Abstract
The hippocampus in Alzheimer’s disease is burdened with amyloid plaques and is one of the few locations where neurogenesis continues throughout adult life. To evaluate the impact of amyloid-β deposition on neural stem cells, hippocampal neurogenesis was assessed using bromodeoxyuridine incorporation and doublecortin staining in two amyloid precursor protein (APP) transgenic mouse models. In 5-month-old APP23 mice prior to amyloid deposition, neurogenesis showed no robust difference relative to wild-type control mice, but 25-month-old amyloid-depositing APP23 mice showed significant increases in neurogenesis compared to controls. In contrast, 8-month-old amyloid-depositing APPPS1 mice revealed decreases in neurogenesis compared to controls. To study whether alterations in neurogenesis are the result of amyloid-induced changes at the level of neural stem cells, APPPS1 mice were crossed with mice expressing green fluorescence protein (GFP) under a central nervous system-specific nestin promoter. Eight-month-old nestin-GFP × APPPS1 mice exhibited decreases in quiescent nestin-positive astrocyte-like stem cells, while transient amplifying progenitor cells did not change in number. Strikingly, both astrocyte-like and transient-amplifying progenitor cells revealed an aberrant morphologic reaction toward congophilic amyloid-deposits. A similar reaction toward the amyloid was no longer observed in doublecortin-positive immature neurons. Results provide evidence for a disruption of neural stem cell biology in an amyloidogenic environment and support findings that neurogenesis is differently affected among various transgenic mouse models of Alzheimer’s disease.
Neurogenesis in the mammalian brain continues through adulthood and into old age primarily in the subventricular zone of the lateral ventricles, the olfactory bulb and the granular cell layer (GCL) of the hippocampus.1,2,3 GCL neurons are generated in high numbers in young animals and despite a significant decrease with progressive age, neurogenesis continues until senescence.4,5 The function of neurogenesis in the hippocampus remains elusive, but growing evidence suggests its importance for certain memory tasks that are also strongly affected in Alzheimer’s disease (AD).2,6
The neuropathological characterization of AD involves a progressive deposition of β-amyloid (Aβ) protein in the brain parenchyma, often accompanied by Aβ deposition in cerebral blood vessels with an accompanying vascular angiopathy.7,8 It has been suggested that other hallmarks of AD, ie, an increased inflammatory response, neurofibrillary tangles, dystrophic neurites, neuron loss, or cognitive deficits are triggered by the aggregation and accumulation of Aβ and that this combined insult influences the cognitive outcome.9
The hippocampus is one of the regions of the AD brain most heavily burdened with amyloid plaque and is also one of the few locations in the adult brain where neurogenesis continues throughout adult life. Several attributes of adult hippocampal neurogenesis suggest that amyloid deposition may influence neurogenesis. Proliferative amplification of neural progenitor cells occurs within the microvascular niche,10 an area prone to amyloidogenesis and affected in virtually all AD cases.11,12,13,14,15 Numerous growth factors are known to be up-regulated in vicinity of amyloid plaques16,17,18 and the same growth factors are also known to be potent modulators of neural stem cell activity.19,20,21,22,23,24 Conversely, inflammation that accompanies amyloid deposition25 may negatively impact neurogenesis given the potent down-regulation of neurogenesis observed in pro-inflammatory environments.26,27
In the present study, two APP transgenic mouse models were used to evaluate the potential impact of Aβ deposition on neural stem cell biology. Extending previous research that reported both increases and decreases in mouse models of cerebral amyloidosis,28,29,30,31,32 we found that neurogenesis is differentially affected dependent on the mouse model studied. In very old APP23 mice a severalfold increase in newborn neurons compared to age-matched wild-type (WT) mice was found while adult amyloid-depositing (APPPS1) mice revealed a decrease in neurogenesis. Cross-breeding of the APPPS1 mouse model with mice expressing nestin-driven green fluorescence protein (GFP) suggested deficits in neurogenesis already at the level of the nestin-positive hippocampal stem cells.
Materials and Methods
Animals
In a first experiment adult (5-month-old) and aged (25-month-old) female APP23 transgenic mice and corresponding non-transgenic, mostly littermate, WT control mice were used.33 APP23 mice express KM670/671NL mutated human APP under a murine Thy-1 promoter element (“Swedish mutation”). Mice have been generated on a B6D2 background and in the following, have been backcrossed with C57BL/6J mice for more than ten generations. Five-month-old mice were selected as pre-depositing mice since amyloid deposition in this line starts at 6 to 8 months of age.33 Twenty-five month-old mice were selected because at this age, robust and high amyloid-deposition is observed in the hippocampus and because this age approximates the mean life span of C57BL/6J mice.34 In a second experiment 8-month-old APPPS1 transgenic mice (line 21) and corresponding WT control mice were used (most of the mice were littermates; male and female mice were included in the study since no gender difference in terms of cerebral amyloidosis is found in this line).35 APPPS1 mice express both KM670/671NL mutated human APP and L166P mutated human presenilin (PS1) under the Thy-1 promoter element. Mice have been generated on a C57BL/6J background. Eight-month-old mice were selected since they revealed qualitatively an amyloid load comparable to that seen in aged APP23 mice. In a third experiment, APPPS1 mice were crossed with transgenic mice expressing enhanced GFP under a CNS-specific nestin promoter element.36 For this latter analysis eight-month-old mice, all females, were selected. Nestin-GFP mice have initially been generated on a B6D2 background but were backcrossed to C57BL/6J for at least three generations before crossing to the APPPS1 mice. All experimental procedures were in accordance with the veterinary office regulations of the Land Baden-Württemberg in Germany.
5-Bromo-2-Deoxyuridine Treatment
5-Bromo-2-deoxyuridine (BrdU) was obtained from Sigma (Taufkirchen, Germany). During the first 7 days of the experiment animals received daily i.p. injections of 50 μg BrdU/g body weight at a concentration of 10 mg/ml in 0.9% NaCl. Mice were sacrificed 3 weeks after the last BrdU injection.
Tissue Preparation
Mice were anesthetized with an overdose of 0.8% ketamine and 1% xylazine in 0.9% NaCl, transcardially perfused with PBS followed by ice-cold 4% paraformaldehyde in 0.1M PBS. Brains were removed and post-fixed at 4°C in 4% paraformaldehyde, dehydrated in 30% sucrose, and frozen. Coronal serial 40 μm sections were cut with a microtome and collected in cryoprotectant (30% glycerol, 45% ethylene glycol in PBS) and stored at −20°C until use.
Immunohistochemistry
Free-floating sections were processed for immunohistochemistry as described elsewhere.10,37 Briefly, sections were washed in tris-buffered saline (TBS) and blocked with 3% goat or donkey serum (Vector Laboratories Inc., Burlingame, CA) in 0.3% Triton-X-100 (Fisher, Fair Lawn, NJ). The sections were incubated overnight with primary antibodies at 4°C in 2% serum and 0.3% Triton-X-100, washed three times with TBS and incubated for 3 hours with biotin-conjugated secondary antibodies. After repeated TBS washing, sections were stained by complexing with avidin-biotin and diaminobenzidine (DAB) or SG blue (Vectastain ABC Elite Kit; Vector Laboratories). Sections were mounted on precleaned glass microscope slides (Superfrost Plus; Langenbrinck, Teningen, Germany), dehydrated with an alcohol series, cleared in xylene, and coverslipped in a xylene-soluble mounting medium (Pertex; medite GmbH, Burgdorf, Germany).
For immunofluorescence, a similar protocol was followed but with fluorophore-coupled secondary antibodies. After 3 hours of incubation with secondary antibodies, sections were repeatedly washed in TBS, mounted, and coverslipped in polyvinyl alcohol with 2,5% 1,4-diazabicyclo(2.2.2)octane (DABCO, Sigma) or Vectashield (Vector Laboratories) for GFP fluorescence.
Primary antibodies were used at the following concentrations: rat anti-BrdU (1:1000; Accurate, Westbury, NY), goat anti-doublecortin (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-NeuN (1:1000; Chemicon Int., Temecula, CA), guinea pig anti-GFAP (1:1000; Advanced ImmunoChemical, Inc., Long Beach, CA), rabbit anti-S100β (1:2000; Swant, Bellinzona, Switzerland), rabbit anti-Ionized calcium binding adaptor molecule 1(Iba-1; Wako Chemicals, Neuss, Germany), and rabbit anti-Aβ (NT11/12;.33). As secondary antibodies Biotin-SP, Cy5, Cy3 (Vector Laboratories), or Alexa 488, Alexa 568 (Molecular Probes, Inc., Eugene, OR) conjugated goat or donkey IgG at a dilution of 1:500 were used.
For BrdU antigen retrieval, sections were treated for 30 minutes with 2 M HCl at 37°C followed by repeated washing with TBS. For immunofluorescence co-labeling experiments with BrdU, sections were first stained for NeuN and GFAP, and then fixed for 10 minutes in 4% paraformaldehyde, followed by antigen retrieval processing and staining for BrdU.
Histochemistry
Fibrillar Aβ was visualized by staining free floating sections with 0.005% thioflavine S (Sigma) for 1 minute. Alternatively, mounted sections were incubated in 0.5% Congo red (Sigma) in NaCl-saturated 80% ethanol.
Quantification of Cell Number and Plaque Load
For the stereological analysis of the total number of BrdU and doublecortin (DCX)-positive cells in the GCL of APP23 mice, every sixth section through the hippocampus was immunohistochemically stained, and BrdU and DCX-positive cell numbers were estimated under light microscopy using the ‘Optical Fractionator’ probe of Microbrightfield Stereo Investigator software (MicroBrightField, Williston, VT). Cells were included in the analysis, if located inside or within one cell diameter of the GCL.
For phenotyping of BrdU-positive cells, double or triple immunofluorescence was used, with anti-BrdU, anti-NeuN, and/or anti-GFAP antibodies. Cells were scored using a Zeiss 510 Meta confocal microscope (Jena, Germany). Careful analysis of the z-plane was used to avoid scoring closely juxtaposed cells as double-positive.38 One hundred BrdU-positive cells per animal were examined. However, in older animals, where BrdU labeling was often very low,4 20 to 100 cells per animal were analyzed. To determine the number of double-labeled cells, the percentage of BrdU-positive cells co-labeled with a specific lineage marker was multiplied with the total number of BrdU-positive cells in the GCL estimated in the stereological analysis.
The number of stem cells in the GCL of GFP-APPPS1 and GFP-wt mice was estimated using a variant of the optical fractionater technique as previously described.4 Every sixth section throughout the entire hippocampus was stained immunohistochemically for GFP and analyzed under the light microscope. The total number of cells was estimated by multiplying the counted cell number with the reciprocal of fraction of the sampled region. GFP-positive cells were classified as quiescent astrocyte-like or transient amplifying cells, if the orientation of the dendritic processes were either perpendicular or horizontal, respectively, and if the cell body was located in the subgranular zone.39
Amyloid load was estimated on every sixth Aβ-immunostained section through the entire dentate gyrus (including the GCL, molecular layer and CA4) using the ‘Area Fraction Fractionator’ probe of Microbrightfield Stereo Investigator software using a similar protocol as previously described.40 The region was determined according to a mouse brain atlas.41
Statistical Analysis
Statistical analysis was performed using analysis of variance (Statview 5.0 for Macintosh). Throughout the paper the mean ± SEM is indicated. Statistical significance was accepted as P < 0.05.
Results
Increased Cell Proliferation and Neurogenesis in the GCL of Aged APP23 Mice
The incorporation of BrdU in the S-phase of mitotic cell division was used to monitor proliferative activity in the GCL. The effect of age (5 months vs. 25 months) and genotype (WT versus transgenic mice) on the number of newly generated cells in the dentate GCL of APP23 mice was estimated using unbiased stereological methods (Figure 1, A and B). Analysis of variance revealed a significant effect of age [F(1,20) = 11.16; P < 0.01] and an age and genotype interaction [F(1,20) = 4.89; P < 0.05; n = 6/group]. In young 5-month-old mice there was no statistical difference between transgenic mice and WT mice, although transgenic mice appeared to have less BrdU-positive cells. In aged 25-month-old mice, a significantly higher number of BrdU-positive cells was found in the GCL of transgenic compared to control mice [t(10) = 4.95; P < 0.001], reaching almost the level of adult 5-month-old APP23 mice (Figure 1, A and B). Stereological estimation of the amyloid load in the dentate gyrus of 25-month-old mice was 14.0 ± 1.9% (see also Figure 2A) while no amyloid deposition was found in the 5-month-old APP23 mice, demonstrating that the amyloidogenic environment of aged APP23 mice was accompanied by increased proliferative activity in the dentate gyrus.
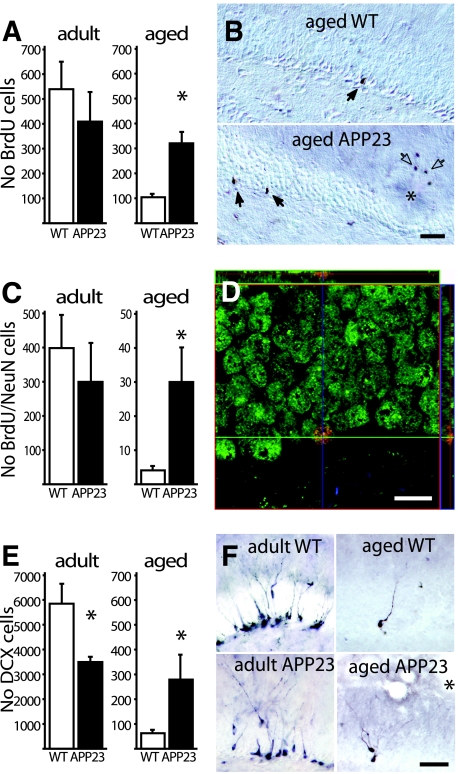
Figure 1.
Neurogenesis in the GCL of adult and aged APP23 mice. A: Three weeks after BrdU injections no difference was found in the number of BrdU-positive cells in the GCL in 5-month-old (adult) APP23 mice (6 mice/group). In contrast, in 25-month-old (aged) APP23 mice, number of BrdU-positive cells were significantly increased compared to aged-matched WT mice (6 mice/group; *P < 0.001). B: Most BrdU-positive cells (closed arrows) were found close to the SGZ of the dentate gyrus. In the aged APP23 mice additional BrdU-positive cells were found in the periphery of amyloid plaques (asterisk) and identified as microglia cells (open arrows). C: When BrdU-positive cells were phenotyped using the neuronal marker NeuN no significant difference was again found between adult APP23 and WT mice (6 mice/group). In aged APP23 mice a significantly higher number of newly generated neurons was found compared to WT (6 mice/group; *P < 0.05). D: This phenotyping was done by capturing multiple confocal images in the Z-dimension, allowing the evaluation of the co-localization of different markers in all three dimensions. (Green: NeuN; Red: BrdU; Blue: GFAP). E: To estimate the numbers of immature neurons adjacent sections were stained for DCX. In adult APP23 mice the pool of DCX-positive cells was smaller compared to WT mice (6 mice/group; *P < 0.05). In aged APP23 mice the population of immature neurons was significantly increased compared to WT mice (6 mice/group; *P < 0.001). This increase in the aged APP23 mice was most pronounced in the caudal pole of the dentate gyrus. F: Representative micrographs of DCX-positive cells in the GCL. Asterisks indicate amyloid plaques. Scale bar: 50 μm in B and F; 10 μm in C.
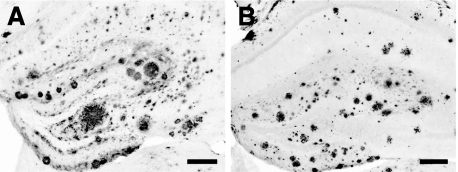
Figure 2.
Amyloid deposition in APP23 and APPPS1 mice. Immunohistochemical staining for Aβ in the hippocampus of a 25-month-old APP23 mouse (A) and of an 8-month-old APPPS1 mouse (B). Note the high amyloid load in the dentate gyrus in both mouse models at that age. Scale bars = 200 μm.
To determine whether this increased proliferation reflects neurogenesis, BrdU labeling was combined with confocal microscopy and fluorescent multiple labeling for the neuronal marker NeuN and the glial marker GFAP. In 5-month-old APP23 mice no difference in the total number of BrdU and NeuN co-labeled cells was found (Figure 1, C and D). There was also no difference in the fractions of BrdU-positive cells co-labeling with NeuN in transgenic and WT mice (71.4% ± 5.3% and 67.0% ± 5.9%, respectively; P > 0.05). In contrast, aged APP23 revealed a six-fold higher number of total newborn neurons compared to WT mice [t(10) = 2.73; P < 0.05; n = 6/group] (Figure 1, C and D). The fraction of BrdU cells expressing NeuN also appeared greater however did not reach significance in aged APP23 mice compared to WT animals (10.9% ± 4.0% vs. 3.7% ± 0.8%; P > 0.05). The percentage of BrdU-positive cells co-labeled for GFAP was low in both 5-month-old (0.9% ± 0.6% and 2.4% ± 1.2% in WT and transgenic, respectively) and 25-month-old animals (1.4% ± 0.9% and 4.3% ± 1.3% in WT and transgenic, respectively) resulting in very few BrdU-labeled astrocytes in the GCL. In 5-month-old APP23 animals no significant difference was observed in newborn glia between transgenic and WT mice (6.5 ± 3.6 and 5.1 ± 3.3 newborn astrocytes per GCL, respectively), while in 25-month-old animals significantly more newborn glia cells were found in transgenic compared to WT control mice (12.3 ± 3.5 vs. 1.3 ± 1.0, newborn astrocytes per GCL, respectively; P < 0.05).
Because the incorporation of BrdU into dividing cells takes place after systemic administration of BrdU, it cannot be excluded that Aβ-induced changes in the vascular system influence the uptake of BrdU. Moreover, BrdU labeling has also been attributed to Aβ-induced cell-cycle processes in post-mitotic neurons rather than neurogenesis.42,43 Thus, we sought to confirm our results with an additional marker of neurogenesis. Neuroblasts committed to the neuronal lineage express DCX during their last divisions but subsequently down-regulate DCX as NeuN is up-regulated during neuronal maturation.44,45 DCX is therefore an excellent marker to monitor the abundance of immature neurons within the hippocampal dentate (Figure 1, E and F). analysis of variance revealed significant effects of age [F(1,20) = 143.6; P < 0.001], genotype [F(1,20) = 8.38; P < 0.01], and age × genotype interaction [F(1,20) = 11.2; P < 0.01; n = 6/group]. Five-month-old APP23 mice showed significantly fewer DCX-positive cells than age-matched controls [t(10) = 3.13; P < 0.05] (Figure 1, E and F). In contrast, aged 25-month-old APP23 mice showed a significant increase in DCX-positive cells compared to age-matched controls [t(10) = 4.23; P < 0.01] (Figure 1, E and F), supporting the conclusion that an amyloidogenic environment stimulates neurogenesis and/or survival of newborn neurons.
Decreased Cell Proliferation and Neurogenesis in the GCL of Adult APPPS1 Mice
Eight-month-old APPPS1 mice and corresponding age-matched WT controls were selected. The amyloid load in the dentate gyrus of APPPS1 mice was 4.16 ± 0.4%. In contrast to the APP23 mouse model with diffuse and compact amyloid, APPPS1 mice revealed predominantly compact amyloid deposits (Figure 2B).
Both, the number of BrdU-positive cells and the number of BrdU-positive cells co-labeled with NeuN were decreased in APPPS1 mice compared to age-matched WT control mice. However both comparisons did not reach statistical significance (P > 0.05; n = 6/group; Figure 3, A and B). In contrast, the decrease in the number of DCX-positive cells reached statistical significance in APPPS1 as mice compared with the control mice [t(13) = 2.17; P < 0.05; n = 7 to 8/group] (Figure 3C). Note that the DCX-positive immature neurons extended dendrites close to and past amyloid plaques without showing significant attraction to the amyloid deposits (Figure 3, D–H).
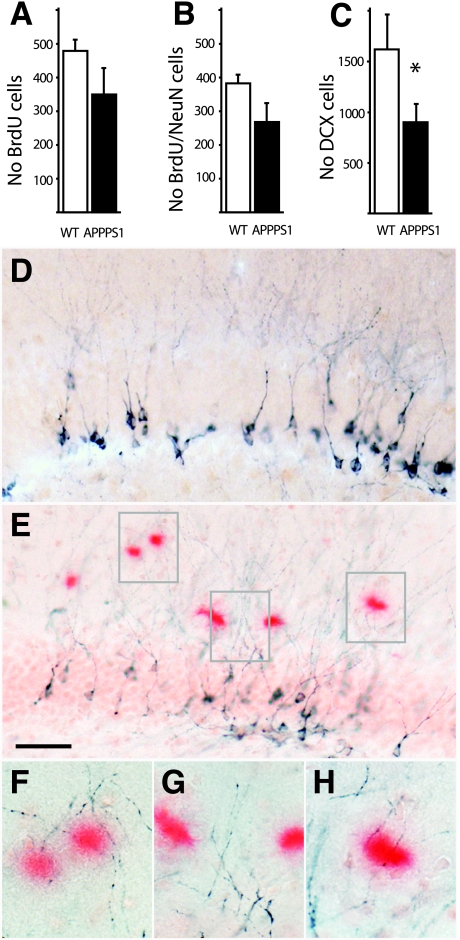
Figure 3.
Neurogenesis in the GCL of adult APPPS1 mice. A: Three weeks after BrdU injections a decrease was found in the number of BrdU-positive cells in the GCL in 8-month-old APPPS1 mice compared to age-matched WT control mice (6 mice/group). However this decrease was statistically not significant. B: When BrdU-positive cells were phenotyped using the neuronal marker NeuN a similar not significant decrease was found. C: In contrast the decrease in the number of DCX-positive immature neurons reached statistical significance (7 to 8 mice/group; *P < 0.05). Distribution and morphology of DCX-positive cells in the control mice (D) and APPPS1 mice (E). Amyloid is stained with Congo red. Note that DCX-positive dendrites are neither attracted nor sprout to the amyloid plaques (E–H). Scale bar = 50 μm.
One disadvantage of this rapidly amyloid-depositing mouse model is that virtually at the time when postnatal development of the dentate gyrus is complete, first amyloid deposits are already present (8 to 12 weeks of age). Nevertheless, we injected 6-week-old APPPS1 mice and WT control mice with BrdU and mice were analyzed 3 weeks later. No decrease in the number of newly generated cells was observed. In fact there was a trend toward an increase in the number of BrdU-positive cells in the GCL of the dentate gyrus in APPPS1 mice compared to the WT control mice (3600 ± 268 vs. 2815 ± 207 cells; n = 4 to 5/group; P > 0.05).
Alterations of Neural Stem Cells in Close Vicinity of Aβ Deposits
Next we intended to study whether these alterations in neurogenesis in the two mouse models are the result of a change in number of nestin-positive neural stem cells. Nestin is an intermediate filament protein expressed in neural stem and progenitor cells.10,46,47 However, consistent with previous reports of a striking age-related decrease in stem cells in the subventricular zone48 and poor detection of mouse nestin with commercially available antibodies we could not detect reliably nestin-positive cells in any of the transgenic mouse models. Thus, we thought to improve detection of stem cells by crossing the faster developing APPPS1 mouse model with mice expressing GFP under a nestin promoter.36 Double transgenic APPPS1 × nestinGFP (APPPS1-GFP) and corresponding WT-nestinGFP littermate mice (WT-GFP) were analyzed again at 8 months of age.
Nestin-positive stem cells, identified with an antibody to GFP, were located at the subgranular zone (SGZ) and often extended small but very dense trees of dendritic-like processes perpendicular to the GCL into the inner molecular layer (Figure 4A).49 These putative neuronal stem cells have been described as radial astrocytes50 or as quiescent astrocyte-like “Type-1” cells,51,52 and are characterized by co-expression of nestin and GFAP, but lack of S100β or DCX expression (results not shown). Remarkably, a striking morphological alteration of these quiescent astrocyte-like stem cells in the molecular layer of GFP-APPPS1 mice as compared with WT-GFP mice was observed. The tree of processes perpendicular to the GCL was strongly attracted toward amyloid deposits with a thickening and hypertrophy of processes aberrantly contacting Aβ plaques (Figure 4, B–D). Estimates of total nestin-GFP-positive quiescent astrocyte-like stem cells in amyloid-depositing 8-month-old GFP-APPPS1 mice showed a significant reduction in number compared to GFP-WT control mice [t(13) = 4.84; P < 0.05; n = 7 to 8 per group] (Figure 4E). In the GFP-APPPS1 mice 34 ± 8% of all nestin-GFP-positive astrocyte-like stem cells revealed an aberrant morphological phenotype.
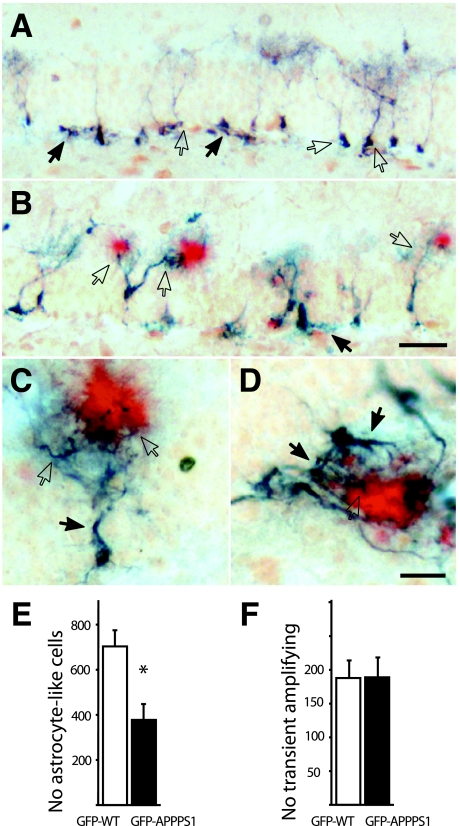
Figure 4.
Neural stem cells in the GCL interact with amyloid plaques. A: Adult nestin-positive stem cells in the GCL of aged 8-month-old GFP-WT mice. Quiescent astrocyte-like stem cells (open arrows) were located along the GCL, with their cell body in the SGZ and dendritic-like processes in the molecular layer perpendicular to the GCL. In contrast, transient amplifying precursor cells (closed arrows) in the SGZ displayed a horizontal orientation. B: In GFP-APPPS1 mice, amyloid deposits (Congo red staining in red) strongly attracted these dendritic-like processes of quiescent astrocyte-like (open arrows) and transient amplifying cells (closed arrow). C: High magnification of the typical morphological alterations of a quiescent astrocyte-like cell attracted to Aβ (red). Note the thickened main branch of the tree of processes (closed arrow) and the thickened fibers in close vicinity to the plaque (open arrows). D: Transient amplifying cells were found to react to the presence of Aβ similar to quiescent astrocyte-like cells with thickened processes (closed arrows) and aberrant dendritic-like processes sheathing the amyloid plaque with dystrophic bouton-like structures (open arrow). E: Stereological estimates of quiescent astrocyte-like stem cells revealed a significant reduction in 8-month-old GFP-APPPS1 as compared with age-matched GFP-WT mice control mice (n = 7 to 8 mice/group; *P < 0.05). F: No difference occurred in the number of transient amplifying progenitor cells in GFP-APPPS1 compared to GFP-WT mice. Scale bars: 50 μm in B; 10 μm in D. A and B and C and D have the same magnification.
As neural stem/progenitor cells begin to differentiate, GFAP expression is extinguished and cells adopt a migratory-like morphology with a major axis tangential to the GCL. These cells have been referred to as “Type-II” cells51,52 or D cells53 and represent a transient amplifying population of neural progenitor cells. These GFAP-negative and nestin-positive cells were also found to extend aberrant processes to amyloid deposits (Figure 4, B–D), but their total number did not differ between GFP-APPPS1 and GFP-WT mice [t(13) = 0.53, P > 0.05] (Figure 4F). In the GFP-APPPS1 mice 27 ± 5% of these transient amplifying cells revealed an aberrant morphological phenotype.
Discussion
In the present study we investigated neurogenesis in the context of cerebral amyloidosis using two transgenic mouse models both on a B6 congenic background. APP23 transgenic mice overexpress APP harboring the Swedish mutation (APPSwe) at levels seven-fold over endogenous mouse APP and develop amyloid deposition in the hippocampus at 8 to 10 months of age.33 APPPS1 mice also overexpress APPSwe at levels three-fold over endogenous mouse APP, but additionally express mutated PS1.35 While APP23 mice generate more Aβ1–40 than Aβ1–42, APPPS1 mice generate severalfold more of the highly amyloidogenic Aβ1–42 compared to Aβ1–40 and thus develop cerebral amyloidosis in hippocampus as early as 8 to 12 weeks. Moreover, APPPS1 mice develop predominant compact parenchymal amyloid deposits while APP23 mice develop diffuse and compact amyloid in both parenchyma and vasculature. The present results reveal a significant increase in neurogenesis in aged amyloid-laden 25-month-old APP23 mice compared to age-matched control mice, whereas in 8-month-old amyloid-depositing APPPS1 mice, a decrease of neurogenesis was found as compared with age-matched control animals.
Previous results on the effect of cerebral amyloidosis on neural progenitor proliferation in the hippocampus of transgenic mice have been inconsistent. Initially, two studies reported a decrease in neurogenesis in APP transgenic mice. However, mice were analyzed before or at the time amyloid deposition starts, namely at 9 months, for the Tg2576 mouse line, and at 12 to 14 months in another APPSwe transgenic mouse line.29,32 Unfortunately, in neither of these studies DCX and/or NeuN were used as markers to determine the neuronal fate of the increased BrdU-labeled cells. A third study reporting decreased neurogenesis used homozygous 12-month-old PDAPP mice harboring the APP V717F mutation (APPInd).28 In these mice the amyloid load in the hippocampus was high, but strikingly, the neurogenic SGZ and the hilus of the dentate gyrus was clear of amyloid deposition. In contrast, a fourth study using transgenic mice that harbor both the APP Swedish and the Indiana mutation (APPSwe Ind) found a two-fold increase in neurogenesis, both in pre-depositing and amyloid-depositing 12-month-old mice.30
Contrary to the mouse models used in these previous studies, aged APP23 mice exhibit robust amyloid deposits in the neurogenic SGZ and its neighboring hilus, GCL, and molecular layer (see Figure 2). Thus, our results suggests that a micro-environment with chronic amyloid deposition can lead to a significant increase in neurogenesis, consistent with investigation of the postmortem AD brain where an increased expression of markers of proliferation and immature neurons in the hippocampus has been reported.54 It is known that amyloid deposits stimulate the local accumulation of several growth factors that are potent regulators of neurogenesis such as brain-derived neurotrophic factor, vascular endothelial growth factor, fibroblast growth factor, and cystatinC.16,17,18,55 A neurogenic effect of Aβ peptide on hippocampal neural stem cells has also been reported.56 The elevation in neurogenesis in a chronic amyloidogenic environment may also represent an endogenous neural replacement response to neurodegeneration and dysfunction rather than a direct response to amyloidogenesis. Indeed, neurogenesis in the hippocampus is stimulated in response to a variety of insults such as cerebral ischemia, seizure or neurotoxic lesions.57,58,59,60 In both AD patients and aged APP23 mice, neuronal cell loss in the hippocampus has been reported.61,62
In light of these results the observation of a decrease in neurogenesis in APPPS1 mice was rather unexpected but confirms a recent study that also used APP/PS1 transgenic mice.63 Possible explanations for the different observations between APP23 and APPPS1 mice include that (i) a more chronic amyloid environment is necessary to stimulate neurogenesis (APP23 mice were analyzed at 25 months of age, vs. 8 months for the APPPS1 mice); (ii) the nature of the deposited amyloid species are important (both Aβ1–40 and Aβ1–42 in APP23, mainly Aβ1–42 in APPPS1); (iii) the amyloid deposition in the vasculature is important (significant amyloid angiopathy in APP23, virtually no vessel amyloid in APPPS1); (iv) the level of APP overexpression is important (seven-fold in APP23, three-fold in APPPS1); and (v) the additional expression of mutated PS1 is the reason for the decrease in neurogenesis in the APPPS1 mice although previous studies using mutated PS1 transgenic mice (in the absence of cerebral amyloidosis) reported either no change or an increase in neurogenesis.31,64
In an effort to study whether the observed changes in neurogenesis are already reflected at the level of nestin-positive stem cells, we found for the amyloid-depositing 8-month-old APPPS1 mice that quiescent astrocyte-like stem cells were reduced in numbers compared to WT mice, while the number of amplifying stem cells remained unchanged. Transient amplifying cells are believed to be the more differentiated and more proliferative progeny of quiescent astrocyte-like cells,51,52,53 suggesting that amyloid deposition in APPPS1 mice promotes an increase in the ratio of transient amplifying/quiescent astrocyte-like cells. Such a relative increase in transient amplifying stem cells may be seen as compensatory response, which however after all still results in a reduced number of DCX-positive immature neurons and neurogenesis in the APPPS1 mice. Thus, our results suggest that neurogenesis in 8-month-old APPPS1 mice is disturbed at the level of the development of immature neurons.
The observation of grossly altered morphology and aberrant attraction of nestin-positive progenitor cells, but no longer of DCX-positive immature neurons, to amyloid deposits was unexpected. Previous research has shown that dendrites become thinned and undergo spine loss in vicinity of amyloid deposits without showing significant attraction, wheras axons reveal aberrant sprouting toward the amyloid with the formation of dystrophic boutons.65,66,67 The present aberrant attraction of the nestin-positive progenitor cell toward the amyloid deposits is however in its nature neither fully typical for axons nor dendrites. It implies and supports the idea that neurogenesis in APPPS1 mice is impaired at the transition of nestin-positive progenitor cells to DCX-positive immature neurons. The latter is also plausible since recent studies have shown that the integration of newborn neurons into functional neural circuits is critically dependent on the appropriate and cell-specific synaptic input signals2,6,68,69 that are disturbed in an amyloidogenic environment.
Such aberrant neurogenic responses of nestin-positive stem cells in an amyloidogenic environment raises cautions about the idea of promoting neurogenesis by stem cell replacement therapy in AD, or by endogenous stimulation of neurogenesis.70 Thus, it may not be enough to stimulate neurogenesis per se in AD but rather to find a strategy to sustain survival and to promote proper development and integration of immature neurons into functional neural networks.
The present work did not allow to firmly establish whether the increased neurogenesis observed in the aged 25-month-old APP23 mice is compensatory or is aberrant and dysfunctional. Previous behavioral experiments have shown impairment in hippocampus-dependent memory tasks in aged APP23 mice and thus neurogenesis apparently is not sufficient to overcome the neural dysfunction induced by cerebral amyloidosis in these mice.71,72 Similar to the APPPS1 mice, neurogenesis in a microenvironment severely affected by amyloid deposition may be dysfunctional despite the expression of NeuN/BrdU, which indicates that the newly generated cells have developed a more mature neuronal phenotype. In future studies, it might be interesting to ablate neurogenesis in aged APP23 mice to study whether such treatment improves or harms hippocampal function in such mice.
Acknowledgments
We thank Yu Chun Tsai, Esther Kohler, Yuliya Golub, Claudia Schäfer, and Michael Calhoun (Tübingen) for experimental support and helpful discussions.
Footnotes
Address reprint requests to Theo D. Palmer, Ph.D., Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA 94305, or Mathias Jucker, Ph.D., Department of Cellular Neurology, Hertie Institute for Clinical Brain Research, University of Tübingen, D-72076 Tübingen, Germany. E-mail: tpalmer@stanford.edu or mathias.jucker@uni-tuebingen.de.
Supported by grants from the Roche Research Foundation (Basel), EU contract LSHM-CT-2003–503330 (APOPIS) in which the Swiss participants are funded by the Swiss State Secretariat for Education and Research, and the German National Genome Network (NGFN2).
References
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA. Amyloid beta peptide 1–42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol (Berl) 2004;107:283–291. doi: 10.1007/s00401-004-0822-6. [DOI] [PubMed] [Google Scholar]
- Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The pathology of ischemic-vascular dementia: an update. J Neurol Sci. 2002;203–204:153–157. doi: 10.1016/s0022-510x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol. 1998;57:353–359. doi: 10.1097/00005072-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Burbach GJ, Hellweg R, Haas CA, Del Turco D, Deicke U, Abramowski D, Jucker M, Staufenbiel M, Deller T. Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J Neurosci. 2004;24:2421–2430. doi: 10.1523/JNEUROSCI.5599-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Arima K, Haga S, Aizawa T, Motoi Y, Otsuka M, Ueki A, Ikeda K. Fibroblast growth factor (FGF)-9 immunoreactivity in senile plaques. Brain Res. 1998;814:222–225. doi: 10.1016/s0006-8993(98)01042-7. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Ingram DK. Murine models of brain aging and age-related neurodegenerative diseases. Behav Brain Res. 1997;85:1–26. doi: 10.1016/s0166-4328(96)02243-7. [DOI] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Holscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Calhoun M, Ermini F, Kuhn HG, Wiederhold KH, Walker L, Staufenbiel M, Jucker M. Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. J Neurosci. 2002;22:515–522. doi: 10.1523/JNEUROSCI.22-02-00515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. San Diego: Academic Press; The Mouse Brain in Stereotaxic Coordinates. 2004 [Google Scholar]
- Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer’s disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression—a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F. Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer disease patients. J Neuropathol Exp Neurol. 2001;60:94–104. doi: 10.1093/jnen/60.1.94. [DOI] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27:6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Phinney AL, Deller T, Stalder M, Calhoun ME, Frotscher M, Sommer B, Staufenbiel M, Jucker M. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J Neurosci. 1999;19:8552–8559. doi: 10.1523/JNEUROSCI.19-19-08552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Bondolfi L, Hunziker D, Schlecht HP, Carver K, Maguire E, Abramowski D, Wiederhold KH, Sturchler-Pierrat C, Jucker M, Bergmann R, Staufenbiel M, Sommer B. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging. 2003;24:365–378. doi: 10.1016/s0197-4580(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Dumont M, Strazielle C, Staufenbiel M, Lalonde R. Spatial learning and exploration of environmental stimuli in 24-month-old female APP23 transgenic mice with the Swedish mutation. Brain Res. 2004;1024:113–121. doi: 10.1016/j.brainres.2004.07.052. [DOI] [PubMed] [Google Scholar]