytes within the intimal lesions at the 10- and 16-week time points compared to ApoE−/− C57BL/6 mice. D: Expression of MHC class II was decreased in ApoE−/− BALB/c mice at all three time points. Both number of T cells counted in the intima as well as MHC class II expression were normalized to the lesion area. Data represent mean ± SEM. P values ≤0.05 were considered significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; n.s., nonsignificant. Original magnifications: ×10 (A); ×4 (C).
Innate Immune Responses in ApoE−/− C57BL/6 and ApoE−/− BALB/c Mice
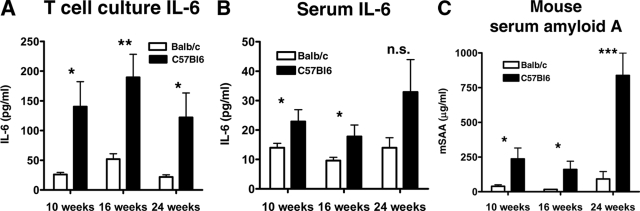
The proinflammatory cytokine IL-6 associates with early atherosclerotic lesion development,23 and drives the acute phase response, a crucial regulator of thrombosis and fibrinolysis. T-cell culture supernatants from ApoE−/− BALB/c and BL/6 mice were analyzed for IL-6 levels by ELISA. T cells from ApoE−/− BL/6 mice secreted significantly more IL-6 than T cells from ApoE−/− BALB/c mice (140.30 ± 41.99 pg/ml versus 26.14 ± 3.32 pg/ml, P < 0.05 at 10 weeks; 189.50 ± 38.80 pg/ml versus 51.85 ± 9.06 pg/ml, P < 0.01 at 16 weeks, 122.0 ± 41.14 pg/ml versus 21.95 ± 3.48 pg/ml, P < 0.05 at 24 weeks; Figure 6A). Additionally, ApoE−/− BL/6 mice had significantly higher serum levels of IL-6 compared to BALB/c mice at 10 and 16 weeks of high-cholesterol diet (22.87 ± 4.05 pg/ml versus 13.96 ± 1.53 pg/ml, P < 0.05 at 10 weeks; 17.78 ± 3.92 pg/ml versus 9.59 ± 1.10 pg/ml, P < 0.05 at 16 weeks; 32.90 ± 11.05 pg/ml versus 13.95 ± 3.44 pg/ml, nonsignificant, at 24 weeks; Figure 6B).
Figure 6.
Differences in IL-6 levels in CD4+ T-cell cultures (A) and serum (B) of ApoE−/− BALB/c and ApoE−/− C57BL/6 mice as well as mouse SAA levels (C) at 10, 16, and 24 weeks of atherogenic diet, measured by ELISA. Data represent mean ± SEM. P values ≤0.05 were considered significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; n.s., nonsignificant.
IL-6 induces the hepatic acute phase response. ApoE−/− BL/6 mice had significantly higher levels of SAA, a sentinel acute phase reactant in the mouse, after 10, 16, and 24 weeks on high-cholesterol diet compared to BALB/c mice, indicating a higher degree of systemic inflammation in these mice (236.0 ± 78.81 μg/ml versus 39.37 ± 10.47 μg/ml, P < 0.05 at 10 weeks; 164.80 ± 59.77 μg/ml versus 16.81 ± 1.21 μg/ml, P < 0.05 at 16 weeks; 837.30 ± 161.50 μg/ml versus 91.76 ± 53.14 μg/ml, P < 0.001 at 24 weeks; Figure 6C).
Discussion
The balance between Th1- and Th2-type immune responses may influence the progression of numerous inflammatory diseases, including atherosclerosis. Previous human and animal studies suggest involvement of Th1 immune responses in atherogenesis. Th1 cytokines such as IFN-γ and IL-12 localize in atherosclerotic lesions of both humans and mice,12,13,14,24,25 whereas Th2 cytokines associate with the development of abdominal aortic aneurysms.26,27 LDL-R−/− mice deficient in the Th1-specific transcription factor T-bet showed significantly reduced plaque development compared to LDL-R−/− controls.28
Various studies support either disease-preventing or -promoting effects of Th2 cytokines. Several findings support an antiatherogenic effect of Th2 responses: 1) atherosclerotic lesions can produce the Th2-related cytokine IL-10;29 2) endogenous IL-10 protects against atherosclerosis in several experimental preparations;30,31,32 and 3) mice, in which Th2 dominates over Th1 responses, showed reduced formation of early fatty streaks.33 However, deficiency in IL-4, the major cytokine directing Th2 differentiation, associates with decreased atherosclerotic lesion formation, indicating a proatherogenic role of Th2.34 A previous study that compared atherosclerotic lesion development in ApoE−/− IL-12−/− and ApoE−/− IL-4−/− mice with those simply deficient in ApoE, reported significantly reduced plaque size in both cytokine-deficient mouse strains, suggesting that both Th1 and Th2 cytokines play roles throughout the development of atherosclerosis in ApoE−/− mice.35
To investigate the effect of genetically programmed biases in T-cell differentiation on advanced atherosclerosis, we compared lesion development and disease-specific immune responses in two mouse strains, C57BL/6 and BALB/c, both ApoE−/−. Our observations revealed clear cut differences in multiple indices of Th1/Th2 bias relevant to atherosclerosis between these strains. ApoE−/− C57BL/6 mice exhibited deficiencies in Th2 responses with reduced production of IL-4 and IL-5, and decreased secretion of IgG1. In contrast, ApoE−/− BALB/c mice showed reduced IFN-γ, increased IL-4 and IL-5 production, as well as decreased secretion of IgG2a, indicating impaired Th1 responses. Measurements of the IgG isotypes to oxLDL provided a disease-relevant assessment of Th1 and Th2 bias. In ApoE−/− C57BL/6 mice, anti-oxLDL IgG1 titers decreased, indicating deficient Th2 responses, whereas ApoE−/− BALB/c animals produced low titers of anti-oxLDL IgG2a. Although hyperlipidemic animals consistently have autoantibodies to oxLDL, the impact of these antibodies on the atherosclerotic process remains unclear. Whereas titers of autoantibodies to selected forms of oxLDL associate with the severity of human carotid atherosclerosis,36 immunization of hypercholesterolemic rabbits and mice with different forms of modified LDL induced robust T-cell-dependent (TD) IgG titers and reduced the progression of atherogenesis, suggesting that components of the adaptive immune response to these antigens can also confer protection.37,38
Th1-deficient ApoE−/− BALB/c mice showed reduced atheroma formation in the aortic root and the abdominal aorta at all time points studied. In contrast to an earlier study33 the high-cholesterol diet fed was cholate-free. Cholate’s nonphysiological nature and potential toxicity have led to the speculation that atherosclerosis induced by cholate-containing diets may not accurately reflect the human disease process.39 We observed no differences in the relative content of macrophages, lipids, collagen, or smooth muscle cells between ApoE−/− BL/6 and BALB/c mice. However, ApoE−/− BL/6 mice displayed significantly higher levels of intimal CD4+ T cells at the 10- and 16-week time points, indicating an important role for T-cell-derived immune responses in these mice. The CD4+ T-cell content trended down in ApoE−/− BALB/c mice at the 24-week time point, although the decline did not reach statistical significance. Also, ApoE−/− BL/6 mice showed significantly more MHC class II expression in the aortic roots at all three time points compared to BALB/c mice, suggesting a higher degree of immune activation. MHC class II levels decreased from 10 to 24 weeks of high-cholesterol diet in both ApoE−/− BL/6 and BALB/c mice consistent with progression to more fibrotic, immunologically more quiescent plaques with time. Of note, the changes in lesion extent and character occurred in the face of similar serum cholesterol levels in ApoE−/− BL/6 and BALB/c mice.
Comparing atherosclerotic lesion development and systemic cytokine production, we found a positive correlation between IFN-γ secreted by splenic CD4+ T cells and plaque size, whereas IL-4 production correlated negatively with lesion size. These findings suggest that systemic production of IFN-γ modulates atherosclerosis extent and underlines the importance of Th1-mediated responses in atherosclerosis progression.
Atherosclerotic lesion formation in ApoE−/− BL/6 mice also associates with higher blood levels of IL-6, a proinflammatory cytokine of the innate immune system, indicating a systemic proinflammatory state in these mice. Increased levels of IL-6 in patients with unstable angina predict worse outcomes.40 In mice, IL-6 enhances fatty lesion development,22 and may increase lesion size through its ability to potentiate CD4+ T-cell differentiation.41 Several mediators, notably IL-6, induce the hepatic acute phase response. The acute phase proteins CRP and SAA associate strongly with increased atherosclerotic risk.42,43 Indeed, measurement of inflammatory biomarkers such as CRP have clinical utility for investigative and risk stratification purposes. Mice use SAA rather than CRP as a major acute phase reactant. A recent study reported a significant correlation between plasma SAA levels and the extent of atherosclerosis in LDL-R−/− mice.44 Similarly, we observed elevated SAA levels in ApoE−/− BL/6 mice that displayed advanced atherosclerotic lesion development compared to ApoE−/− BALB/c mice. Besides Th1/Th2 slant, BL/6 and BALB/c mouse strains have other differences that influence atherogenesis. ApoE−/− BALB/c mice exhibit lower levels of the circulating adhesion molecules sVCAM-1 and sP-selectin compared to ApoE−/− BL/6 mice.45 Several genes may account for differences in atherogenesis between ApoE−/− BL6 and BALB/c mouse strains. Ath1 was identified as a quantitative trait locus on mouse chromosome 1 that renders C57BL/6 mice susceptible and C3/H/He and BALB/c mice resistant to atherosclerosis.46 Tnfsf4, which encodes Ox40L, was recognized as a gene at the Ath1 locus that influences atherosclerosis susceptibility.47 The functions of Ox40L link to atherogenesis. Activation of the Ox40L-Ox40 pathway enhances the proliferation and survival of T cells, which play an important role in the development of atherosclerotic lesions. Taken in context with the other observational and gain and loss of function findings in the literature, the present results reinforce the importance of Th1/Th2 balance as a modulating influence during atherogenesis.
In conclusion, our study illustrates the effect of genetically programmed biases in Th1 and Th2 immune responses on plaque size at all stages of atherogenesis, independent of cholesterol levels. A Th1 immune response promotes atherosclerosis progression, whereas Th2 associates with decreased lesion size. Our results shed new insights into the mechanism of these T-cell influences on atherogenesis, regulating the humoral immune response to a disease-related antigen. Moreover, disease progression not only involves adaptive T-cell responses to atherosclerosis antigens, but also associates with increased cytokines and proteins of the innate immune system such as IL-6 and SAA. Hence, our study indicates that the altered balance in adaptive immune cells influences both humoral and adaptive immunity. The present observations not only support the relevance of Th1/Th2 balance for atherogenesis, but shed new light on the regulation of inflammatory biomarkers, increasingly used in clinical practice. Additionally, these results illustrate that genetically programmed immune and inflammatory factors can modulate vascular consequences of risk factors such as dyslipidemia.
Acknowledgments
We thank Lindsey McFarlane, Eugenia Shvartz, and Elissa Simon-Morrissey for their skillful technical assistance; and Dr. Jan Breslow for generously providing the apolipoprotein E-deficient mice on the BALB/c background for these studies.
Footnotes
Address reprint requests to Peter Libby, M.D., Brigham and Women’s Hospital, 77 Avenue Louis Pasteur, NRB 741, Boston, MA 02115. E-mail: plibby@rics.bwh.harvard.edu.
Supported by the National Heart, Lung, and Blood Institute (grant HL-34636 to P.L.) and the German Academic Exchange Service (to S.S.).
References
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer OJ, van der Wal AC, Houtkamp MA, Ossewaarde JM, Teeling P, Becker AE. Unstable atherosclerotic plaques contain T-cells that respond to Chlamydia pneumoniae. Cardiovasc Res. 2000;48:402–408. doi: 10.1016/s0008-6363(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Stemme S, Fager G, Hansson GK. MHC class II antigen expression in human vascular smooth muscle cells is induced by interferon-gamma and modulated by tumour necrosis factor and lymphotoxin. Immunology. 1990;69:243–249. [PMC free article] [PubMed] [Google Scholar]
- Warner SJ, Friedman GB, Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis. 1989;9:279–288. doi: 10.1161/01.atv.9.3.279. [DOI] [PubMed] [Google Scholar]
- Wedgwood JF, Hatam L, Bonagura VR. Effect of interferon-gamma and tumor necrosis factor on the expression of class I and class II major histocompatibility molecules by cultured human umbilical vein endothelial cells. Cell Immunol. 1988;111:1–9. doi: 10.1016/0008-8749(88)90046-9. [DOI] [PubMed] [Google Scholar]
- Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Libby P, Ross R. Cytokines and growth regulatory molecules. Fuster V, Ross R, Topol E, editors. New York: Lippincott-Raven,; Atherosclerosis and Coronary Artery Disease. 1996:pp 585–594. [Google Scholar]
- Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Mach F, Schönbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in apoE knockout mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apoE-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987;138:744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- Smith JD, James D, Dansky HM, Wittkowski KM, Moore KJ, Breslow JL. In silico quantitative trait locus map for atherosclerosis susceptibility in apolipoprotein E-deficient mice. Atheroscler Thromb Vasc Biol. 2003;23:117–122. doi: 10.1161/01.atv.0000047461.18902.80. [DOI] [PubMed] [Google Scholar]
- Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signaling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci USA. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–170. [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, Conze D, Hardin N, Tracy RP. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci USA. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Heymes C, Ohan J, Faggin E, Lesèche G, Tedgui A. Expression of interleukin-10 in human atherosclerotic plaques. Relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19:611–616. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- Von Der Thüsen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssönen K, Palinski W, Witztum JL. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Clinton SK, Iiyama K, Connelly PW, Libby P, Cybulsky MI. Hyperlipidemia and atherosclerotic lesion development in LDL receptor-deficient mice fed defined semipurified diets with and without cholate. Atheroscler Thromb Vasc Biol. 1999;19:1938–1944. doi: 10.1161/01.atv.19.8.1938. [DOI] [PubMed] [Google Scholar]
- Biasucci L, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;96:2099–2101. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. The association of C-reactive protein, serum amyloid A and fibrinogen with prevalent coronary heart disease: baseline findings of the PAIS project. Atherosclerosis. 2001;156:451–456. doi: 10.1016/s0021-9150(00)00681-x. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O'Brian KD, Chait A. Increase in serum amyloid A evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- Tian J, Pei H, James JC, Li Y, Matsumoto AH, Helm GA, Shi W. Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochem Biophys Res Commun. 2005;329:1102–1107. doi: 10.1016/j.bbrc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- Paigen B, Mitchell D, Holmes PA, Albee D. Genetic analysis of strains C57BL/6J and BALB/cJ for Ath-1, a gene determining atherosclerosis susceptibility in mice. Biochem Genet. 1987;25:881–892. doi: 10.1007/BF00502607. [DOI] [PubMed] [Google Scholar]
- Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samnegard A, Petros C, Rollins J, Bennet AM, Wiman B, de Faire U, Wennberg C, Olsson PG, Ishii N, Sugamura K, Hamsten A, Forsman-Semb K, Lagercrantz J, Paigen B. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]