Abstract
Duchenne muscular dystrophy (DMD) is a lethal, X-linked disorder associated with dystrophin deficiency that results in chronic inflammation, sarcolemma damage, and severe skeletal muscle degeneration. Recently, the use of l-arginine, the substrate of nitric oxide synthase (nNOS), has been proposed as a pharmacological treatment to attenuate the dystrophic pattern of DMD. However, little is known about signaling events that occur in dystrophic muscle with l-arginine treatment. Considering the implication of inflammation in dystrophic processes, we asked whether l-arginine inhibits inflammatory signaling cascades. We demonstrate that l-arginine decreases inflammation and enhances muscle regeneration in the mdx mouse model. Classic stimulatory signals, such as proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α, are significantly decreased in mdx mouse muscle, resulting in lower nuclear factor (NF)-κB levels and activity. NF-κB serves as a pivotal transcription factor with multiple levels of regulation; previous studies have shown perturbation of NF-κB signaling in both mdx and DMD muscle. Moreover, l-arginine decreases the activity of metalloproteinase (MMP)-2 and MMP-9, which are transcriptionally activated by NF-κB. We show that the inhibitory effect of l-arginine on the NF-κB/MMP cascade reduces β-dystroglycan cleavage and translocates utrophin and nNOS throughout the sarcolemma. Collectively, our results clarify the molecular events by which l-arginine promotes muscle membrane integrity in dystrophic muscle and suggest that NF-κB-related signaling cascades could be potential therapeutic targets for DMD management.
Duchenne muscular dystrophy (DMD) is the most common muscle wasting disease and it leads to early disability and death in young boys.1 The absence of dystrophin is a key factor in developing this disease.2 This protein is the central component of the dystrophin-glycoprotein complex that links the actin cytoskeleton to the extracellular matrix, thus maintaining muscle fiber membrane integrity.3 Although the primary genetic defect is known, the dystrophic process (eg, necrosis, exhaustible regeneration, and secondary fibrosis) has not been definitively identified. The mdx mouse, a genetically homologous DMD model, is frequently used to study the disease pathogenesis, despite relevant clinical and pathological differences.4,5 Compared to human disease, the murine model shows slower disease progression with repetitive degeneration regeneration cycles occurring between 2 and 12 weeks of age, particularly in the diaphragm that closely reflects the human pathology.6 On the other hand, utrophin (a fetal homolog of dystrophin) is overexpressed in mdx muscles whereas its level seems lower in DMD. These observations suggest that genetically or pharmacologically utrophin induction could be an interesting therapeutic strategy to compensate for dystrophin deficiency.
Several lines of evidence suggest the involvement of oxidative stress in the dystrophic process7 and underline the fact that dystrophic muscle cells are more susceptible to reactive oxygen intermediates. Oxidants are traditionally considered to exert their effects via a direct toxic action on target cells, but recent studies have suggested their contributory role in gene induction.8,9 Nuclear factor (NF)-κB is a pleiotropic transcription factor activated by low reactive oxygen intermediate levels and inhibited by antioxidants.10 This factor regulates the expression of a plethora of genes involved in the inflammatory, immune, and acute stress response. In fact, NF-κB, after proteosomal degradation of the inhibitory protein I-κ-B (IκB-α), translocates to the nucleus and binds target DNA elements in the promoter of genes expressing cytokine, chemokine, cell adhesion molecules, immunoreceptors, and inflammatory enzymes such as nitric oxide synthase (nNOS), matrix metalloproteinase (MMPs), and phospholipase A2.11 Several recent observations have suggested a possible role of NF-κB in the muscle-wasting process.12 NF-κB activity and level have been demonstrated to be increased in muscle of either DMD patients or mdx mice.13,14,15 Monici and colleagues16 observed increased immunoreactivity for NF-κB in the cytoplasm of all regenerating fibers and 20 to 40% of necrotic fibers in DMD as well as in inflammatory myopathies. NF-κB is activated in response to several secreted inflammatory molecules such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), whose levels have been found to be elevated in dystrophin-deficient muscle and other types of muscular dystrophies.17 On the other hand, NF-κB also regulates myogenesis, and systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury, suggesting a beneficial effect of NF-κB blockade on muscle repair.18 Moreover, reduced necrosis was observed in mdx muscle when TNF-α action was blocked with etanercept.19
l-arginine, the substrate of nNOS, was proposed as a powerful pharmacological tool by reducing the necrotic zone in mdx lower limbs.20 Nevertheless, the mechanistic effect of the l-arginine on dystrophic muscle and its impact on necrotic associated pathways has not yet been investigated. Here we assessed whether l-arginine treatment of 5-week-old mice could affect the NF-κB pathway and investigated the impact of this regulation on the protein stability of mdx sarcolemma. Our study showed that l-arginine treatment inhibited IL-1β, IL-6, and TNF-α secretion by macrophages and other inflammatory infiltrating cells in mdx diaphragm muscle, leading to down-regulation of the NF-κB/MMP cascade. This effect decreased 43-kDa β-dystroglycan cleavages into a ∼30-kDa form and significantly improved dystrophic patterns. Full-length β-dystroglycan anchors and stabilizes utrophin within a glycoprotein-complex located at the plasmatic membrane, thus relocating nNOS to the subsarcolemmal compartment.
Materials and Methods
Animals
Five-week-old male dystrophin-deficient (mdx) mice were purchased from Jackson Laboratory, Bar Harbor, ME (S/C57 BL/10 SC MAJAX SN 1801), and bred in our animal facilities. Mice were housed in plastic cages in a temperature-controlled environment with a 12-hour light/dark cycle and free access to food and water. The investigation complied with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The animals were euthanized by rapid cervical dislocation and experiments were carefully designed to minimize the number of animals and their suffering. Animals (mdx control, n = 7; and mdx treated, n = 7) were treated for 2 weeks with intraperitoneal injections. The control group was injected with (SS) physiological saline solution (mdx-SS). The treated group was injected with l-arginine, (Sigma, St. Louis, MO), 20 μl vol/g at a cc of 200 mg/kg (mdx-l-Arg). After beheading, the diaphragm was dissected into two parts. One part was immediately frozen in liquid nitrogen-cooled isopentane and stored at −80°C and the other part was analyzed immediately to determine the enzymatic activities.
Antibodies
Rabbit polyclonal antibodies that detect the macrophage α/CCL3 protein, IL-1β, IL-6, and TNF-α were purchased from Abcam, Cambridge, UK. Polyclonal antibodies against NF-κB, IκB, and BcL2 protein family (BAX and BcL2) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Polyclonal antibodies against β-dystroglycan (LG5) and utrophin (K7) were produced and determined to be specific after testing in competition with their corresponding peptides, as previously described.21 The polyclonal anti-actin antibody was from Sigma and the nNOS commercial antibody was from BD Transduction Laboratories (Lexington, KY). Pan- and phospho-monoclonal antibodies which, respectively, recognize the nonphosphorylated and phosphorylated forms of p38 MAPK, ERK1/2, and JNK1/2, were purchased from R&D Systems (Minneapolis, MN). All antibodies were tested according to the manufacturer’s recommendations using positive protein controls.
Morphometric Analysis
Ten-μm transverse cryostat sections of mdx and wild-type muscles were stained by hematoxylin and eosin (H&E). Morphometric analysis was performed on six cross-sections of each muscle. The histological parameters were evaluated and treated according to previously described suitable parameters.21,22,23 Stained sections were viewed under a Nikon optiphot-2 microscope and images were analyzed using Histolab program version 5-13-1 (license number 2497; Microvision Instruments, Evry, France). All diaphragm images were obtained under identical conditions and at the same magnification. The shape of each muscle fiber was accurately defined (on images ×400%) to obtain a schematic diagram of each H&E-stained diaphragm section, assigning black or white colors to fibers with peripheral or internalized nuclei, respectively. These schematic representations of each muscle section were later analyzed using Histolab software, as described above. On each schematic diagram, the percentage of nonmuscle area (colored in light gray) and black or white fibers were analyzed to obtain related surfaces, relative fiber percentages, and variance of Feret’s diameters, respectively. Data were averaged per ∼1500 muscle fibers from each dissected diaphragm obtained after l-arginine treatment of mdx mice and statistically compared using the Mann-Whitney test. Displayed data (mean ± SD) were normalized to the staining intensity of normal diaphragm muscle fibers from untreated mdx mice and comparisons were considered significant when *P < 0.05.
Immunofluorescence
Ten-μm unfixed cryostat sections were incubated with the primary antibody used at the appropriate dilution for 1 hour at room temperature or O/N at 4°C, according to the manufacturer’s recommendations (Santa Cruz Biotechnology, Santa Cruz, CA). After washing in phosphate-buffered saline solution, sections were incubated with a secondary antibody (Cy3-goat or fluorescein isothiocyanate-goat anti-rabbit IgG; Chemicon International, Temecula, CA). For negative controls, only the second antibody was applied.
Total Protein Extract and Western Blotting
Muscles (0.1 g) were homogenized in 150 μl of 5% sodium dodecyl sulfate (SDS) buffer (50 mmol/L Tris/HCl, pH 8.0, 10 mmol/L ethylenediaminetetraacetic acid, 5% SDS) supplemented with 1% trypsin inhibitor, 1% saponin, and 15 μg/ml of leupeptin. After centrifugation (10 minutes at 13,000 × g), the protein concentration was estimated in the supernatant using the BCA protein assay kit (Pierce, Rockford, IL). Protein homogenates recovered from the supernatant obtained from each sample were denatured for 5 minutes at 95°C in reducing buffer (50 μl of SDS buffer containing 5% SDS, 0.01% bromophenol blue, 10% glycerol, and 5% β-mercaptoethanol). Protein extracts were submitted, in duplicate, to SDS-polyacrylamide gel electrophoresis (3 to 10% or 5 to 15%) with prestained standard proteins (Bio-Rad, Hercules, CA) to achieve a more accurate molecular weight determination. The resulting gel was transferred onto a 0.2-μm nitrocellulose membrane using the transfer buffer (25 mmol/L Tris-HCl, pH 8.3, 192 mmol/L glycine, and 20% methanol). The membranes were stained with Ponceau S (0.005% in 1% acetic acid) to confirm that equal amounts of protein had been loaded and were blocked with Tris-buffer, 0.1% Tween 20 (TBST) containing 3% bovine serum albumin (w/v) for 1 hour at room temperature. All membranes were incubated with primary antibodies followed by several washings in Tris-buffer and 0.1% Tween 20 (TBST). The membranes were incubated with peroxidase-conjugated antibodies (Chemicon International) for 1 hour and washed several times with the washing buffer, as described above, and finally revealed by the enhanced chemiluminescence system according to the manufacturer’s protocol (Amersham, Little Chalfont, Buckinghamshire, UK). The protein signals were quantified by scanning densitometry using the National Institutes of Health (Bethesda, MD) program package. The results from each experimental group were expressed as integrated intensities relative to the control samples. Equal loading of proteins was assessed on stripped blots by immunodetection using the β-actin antibody.
Zymography
Zymography was performed as described by Heussen and Dowdle.24 Zymogram gel (5 to 15% polyacrylamide) was impregnated with gelatin (1 mg/ml). After electrophoresis, the gel was washed in 2.5% Triton X-100 solution at room temperature and incubated for 24 hours in a substrate buffer (50 mmol/L Tris-HCl, pH 8.0, 5 mmol/L CaCl2, 0.02% NaN3) at 37°C. MMPs are secreted in a latent form and require cleavage of a peptide from their NH2 terminus for activation. However, exposure of proenzymes of tissue extracts to SDS during the gel separation procedure leads to activation without proteolytic cleavage. The gel was stained in Coomassie Blue R250 for 1 hour and destained in water overnight. Gelatin-degrading enzymes were visualized as clear bands, indicating proteolysis of the substrate protein. The gel was treated in black/white and the MMP bands were quantified using the National Institutes of Health image analysis program.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from muscle with an SV total RNA isolation kit (Promega Corporation, Madison, WI) according to the manufacturer’s instructions. We used 0.1 μg of total RNA to generate first strand cDNA sequences with random hexanucleotide primers and the M-MLV reverse transcriptase (Invitrogen Corporation, Carlsbad, CA). Twelve μl of the RT reaction was subsequently used for each PCR reaction using the Taq polymerase (Qbiogene SA, Illkrich, France). Three PCR reactions were performed to amplify the mouse Up395 isoform using the primers (mUp-ex17/20-F and mUp-ex17/20-F) described previously.25,26 The mouse β-dystroglycan (β-DG) gene fragment was amplified using the following primers: Fdag1, 5′-GGAGGCTGTTCCCACCGTGGT-3′ and Rdag1, 5′-CTCTGCATTCTGTTCAACAGATCG-3′. The obtained PCR products were 383 bp (mUp395) and 474 bp (dag1), respectively. The PCR conditions included 94°C for 5 minutes, 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 1 minute, and a final extension of 72°C for 8 minutes. Experiments were performed in duplicate using the housekeeping gene GAPDH to normalize the expression level of Up395 and β-DG mRNA, as described previously.25,27 Negative controls were performed, with total RNA replaced by either RNAase-free water or reaction mixtures without RT (for DNA contaminations). PCR products were visualized on 1.5% agarose gel. One hundred-bp molecular mass markers (Promega) were used to estimate the molecular mass of the PCR products.
Statistical Analysis
The results are expressed as mean ± SD. Statistical analysis was performed using the unpaired Student’s test and multiple statistical comparisons between groups were performed by one-way analysis of variance followed by Bonferroni’s t-test posthoc correction to obtain a better evaluation of the intra- and intergroup variabilities and avoid false-positives using the Statview program (version 5.0; SAS Institute Inc., Cary, NC). Statistical significance was set at *P < 0.01.
Results
l-Arginine Improves Dystrophic Pattern and Promotes Regeneration
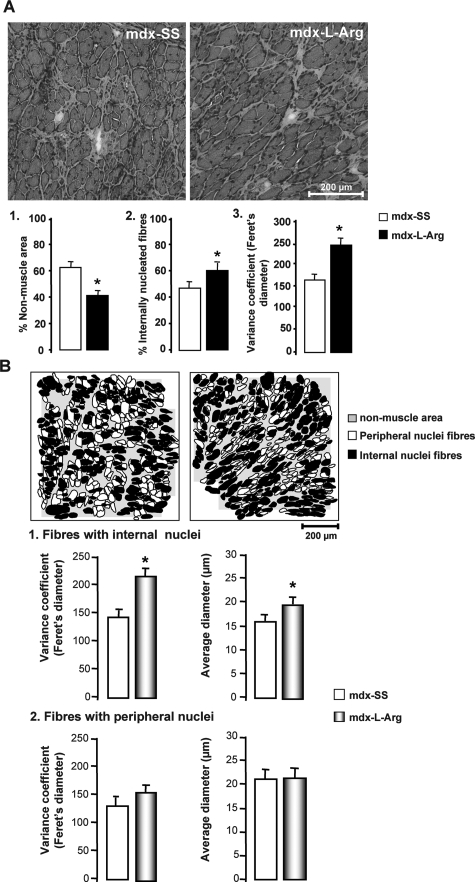
In accordance with previous studies,28,29 the morphological aspect of l-arginine-treated mdx diaphragm is significantly improved. The analyzed muscles share also a significant decrease in the number of infiltrated cells (macrophages and lymphocytes). Analysis of the H&E-stained section (Figure 1A) shows a decrease in the percentage (∼20%) of nonmuscle area in treated mdx diaphragm sections (Figure 1A, histogram 1, panel 1). However, there was an overall increase in the number of fibers with internalized nuclei (more than 20%), with a concomitant increase in the variance coefficient of the Feret’s diameter (Figure 1A, 2 and 3). To determine the specific impact of the treatment on histomorphological features of muscle fibers, we measured the variance coefficient for the Feret’s diameter and the average diameter in two different fiber populations: fibers with internal nuclei, ie, regenerating fibers (desmin-positive fibers, not shown) and fibers with peripheral nuclei (drawing boards; Figure 1B, 1 and 2, respectively). We noted in the first population (regenerating fibers) that both the Feret’s diameter variance coefficient and the average diameter were significantly increased after treatment. However, these two parameters were not affected in the second population (fibers with peripheral nuclei). This indicated, in accordance with the results presented above, that the treatment enhances the regenerative process in mdx muscle while also protecting new muscle fibers from degeneration or/and necrosis.
Figure 1.
A: H&E staining on 10-μm cryostat sections showing morphological features of mdx diaphragm. Histograms 1, 2, and 3 represents morphometric parameters analyzed in the l-arginine-treated (mdx-l-Arg) and control groups (mdx-SS): percent of nonmuscle area, percent of internally nucleated fibers, and the Feret’s diameter variance coefficient, respectively. B: The schematic drawing of cryostat section of mdx-SS and mdx-l-Arg after H&E staining and specific labeling of regenerative fibers with anti-desmin antibody (not shown). Nonmuscle areas are stained light gray; the fibers are white when nuclei are peripheral and black when internalized. Histograms 1 and 2 represent the Feret’s diameter variance coefficient and the average diameter of each of fiber type (with internal nuclei, 1; and with peripheral nuclei, 2) in both the treated (mdx-l-Arg) and control group (mdx-SS). Data are presented as mean ± SD. Statistical significance was *P < 0.01. Scale bar = 200 μm (B).
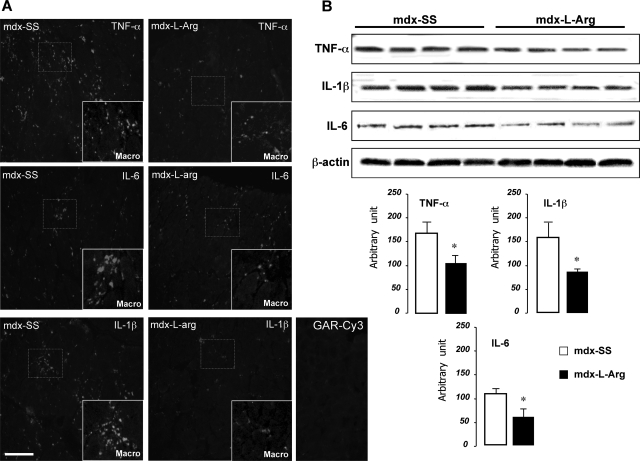
Figure 2.
A: TNF-a, IL-1β, and IL-6 staining of mdx diaphragm sections and co-localization with macrophages (inset). The enlarged views (macro) correspond to specific fields with high fluorescent staining. Comparison between untreated mdx diaphragms (left) and l-arginine-treated samples (right) are relatively similar when stained with TNF-α (first lane), IL-1β (middle lane), and IL-6 (last lane). B: Western blot detection of TNF-α, IL-1β, and IL-6, in mdx diaphragm samples from mdx-SS (control) and mdx-l-Arg groups. β-Actin revelation is shown at the bottom of the blots to indicate equivalent protein concentrations between all samples. The results are presented as a histogram (bottom) and allow comparison of treated (black) and untreated (white) mdx diaphragms. Data are presented as mean ± SD. Statistical significance was *P < 0.01.
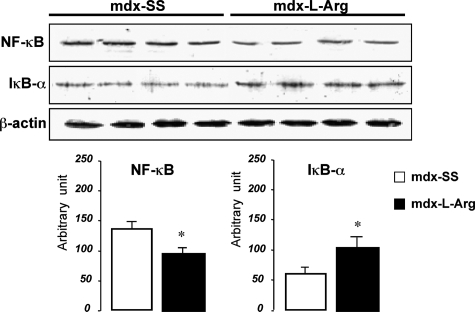
Figure 3.
Western blot detections of NF-κB and IκB-α from treated (mdx-l-Arg) and control (mdx-SS) mdx diaphragm. Immunoblot detection of β-actin on stripped nitrocellulose membranes was recorded to show the equivalent protein quantity between samples. Blots were analyzed and the results are presented as a histogram (bottom) and allow comparison of treated (black) and untreated (white) muscle extracts. Data are presented as mean ± SD. Statistical significance was *P < 0.01.
Decreased Proinflammatory Cytokine and Infiltrating Macrophage after Treatment
It was suggested that proinflammatory cytokines secreted by macrophages and other immune cells are involved in the necrotic phase in dystrophic muscle. It is also demonstrated that muscle regeneration occurs in response to injury and inflammation and that modulation of this inflammatory process would predictably decrease, rather than increase, the regenerative capacity of skeletal muscle. We thus focused on the impact of l-arginine on these proinflammatory cytokines. Co-staining of macrophage markers with TNF-α, IL-1β, and IL-6, showed a decrease in the levels of these inflammatory cytokines in mdx-l-Arg diaphragms (Figure 2A, and corresponding inset) with a concomitant reduction in the number of macrophages in necrotic zones. This result was confirmed by Western blot analyses, showing a decreased total level of TNF-α (∼62%), IL-1β (∼56%), and IL-6 (∼54%), as shown in Figure 2B (blots and associated histograms). We next sought to determine the mechanism by which l-arginine mediates its action on dystrophic myofibers.
l-Arginine Down-Regulates NF-κB Activity in Mdx Muscle
Several lines of evidence have suggested that NF-κB-mediated inflammation has an important role in mdx and DMD muscles. Our results showed that l-arginine treatment has an inhibitory effect on the total NF-κB level in mdx diaphragm and concomitant inhibition of IκB-α degradation (Figure 3). In line with previous reports,30 we suggest that the decreased level of inflammatory cytokines (TNF-α, IL-1β, and IL-6) after l-arginine treatment could directly affect the expression level and the activity of NF-κB in dystrophic muscle myofibers. Moreover, one of the mechanisms by which NF-κB signaling appears to contribute to dystrophy is by promoting chronic inflammation. Because of the wide range of NF-κB target genes, including cytokines and chemokines, we speculate that NF-κB-mediated transcription might serve as an amplification signal for a persistent immune response in dystrophic muscle, as supported by the noted reduction in the inflammatory response in dystrophic muscle after l-arginine administration. Besides immune cells, injured or necrotic muscle fibers could also act as a source of cytokines and chemokines involved in immune cell chemotaxis. Having gained insight into the role of l-arginine in NF-κB signaling in the immune cell compartment in dystrophin-deficient diaphragm, we have turned our attention to the impact of this treatment on modulating NF-κB signaling in mdx diaphragm myofibers.
NF-κB Down-Regulation Decreases MMP-2/MMP-9 Level in Mdx Muscle Fibers
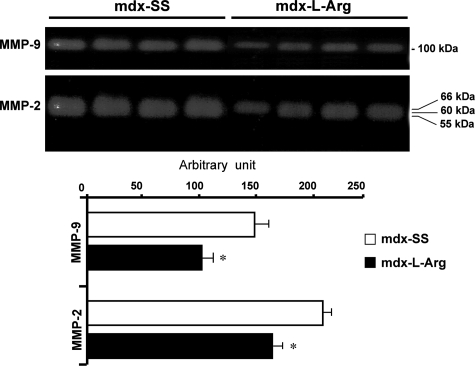
It was suggested that MMPs play a crucial role in many muscular dystrophies and that their activities mediate the processing of several sarcolemmal proteins. β-DG, a trans-membrane component belonging to the dystrophin-glycoprotein complex, anchors utrophin in the plasma membrane in dystrophin-deficient muscle. It has now been demonstrated that β-DG is a target of two active metalloproteinases in muscle, ie, MMP-2 and MMP-9. These proteinases cleave the extracellular domain of β-DG and disrupt its interaction with α-DG, which is the second component of the dystroglycan complex. Interestingly, it was shown that NF-κB activates the transcriptional level of these MMPs in several cell types after induced stress. In our case, the zymography experiment showed that l-arginine treatment decreased the MMP-2 and MMP-9 activities (Figure 4), which suggested that l-arginine could directly influence the transcriptional activity of NF-κB-dependent genes in dystrophic muscle fibers.
Figure 4.
Zymogram gel analyses of MMP-9 and MMP-2 activities. As indicated, control (mdx-Ss) and l-arginine-treated (mdx-l-Arg) samples were compared in relation to endogenous MMP-9 and MMP-2 abilities to degrade gelatin. The results are presented as histograms (bottom) and allow comparisons of treated (black) and untreated (white) muscle extracts. Data are presented as arbitrary units with mean ± SD. Statistical significance was *P < 0.01.
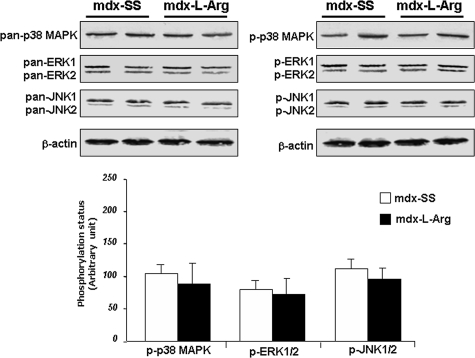
Impact of the Treatment on MAP Kinase Cascades
Several components of the MAP kinase cascade were differentially expressed and activated/phosphorylated in mdx diaphragm, in agreement with the hypothesis that different signaling pathways are distinctly activated depending on the severity of the dystrophic phenotype.31,32,33 We analyzed the impact of l-arginine on three components involved in the MAP kinase cascade (p38 MAP kinase, ERK1/2, and JNK1/2). No changes were observed in either core proteins (nonphosphorylated forms, pan−) or in activity/phosphorylation (p−) status (Figure 5, left and right, respectively, and corresponding histograms). The biological significance of these results could be partially explained by the fact that these kinases were tightly associated to other signaling cascades that were not affected by the treatment. The fact that most of these kinases are important in controlling programmed cell death led us to speculate that they are involved in apoptotic cascades. In addition, in line with this hypothesis, l-arginine did not affect the level of BAX and BcL2 (not shown).
Figure 5.
Western blot detections of p38MAPK, ERK1/2, and JNK1/2 pan and phosphorylated (p−) forms were analyzed using control (mdx-SS) and l-arginine-treated diaphragms (mdx-l-Arg). As shown in the histogram representation (bottom), no significant differences were noted between the two groups. Data are presented as arbitrary units with mean ± SD. Statistical significance was *P < 0.01.
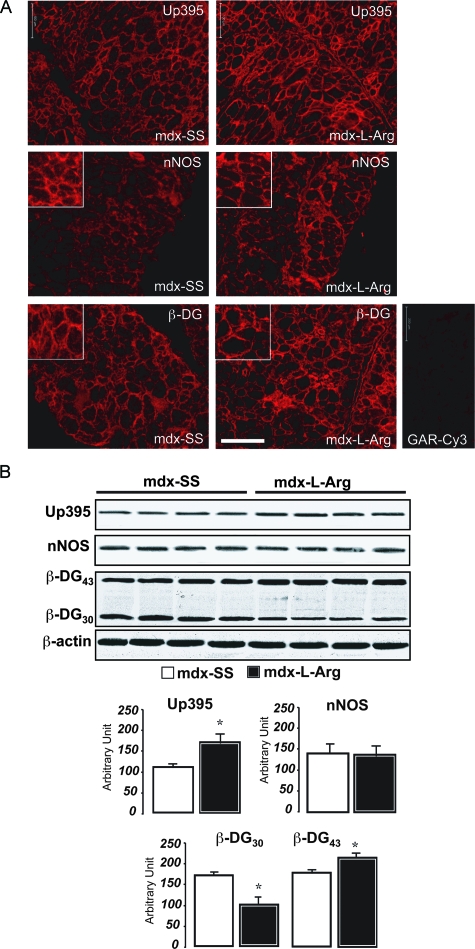
l-Arginine Stabilizes Utrophin/β-Dystroglycan Interactions and Recruits nNOS in the Sarcolemma
Previous studies have showed that l-arginine stimulates NO production, thus promoting nNOS re-localization in mdx sarcolemma. nNOS associates with the dystrophin-glycoprotein complex via the PDZ domains of syntrophins (α and β). A more recent study showed, by immunofluorescence staining, that l-arginine treatment promotes β-DG expression and localization in mdx sarcolemma.29 To confirm these observations, diaphragm sections from l-arginine-treated mdx mice (mdx-l-Arg) and control (mdx-SS) were stained with anti-utrophin, anti-nNOS, and anti-β-dystroglycan antibodies. In agreement with a previous report,29 utrophin was more localized in several fibers after the treatment (Figure 6A, top). nNOS staining was less diffuse in the cytoplasm and relocalized in the sarcolemma (Figure 6A, middle). Similarly, β-DG staining in mdx-l-Arg showed more sarcolemmal staining compared to the control (Figure 6A, bottom). In line with these observations, we investigated protein levels of utrophin, nNOS, and β-dystroglycan by Western blot. Our results showed that l-arginine treatment increased the level of utrophin in mdx diaphragm by ∼1.5- to 2-fold (Figure 6B), but the total nNOS level remained unchanged in the mdx-SS and the mdx-l-Arg groups. Interestingly, Western blot detection of β-DG showed a significantly lower level of the cleaved form of β-DG (β-DG30), with a concomitant increase in the full-length form (β-DG43) (Figure 6B, histograms at the bottom).
Figure 6.
A: Immunofluorescence labeling of utrophin, nNOS, and β-DG on control (left) and l-arginine-treated diaphragms (right) using specific antibodies directed against utrophin (top), nNOS (middle), and β-dystroglycan (β-DG, LG5 antibody; bottom). The inset corresponds to enlarged views focusing on labeling at the fiber membrane where there is more relocalized utrophin, β-DG, and nNOS in the subsarcolemmal compartment. B: Western blot detection of utrophin, nNOS, and β-DG on both control (mdx-SS) and l-arginine-treated diaphragms (mdx-l-Arg). Immunoblot detections of β-actin on stripped nitrocellulose membranes were recorded to show the equivalent protein quantity between samples. The results are presented in a histogram. Scale bar = 200 μm.
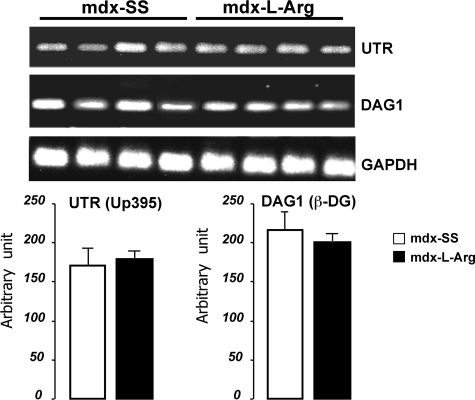
When assessing the level of utrophin and β-DG expression, we analyzed their respective mRNA levels to determine whether l-arginine treatment has a transcriptional effect in muscle cells. We performed semiquantitative RT-PCR using specific primers for cDNA encoding fragments of β-DG and full-length utrophin (Figure 7). Our data showed that there was no modification in the mRNA level in utrophin or β-DG after l-arginine treatment, suggesting that this treatment acts on utrophin and β-DG on a posttranslational level and/or by increasing the protein/protein affinity and relocalization in the subsarcolemmal compartment of mdx fibers.
Figure 7.
Semiquantitative RT-PCR of utrophin (UTR) and β-DG (DAG1) in control (mdx-SS) and treated samples (mdx-l-Arg). The relative levels of Up395 and β-DG transcripts did not differ between the two groups. One hundred-bp molecular mass markers (Promega) were used to estimate the molecular mass of the PCR products. Amplification of the ubiquitously expressed GAPDH gene was recorded as a positive control of the amplification level. Graphical representations show the PCR band quantification. Data are presented as mean ± SD. Statistical significance was *P < 0.01.
Discussion
The data presented here provides new evidence in support to the beneficial role of l-arginine in improving muscle pathogenesis in the mdx mouse model and indicates that NF-κB-related signaling cascades could be potential therapeutic targets for DMD management. Our results showed that l-arginine down-regulates the NF-κB level in mdx muscle and leads to decreased expression of several components of the NF-κB pathway in muscle cells. These proteins and/or cytokines cause substantial damage and promote inflammation and necrosis in mdx muscle. In accordance with previous studies, we also found that l-arginine regulates utrophin and β-dystroglycan levels and localizations in dystrophin-deficient muscle.29 Moreover, the current study shows that l-arginine decreases the activities of two muscle-specific metalloproteinases, MMP-2 and MMP-9. These metalloproteinases cleaved β-DG into a form of ∼30 kDa, which destabilized the dystroglycan interaction and its affinity with utrophin in the subsarcolemmal compartment.27
NF-κB Pathway: A Target of l-Arginine in Mdx Muscle
Our experiment shows that l-arginine administration has a negative effect of the on NF-κB level and associated signaling cascades in dystrophic muscle fibers. Since many reports have shown that NF-κB signaling is directly involved in the dystrophic process by repressing muscle regeneration, our data suggest that blocking the NF-κB function genetically34 or by l-arginine and/or other specific pharmacological products could promote the growth of new myofibers in response to degeneration. This is also consistent with recent observations showing that IKK deletion (the IκB kinase complex responsible for IκB degradation by the 26S proteasome unit) in muscle in response to acute injury can also lead to increased regeneration.35,36 However, Acharyya and colleagues,34 in a recent study, showed that endogenous IκBα phosphorylation and degradation were not associated with the dystrophic phenotype in mdx gastrocnemius muscle or in primary mdx myotube cultures. They also found no difference between nonphosphorylated protein level when comparing wild-type and mdx samples. Our data revealed significant activation of IκBα after l-arginine treatment, thus suggesting that this molecule could act specifically on IκBα stability in mdx diaphragm muscle. The mechanisms of each l-arginine act on IκBα, and the potential specific regulation of this kinase in different muscle requires further investigation.
Anti-Inflammatory Property of l-Arginine Promotes Muscle Repair
The fact that l-arginine is able to down-regulate cytokine secretion, ie, TNF-α, IL-1β, and IL-6, by macrophages in mdx muscle suggests that this molecule could interfere with inflammatory cascades activated during the dystrophic process. Furthermore, we noted a decrease in the number of these infiltrating macrophages after l-arginine administration, suggesting that this molecule has an inhibitory effect on immune cell proliferation in dystrophic muscle. The second possibility is that when the dystrophic pattern is improved after treatment and the necrotic fiber zone is much more limited, the infiltrating immune cells would consequently decrease. The hypothesis that there is interplay between cytokine/NF-κB signaling in immune cells and myofibers in dystrophic muscle is interesting since it is now accepted that these pathways could affect muscle cell growth and regeneration. In line with this, previous in vitro observations have showed that TNF-α inhibits myogenesis through repression of MyoD, a muscle-specific transcriptional factor.37 More recently, a new mechanism was suggested whereby the NF-κB signaling pathway regulates the early initiating steps of regenerative myogenesis by modulating the progenitor cell number in dystrophic muscles. Although, exactly how NF-κB mediates this action on the progenitor population, satellite cells, or myoblasts during regeneration remains to be further explored. Based on our results and in agreement with above data, it is tempting to speculate that NF-κB signaling cascade and inflammatory cytokine could potentially limit the activation of progenitor populations and interfere with their participation in repair. In this context, muscle metalloproteinases MMP-2 and MMP-9 are NF-κB targeted genes. When activated in dystrophic muscle, these MMPs could be interesting candidates by destabilizing satellite cell adhesion and/or myoblast fusion. The role of these MMPs in dystrophic muscle has not been substantially investigated, but the fact that they are able to cleave the extracellular part of β-DG and destabilize the dystroglycan complex suggests that these proteinases play an important role in maintaining the link between muscle cells and the extracellular matrix. The exact impact of l-arginine treatment on these metalloproteinases in mdx muscle and their exact role in satellite cell proliferation and myoblast differentiation should also be investigated in detail. However, because satellite cells are exhausted very early because of futile degeneration and regeneration cycles in muscle of DMD patients, any pharmacological agent that blocks NF-κB and maintains or replenishes the progenitor population, even partially, could possibly improve muscle pathology and function. Furthermore, if NF-κB inhibition in the early stage of dystrophy can reduce the inflammation burden, this might in turn slow initial exhaustion of the regenerative capacity in dystrophic muscles.34 Sustained inhibition of the NF-κB pathway by l-arginine could in turn allow dystrophic muscles to recuperate and reinitiate muscle repair in the later phase of the disease.
l-Arginine Modulates NO-Targeted Cascades in Dystrophic Process
It was demonstrated that l-arginine supplementation in cell culture increases cell proliferation and fusion,38 which is in line with recent reports highlighting the importance of NO in the control of myoblast fusion.39 NO is generated via a five-electron oxidation of a terminal guanidine nitrogen on l-arginine. The reaction is both oxygen- and NADPH-dependent and yields l-citrulline in addition to NO, in a 1:1 stoichiometric ratio. One of the most important regulators of nNOS activity is free cytosolic Ca2+, which stimulates nNOS through interaction with calmodulin. It is now accepted that in dystrophic fibers the high cytosolic level of Ca2+ disrupts several signaling pathways. This elevates the cytosolic Ca2+ concentrations required for calmodulin binding to nNOS, thereby activating the enzyme. When the Ca2+ concentration falls, it dissociates from calmodulin, which in turn dissociates from nNOS, thus acting as a switch that turns the enzyme on and off. l-Arginine administration seems to regulate this cascade but also Ca2+-dependent calpain degradation of nNOS, and finally muscle endurance and force (K. Hnia, A. Lacampagne, S. Matecki, and D. Mornet, personal communication, study submitted for publication).29 NO and its related oxidation products are also implicated in the pathophysiology and many inflammatory conditions and degenerative diseases.40 However, the pathology associated with dystrophin deficiency appears to be independent of nNOS activity41,42 but depends on its localization in the dystrophin-associated protein complex. However it appears that when using a nNOS transgene there is amelioration of the muscular dystrophy in mdx mice.43 Taken together, this suggests to expend our investigation in the future (study in progress) to measure the activity and level of nNOS and NO in different compartment of the mdx muscle fibers but also in DMD muscle cells after l-arginine treatment. This will be helpful to determine also the ratio/dose effect of this molecule in vitro. However using the dose indicated in this study we have not observed any animal premature death during the injections. Then, using physiological amounts of l-arginine, according to the best dose determined in healthy humans,44 it is assumed that this molecule does not have any toxic effect on humans.
Use of l-Arginine in Duchenne Muscular Dystrophies
Utrophin overexpression has been proposed as a therapeutic approach to rescue muscle dystrophy by miming the dystrophin role and function through dystrophin-deficient sarcolemma,45 and could provide a significant therapeutic advantage over dystrophin gene replacement.46 Because utrophin and β-dystroglycan levels and their stability were also increased after l-arginine administration, this could be more beneficial for assembling other associated proteins such as nNOS and syntrophins, while also improving membrane adhesion-associated proteins such as integrin α721 to avoid sarcolemma injury. Our data showed that l-arginine administration leads to moderately increased utrophin at a protein level in comparison to previous study.21 This could be explained by the fact that the mice used in this study were younger (5 weeks old). The expression level of utrophin increases during mdx mouse development in particular in diaphragm and seems to stabilize up to 6 months. This variation could also explain the difference between DMD and mdx muscle phenotype. However, utrophin is less expressed in DMD muscle patients compared to the mdx mouse model and the use of pharmacological molecules such as l-arginine could enhance utrophin level through the sarcolemma. In this case it is interesting to ask if the level of utrophin could be sufficient to improve muscle function in DMD patients. In this context, a recent published study shows that a dystrophin level as low as 30% is sufficient to avoid muscular dystrophy.47 Utrophin shares ∼80% similarity to dystrophin in the cysteine-rich domain (CR), which is responsible for the interaction with the β-dystroglycan.48 In line with these observations, the fact that l-arginine stabilizes the β-dystroglycan in sarcolemma suggests that the same up-regulation of utrophin as after the treatment could provide more efficient utrophin/dystroglycan stability in mdx sarcolemma. Moreover, we and others49,50 have demonstrated the important role of CR domain and particularly the ZZ motif of the human dystrophin/utrophin CR in anchoring β-dystroglycan. This domain contains several amino acids that are mutated in DMD and BMD patients with severe and moderate phenotypes. The major point mutations observed in this part of the dystrophin protein allows a disruption of the interaction with the β-dystroglycan. Utrophin shares a strong similarity in these domains with dystrophin and could link β-dystroglycan similarly. Accordantly, the stability of link between utrophin and β-dystroglycan (as demonstrated by the l-arginine treatment) is one of the most important steps to reorganize dystrophin-associated protein complex in sarcolemma and rescue muscle function in a large spectrum of the DMD phenotypes.
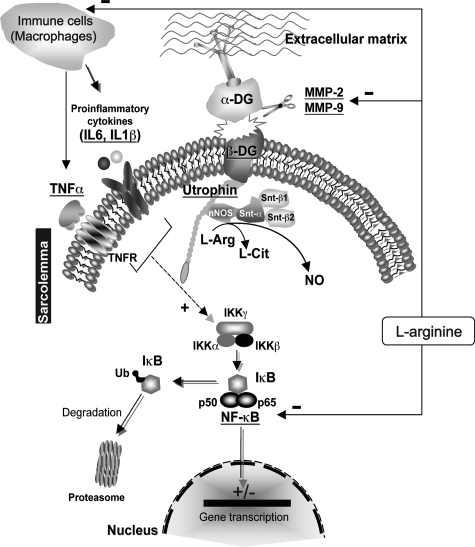
In conclusion, this study demonstrated the involvement of the NF-κB pathway in response to l-arginine treatment on dystrophic muscle (Figure 8, hypothetical schema). Because NF-κB regulation maintains a delicate balance between cell survival and apoptosis and is very important in host defense mechanisms, the specificity and duration of the NF-κB-inhibiting agent is highly relevant for designing pharmacological therapies against chronic diseases. Although it has been shown that long-term inhibition of NF-κB is associated with a vulnerability to bacterial infections and mounting immune response, it is noteworthy that moderate inhibition of NF-κB by l-arginine or other strategies based the cell-permeable NEMO-binding domain engineered from the C terminus of IKKβ and shown to function as a specific IKΚ inhibitor, could be more efficient for improving the dystrophic pattern without blocking basal NF-κB activity.
Figure 8.
Hypothetical scheme illustrating the potential mechanisms by which l-arginine acts on dystrophic muscle. The activation of NF-κB is thought to be part of a stress response because it is activated by a variety of stimuli that include cytokines, such as TNF-α, IL-1β, and IL-6. The stimulus and the mechanism of activation involve overlapping and nonoverlapping steps. The inactive form of NF-κB is sequestered in the cytoplasm, bound by members of the I-κB family of inhibitor proteins. The activity of NF-κB is regulated by inhibitory proteins, which include IKK-α, IKK-β, and IKK-γ. The IKK complex phosphorylates IKK-β, which leads to ubiquitination and then induces the degradation of IKK-α by the proteasome, resulting in the translocation of NF-κB to the nucleus. In the nucleus, NF-κB binds to a specific consensus sequence and positively regulates the transcription of several genes involved in immune and inflammatory responses (cytokines, MMPs). l-Arginine could act on these cascades that lead to decreasing the active cytoplasmic NF-κB level and in consequently its transcriptional activity. After l-arginine administration, nNOS is more targeted to the sarcolemma via syntrophins (Snt-α1, Snt-β1, Snt-β2). l-Arginine is converted via nNOS into a higher amount of NO production, thus reducing oxidative stress and inflammatory responses leading to decreased NF-κB level and activity. This acts negatively on MMP9 and MMP2 resulting in a stabilization of the 43-kDa form of β-DG to anchor utrophin, which promotes a better membrane integrity.
Footnotes
Address reprint requests to Dominique Mornet, INSERM ERI 25 Muscle et Pathologies, CHU A. de Villeneuve, Université de Montpellier1, EA 4202, 34295 Montpellier Cedex 5, France. E-mail: dominique.mornet@univ-montp.fr.
Supported by the “Association Française contre les Myopathies,” INSERM, and the Centre National de la Recherche Scientifique.
S.M. and D.M. contributed equally in this study.
References
- Deconinck N, Dan B. Pathophysiology of Duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007;36:1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Koenig M, Hoffman PE, Bertelson JC, Monaco PA, Feener C, Kunkel ML. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti MJ, Leveille JC, Slaughter AC, Sernett WS, Campbell PK. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller GW, Wight AP, Moore JK. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook SA, Barnard AE, Darlison GM, Barnard JP. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney LH, Shrager BJ, Maguire CH, Panettieri AR, Petrof B, Narusawa M, Leferovich MJ, Sladky TJ, Kelly MA. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Tidball GJ, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- Yang XY, Muqit MM, Latchman SD. Induction of parkin expression in the presence of oxidative stress. Eur J Neurosci. 2006;24:1366–1372. doi: 10.1111/j.1460-9568.2006.04998.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Oumi S, Nagasawa T, Nishizawa N. Oxidative stress induces phosphoenolpyruvate carboxykinase expression in H4IIE cells. Biosci Biotechnol Biochem. 2006;70:2191–2198. doi: 10.1271/bbb.60135. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier RA. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2. NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Kramer FH, Goodyear JL. Exercise, MAPK, and NF-{kappa}B signaling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici CM, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry BD, Boriek MA. Mechanical stretch activates nuclear factor-kappaB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma. FASEB J. 2003;17:1800–1811. doi: 10.1096/fj.02-1148com. [DOI] [PubMed] [Google Scholar]
- Kumar A, Boriek MA. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- Monici CM, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- Stuerenburg HJ, Jung R, Schoser GB. Age effects on interleukin-6 and interleukin-1beta responses to endurance exercise in patients with neuromuscular diseases. Arch Gerontol Geriatr. 1999;29:21–27. doi: 10.1016/s0167-4943(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Durham JW, Arbogast S, Gerken E, Li PY, Reid BM. Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34:298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- Hodgetts S, Radley H, Davies M, Grounds DM. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Archer DJ, Vargas CC, Anderson EJ. Persistent and improved functional gain in mdx dystrophic mice after treatment with L-arginine and deflazacort. FASEB J. 2006;20:738–740. doi: 10.1096/fj.05-4821fje. [DOI] [PubMed] [Google Scholar]
- Chazalette D, Hnia K, Rivier F, Hugon G, Mornet D. Alpha7B integrin changes in mdx mouse muscles after L-arginine administration. FEBS Lett. 2005;579:1079–1084. doi: 10.1016/j.febslet.2004.12.081. [DOI] [PubMed] [Google Scholar]
- De Luca A, Nico B, Liantonio A, Didonna PM, Fraysse B, Pierno S, Burdi R, Mangieri D, Rolland FJ, Camerino C, Zallone A, Confalonieri P, Andreetta F, Arnoldi E, Courdier-Fruh I, Magyar PJ, Frigeri A, Pisoni M, Svelto M, Camerino CD. A multidisciplinary evaluation of the effectiveness of cyclosporine A in dystrophic mdx mice. Am J Pathol. 2005;166:477–489. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar PJ. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004;14:675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle BE. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hnia K, Hugon G, Masmoudi A, Mercier J, Rivier F, Mornet D. Effect of beta-dystroglycan processing on utrophin/Dp116 anchorage in normal and mdx mouse Schwann cell membrane. Neuroscience. 2006;141:607–620. doi: 10.1016/j.neuroscience.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Putt W, Jimenez C, Edwards YH. Up71 and up140, two novel transcripts of utrophin that are homologues of short forms of dystrophin. Hum Mol Genet. 1999;8:1271–1278. doi: 10.1093/hmg/8.7.1271. [DOI] [PubMed] [Google Scholar]
- Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am J Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubourt E, Voisin V, Fossier P, Baux G, Israel M, Porte DLS. The NO way to increase muscular utrophin expression? C R Acad Sci III. 2000;323:735–740. doi: 10.1016/s0764-4469(00)01219-1. [DOI] [PubMed] [Google Scholar]
- Voisin V, Sebrie C, Matecki S, Yu H, Gillet B, Ramonatxo M, Israel M, Porte DLS. L-arginine improves dystrophic phenotype in mdx mice. Neurobiol Dis. 2005;20:123–130. doi: 10.1016/j.nbd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kabouridis SP, Hasan M, Newson J, Gilroy WD, Lawrence T. Inhibition of NF-kappa B activity by a membrane-transducing mutant of I kappa B alpha. J Immunol. 2002;169:2587–2593. doi: 10.4049/jimmunol.169.5.2587. [DOI] [PubMed] [Google Scholar]
- Lang MJ, Esser AK, Dupont-Versteegden EE. Altered activity of signaling pathways in diaphragm and tibialis anterior muscle of dystrophic mice. Exp Biol Med (Maywood) 2004;229:503–511. doi: 10.1177/153537020422900608. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek MA, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid BM, Boriek MA. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Villalta AS, Bakkar N, Bupha-Intr T, Janssen MP, Carathers M, Li WZ, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner LK, Rafael-Fortney AJ, Karin M, Tidball GJ, Baldwin SA, Guttridge CD. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé BS, Rudnicki AM. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Guttridge CD, Mayo WM, Madrid VL, Wang YC, Baldwin SA., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Long HJ, Lira AV, Soltow AQ, Betters LJ, Sellman EJ, Criswell SD. Arginine supplementation induces myoblast fusion via augmentation of nitric oxide production. J Muscle Res Cell Motil. 2006;27:577–584. doi: 10.1007/s10974-006-9078-1. [DOI] [PubMed] [Google Scholar]
- Pisconti A, Brunelli S, Padova DM, Palma DC, Deponti D, Baesso S, Sartorelli V, Cossu G, Clementi E. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Buchwalow BI, Minin AE, Muller UF, Lewin G, Samoilova EV, Schmitz W, Wellner M, Hasselblatt M, Punkt K, Muller-Werdan U, Demus U, Slezak J, Koehler G, Boecker W. Nitric oxide synthase in muscular dystrophies: a re-evaluation. Acta Neuropathol (Berl) 2006;111:579–588. doi: 10.1007/s00401-006-0069-5. [DOI] [PubMed] [Google Scholar]
- Crosbie HR, Straub V, Yun YH, Lee CJ, Rafael AJ, Chamberlain SJ, Dawson LV, Dawson MT, Campbell PK. Mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet. 1998;7:823–829. doi: 10.1093/hmg/7.5.823. [DOI] [PubMed] [Google Scholar]
- Zhuang W, Eby CJ, Cheong M, Mohapatra KP, Bredt SD, Disatnik HM, Rando AT. The susceptibility of muscle cells to oxidative stress is independent of nitric oxide synthase expression. Muscle Nerve. 2001;24:502–511. doi: 10.1002/mus.1033. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer JM, Tidball GJ. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti F, Huneau JF, Szezepanski I, Petzke KJ, Aggoun Y, Tome D, Bonnet D. Meal amino acids with varied levels of arginine do not affect postprandial vascular endothelial function in healthy young men. J Nutr. 2007;137:1383–1389. doi: 10.1093/jn/137.6.1383. [DOI] [PubMed] [Google Scholar]
- Gillis MJ. An attempt of gene therapy in Duchenne muscular dystrophy: overexpression of utrophin in transgenic mdx mice. Acta Neurol Belg. 2000;100:146–150. [PubMed] [Google Scholar]
- Ebihara S, Guibinga HG, Gilbert R, Nalbantoglu J, Massie B, Karpati G, Petrof JB. Differential effects of dystrophin and utrophin gene transfer in immunocompetent muscular dystrophy (mdx) mice. Physiol Genomics. 2000;3:133–144. doi: 10.1152/physiolgenomics.2000.3.3.133. [DOI] [PubMed] [Google Scholar]
- Neri M, Torelli S, Brown S, Ugo I, Sabatelli P, Merlini L, Spitali P, Rimessi P, Gualandi F, Sewry C, Ferlini A, Muntoni F. Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord. 2007;17:913–918. doi: 10.1016/j.nmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Rentschler S, Linn H, Deininger K, Bedford TM, Espanel X, Sudol M. The WW domain of dystrophin requires EF-hands region to interact with beta-dystroglycan. Biol Chem. 1999;380:431–442. doi: 10.1515/BC.1999.057. [DOI] [PubMed] [Google Scholar]
- Hnia K, Zouiten D, Cantel S, Chazalette D, Hugon G, Fehrentz AJ, Masmoudi A, Diment A, Bramham J, Mornet D, Winder JS. ZZ domain of dystrophin and utrophin: topology and mapping of a beta-dystroglycan interaction site. Biochem J. 2007;401:667–677. doi: 10.1042/BJ20061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Sakurai M, Yoshida M, Imamura M, Davies EK, Ozawa E. ZZ domain is essentially required for the physiological binding of dystrophin and utrophin to beta-dystroglycan. Hum Mol Genet. 2004;13:693–702. doi: 10.1093/hmg/ddh087. [DOI] [PubMed] [Google Scholar]