Abstract
Activated lymphocytes and lymphoid-tissue inducer cells express lymphotoxins (LTs), which are essential for the organogenesis and maintenance of lymphoreticular microenvironments. Here we describe that T-cell-restricted overexpression of LT induces fulminant thymic involution. This phenotype was prevented by ablation of the LT receptors tumor necrosis factor receptor (TNFR) 1 or LT beta receptor (LTβR), representing two non-redundant pathways. Multiple lines of transgenic Ltαβ and Ltα mice show such a phenotype, which was not observed on overexpression of LTβ alone. Reciprocal bone marrow transfers between LT-overexpressing and receptor-ablated mice show that involution was not due to a T cell-autonomous defect but was triggered by TNFR1 and LTβR signaling to radioresistant stromal cells. Thymic involution was partially prevented by the removal of one allele of LTβR but not of TNFR1, establishing a hierarchy in these signaling events. Infection with the lymphocytic choriomeningitis virus triggered a similar thymic pathology in wt, but not in Tnfr1−/− mice. These mice displayed elevated TNFα in both thymus and plasma, as well as increased LTs on both CD8+ and CD4−CD8− thymocytes. These findings suggest that enhanced T cell-derived LT expression helps to control the physiological size of the thymic stroma and accelerates its involution via TNFR1/LTβR signaling in pathological conditions and possibly also in normal aging.
The efficient output of lymphocytes from the thymus following positive and negative selection is crucial for efficient adaptive immune responses. The thymus generates a T-cell repertoire essential for adaptive immunity during embryogenesis and throughout life.1 T-cell precursors generated from hematopoietic stem cells in the bone marrow (BM) enter the thymus under chemotactic guidance. A complex maturation process involving interactions between thymocytes and the thymic stroma ensures self-tolerance: most autoreactive thymocytes are eliminated by negative selection before exiting the thymus.2
Impaired responses to pathogens and to altered self-antigens have been recognized as contributing to the aging of the immune system in many mammalian species.3,4,5,6,7 Age-dependent involution of the thymus parallels the age-related deterioration of immune responses.8 In male C57BL/6 mice, thymic involution starts at the age of 8 to 12 weeks and results in a thymic rudiment after 6 to 8 months. In females this process is delayed.7,9 In humans, thymic involution occurs between the first and fourth decades of life.10 The key factors and cellular mechanisms driving involution are poorly understood.11,12
Lymphotoxins (LTs) are expressed by activated T, B, and NK cells,13,14 as well as by CD45+CD3−CD4+CXCR5+ lymphoid tissue inducer cells (LTi cells).15 LTs and related tumor necrosis factor (TNF)-family cytokines are essential for the development and maintenance of secondary lymphoid organs and for appropriate immunological functions.16,17,18,19,20,21,22
Membrane-bound LT consists of heterotrimeric complexes of LTα and LTβ (LTα1β2 or LTα2β1), whereas secreted LT (sLTα) is homotrimeric.23,24,25 LT heterotrimers signal via LTβ receptor (LTβR), whereas sLTα signals mainly via the TNF receptors, TNFR1 and TNFR2.14,26 LTα is inducible in B and T cells, whereas LTβ mRNA is constitutively expressed.14,23,27 LT signaling is essential to the differentiation and function of high endothelial venules28,29 and ectopic LT expression can induce lymphoid neogenesis at atypical sites including kidney, pancreas, and liver.30,31 The cellular and molecular requirements for the establishment of lymph nodes and inflammation-associated neolymphoid structures are similar. In the latter, hyperactivated lymphocytes appear to be able to fulfill the role of LTi cells.15
LT signaling deficiencies can lead to alymphoplasia, yet do not significantly prevent thymic development, thymic structure, T cell differentiation, and negative selection.16,17,18,19,20,21,22,32,33,34,35 However, LTβR signaling was found to control normal differentiation of thymic medullary epithelial cells via cross talk between thymocytes and thymic medullary epithelial cells,36 and to regulate γδ T cell differentiation in concert with other factors.37 LTβR plays an important role in thymic negative selection of organ-specific thymocytes through thymic medullary chemokine regulation.38 Thymic homeostasis and atrophy might be controlled or influenced by changes in homeostatic chemokines and cytokines.6,7,39,40 Administration of pro-inflammatory cytokines and steroids can drive thymic involution.4,5,6,7,12,39 T-cell specific expression of the ligand for herpesvirus entry mediator and LTβR induces lymphoid tissue abnormalities that affect the thymus.41 Enhanced levels of TNF after lipopolysaccharide or concanavalin A treatment may cause a similar phenotype,40 whereas Tnfr1−/− ablation delays age-dependent thymic involution.42
Naïve T cells do not express membrane-bound or soluble LTα,21,43 yet up-regulate both forms on activation. LT receptors are expressed by thymic stroma, suggesting a role for LT signaling in lymphocyte-stromal interactions. We tested the latter hypothesis by constitutively expressing LTs on T cells. This conferred a CD44high phenotype to T cells, indicative of an activated state, and induced fulminant thymic involution. A similar phenotype was detected in wt mice, but not in Tnfr1−/− or Ltβr−/− mice, on adoption of tgLtαβ BM. Hence this thymic pathology resulted from signaling to thymic stroma rather than from impaired thymocyte homing.44,45 We further investigated the effects of a lymphocytic choriomeningitis virus (LCMV) infection with strain WE (LCMV-WE) or strain Docile (LCMV-DOCILE) on thymic homeostasis. Infection of wt but not Tnfr1−/− mice with LCMV also led to precocious thymic involution and up-regulation of LT on CD8+ and CD4−CD8− thymocytes. This suggests that LTβR and TNFR1 signaling by T cells contributes to thymic involution both during physiological aging and in inflammatory conditions.
Materials and Methods
Mice
Animals were free of all bacterial, viral, and parasitic pathogens listed in the Federation of European Laboratory Animal Science Associations recommendations and were maintained under specific pathogen-free conditions. Housing and experimental protocols were in accordance with the Swiss Animal Protection Law and mice were held according to the law of the Veterinäramt, Kanton Zürich. Ltα−/−, Ltβ−/−, Tnfr1−/−, Tnfr2−/−, Ltβr−/−, and JH−/− mice were published previously.19,20,34,35,46
Generation of tg Constructs
A pBluescript II KS (3.0 kbp) vector with a deleted KpnI restriction site in the multiple cloning site was used. The vector was linearized with BamHI/SalI and a BamHI/SalI restriction product (∼5.6 kbp) of the PrP 5′HG construct47 was cloned in (ΔKpNI pBluePrP 5′HG construct). Lck-promoter cassette (∼3.1 kbp) was isolated by digest with BamHI and NotI of the pPrP5′HGLckSalI construct47 and subsequent purification on a 0.7% agarose gel by gel extraction (Qiagen). The full Ltα and Ltβ open reading frames were amplified from a pLtα and pLtβ plasmids using primers that incorporate 50 or 15 bp overhangs with homologous sequences to the Prnp cDNA, incorporating the genuine anti-thymocyte globulin of the Prnp locus of the PrP 5′HG construct47 and a restriction site for MfeI and KpnI. PCR products were 610 bp for Ltα and 921 bp for Ltβ.
Primers used for LT-Prnp overhangs are as follows. LTα primer forward: 5′-CGGGGTACCAGTCCAATTTAG GAGAGCCAAGCAGACTATCATCATGACACTGCTCGGCCGTCTCC-3′. LTβ primer forward: 5′-CGGGGTACCAGTCCAATTTAGGAGAGCCAAGCAGACTATCATCATGGGGACAGGGGACTGCAGG-3′. LTα primer reverse: 5′-CCACCTCAATTGAATCTACAGTGCAAAGGCTCC-3′. LTβ primer reverse: 5′-CCACCTCAATTGAATTCACCCCACCATCACCGC-3′. Ten PCR products of each cDNA were pooled, purified by gel extraction (Qiagen, according to the manufacturer’s manual), and partially digested with KpnI and MfeI. Digested PCR products were repurified and used for subcloning into a MfeI/KpnI linearized ΔKpNI pBluePrP 5′HG vector. After successful subcloning of both LT cDNAs, control digests and sequencing of LTα and Ltβ open reading frames and flanking regions (ABI Prism Sequencer) were performed. These ΔKpNI pBluePrP 5′HGLtα or Ltβ vectors were linearized with BamHI and NotI for the insertion of the promoter cassette. The purified lck-promoter47 was cloned into the ΔKpNI pBluePrP 5′HGlck Ltα (13.0kpb) or ΔKpNI pBluePrP 5′HGlck Ltβ (13.3 kbp) vectors. All restriction enzymes used were purchased from Roche and New England Biolabs.
The LT open reading frame and insertion sites of the lck-promoter were sequenced. Both vectors were linearized with a SalI, NotI digest leading to linearized PrP 5′HGlck Ltα (9.0 kpb), or HG-lck Ltβ (10 kbp). Fragments were isolated on a 0.7% agarose gel by the gel extraction method (Qiagen, according to the manufacturer’s manual) and used for male pronucleus micro-injection.
Genomic Southern Blot
Mouse tail biopsies were received in liquid nitrogen and digested in a lysis buffer containing 100 mmol/L Tris-HCl (pH 8.5), 5 mmol/L EDTA, 0.2% SDS, 20 mmol/L NaCl, 100 μg proteinase K/ml overnight at 55°C with constant rotation. Twenty μg of purified genomic DNA were digested overnight (EcoRI 100 units), and separated overnight on a 0.7% agarose (GIBCO BRL) gels. Genomic digest and DNA separation was checked by ethidium bromide staining. Genomic DNA was depurinated by treatment with 1M HCL for 15 minutes and 0.4M NaOH for 30 minutes and transferred to Hybond-N+ membranes (Amersham) in 0.4M NaOH overnight by capillary blotting technique. Optionally, vacuum blotting was performed at 50 to 55 mbar with a Vacu Gene Pump and a Gene XL blotting apparatus (Pharmacia Biotech) and solutions were used according to the manufacturer’s manual (Pharmacia). After the agarose gel was examined for residual DNA, the blot was marked, washed for 5 minutes in 2× standard saline citrate buffer, UV-crosslinked, and prehybridized according to Church and Gilbert.48 After hybridization overnight at 65°C with a radioactively labeled probe (LTα or LTβ open reading frame), the blot was washed and subjected either to autoradiography using X-ray films (Kodak) or a PhosphorImager (Molecular Dynamics). Exposed images from the PhosphorImager were analyzed with a scanner (Aida) and signals were quantified with the Aida 2.41 imaging analysis program.
Probe Labeling
Template DNA (50 ng) was diluted in sterile water, and random primer (Nonamers or Hexamers) were added (Stratagene). After gentle mixing and incubation at 95°C for 5 minutes, short centrifugation was followed by 3 minutes incubation on ice. This was followed by addition of 5× Primer-buffer (dCTP), radioactive labeled α32P-dCTP (corresponding to the 25 μCi/Amersham Biosciences), and Klenow 5 u/μl (Prime-IT II). For primer elongation, the probe was incubated on 37°C for 75 minutes. All work with radioactivity was performed in an area designated for radioactive work.
Probe Purification
For purification a QIAquick PCR-purification kit was used, according to the manufacturer’s manual (Qiagen).
Adding the Radioactive Probe to the Membrane
Ten μl sonicated salmon sperm (Stratagene) were added to the radioactively labeled, purified probe, per ml hybridization solution (final concentration 100 μg/ml). The mixture was incubated at 95°C for 5 minutes, briefly centrifuged, and chilled on ice for 3 minutes before being added to the prehybridized membrane (2 hours at 65°C).
PCR Specific for tg(Ltαβ), tg(Ltα), and tg(Ltβ)Mice
Mouse tail-lysates (2.5 μl) were used. For tg LTα the following primers were used: Forward primer (Primer 1): 5′-CTGAGTATATTTCAGAACTG-3′, and reverse primer (Primer 2): 5′-CAGAGAAAACCACCTGGGAG-3′. For tg LTβ the following primers were used: Forward primer (Primer 4): 5′-CTGAGTATATTTCAGAACTG-3′ and reverse primer (Primer 5): 5′-GAGTCTCTGAGAGGCTAGAG-3′. The following PCR conditions were established on a Gene Amp PCR System 9700 PCR machine (Applied Biosystems): 95°C 60 seconds denaturation; 55°C 50 seconds annealing; and 72°C 50 seconds elongation; for 35 cycles; followed by 72°C 7 minutes shoot out, and 4°C forever cool down.
RNase Protection Assays
Mice were sacrificed with CO2. Thymi, spleens, Peyer’s patches, and lymph nodes were removed. Total RNA was isolated using TRIzol reagent (Life Technologies) following the manufacturer’s recommendations. Chemokine mRNA levels were determined using the Riboquant Multiprobe RNase Protection Assay system (BD PharMingen). 32P-labeled riboprobes were synthesized from a customized plasmid template set using T7 polymerase. The DNA template was digested with DNase and total RNA (15 μg) was hybridized with the riboprobes overnight at 56°C. Single-stranded RNA species were removed by digestion with RNase A. The protected RNA species were phenol/chloroform extracted, ethanol precipitated, and electrophoresed on a 5% polyacrylamide gel. Protected chemokine probes were visualized by autoradiography of the dried gel.
Preparation of Mouse-Tail Lysates for PCR Analysis
For PCR screening of mice, 5 mm of the tail were cut and lysed in 250 μl lysis buffer containing 50 mmol/L Tris-HCl (pH 9.0), 0.5% Nonidet P-40, 0.5% Tween 20, and 0.1 mg/ml proteinase K, at 55°C with agitation overnight at 600 rpm (Thermomixer 5436, Eppendorf). Following complete digestion, proteinase K was then inactivated by incubation at 95°C for 10 minutes. After centrifugation (12,000 rpm/15 minutes/room temperature) the lysates were immediately used for PCR (2.5 μl/PCR reaction) or stored at −20°C.
Histology and Immunohistochemistry
Frozen sections from thymus (5 μm), lymph nodes, or spleen were stained with H&E. Antibodies FDC-M1 (clone 4C11; 1:50, Becton Dickinson), CD35 (8C12, PharMingen, San Diego, CA), and anti-CD45RO/B220 (RA3-6B2, PharMingen) were used. CD4 for T-helper cells (clone YTS 191; 1:200) and CD8 for cytotoxic T cells (clone YTS 169; 1:50), both rat anti-mouse, were kindly provided by R. Zinkernagel.49 Nonlymphocytic dendritic cells-145 for dendritic cells (Biomedic AG; BMA, Switzerland, T-2013; 1:1000), peanut agglutinin for germinal-center B cells (Vector L-1070; 1:100), and F4/80 (Serotec, 1:50) for macrophages were visualized using standard methods. CK5, CK18, Ulex europeaus agglutinin-1 (UEA-1), and mouse thymus stroma-10 and 24 (MTS10 and MTS24) for confocal microscopy were used as described.50 Terminal deoxynucleotidyl transferase-mediated dUTP staining was performed according to the manufacturer’s protocol (Oncor).
BM Chimeric Mice
BM cells were isolated from tibiae and femurs, and 5 to 10 × 106 BM cells were injected into tail veins of 8 to 10-week-old recipients conditioned by whole-body irradiation (1100 rad) 24 hours earlier as described.51 Six to eight weeks after grafting, reconstitution was assessed by fluorescence-activated cell sorting (FACS) analysis of peripheral blood taken from the retro-orbital plexus of ether-anesthetized mice. Blood samples were prepared at 4°C in buffer solution (PBS containing 2% fetal calf serum and 0.2% NaN3). Reconstitution efficiency was checked in parallel reconstitutions where wt mice received BM from mice expressing the green fluorescent protein under the chicken albumin promoter.52
FACS Analysis
Detection of surface LTα1β2 on B and T lymphocytes: 1 to 5 million lymphocytes from spleen, mesenteric lymph nodes (MLN), or thymus were centrifuged at 1500 rpm for 10 minutes and washed in FACS buffer (PBS, 1 mmol/L EDTA, 0.1% Na Azide, 2% fetal calf serum) followed by incubation with 25 μl of Fc-block (anti-CD16/32 1:100 from PharMingen) in FACS buffer for 15 minutes on ice. Twenty-five μl of diluted LTβR-Fc fusion protein were added directly to the Fc-block and incubated for 30 minutes on ice. This was followed by a washing step (twice) in 200 μl of FACS buffer. The anti-human-Fc antibody (biotin: 109-0635-008; Jackson Immunoresearch) was preabsorbed for 30 minutes with 2% normal mouse and 2% normal rat serum (Sigma) to reduce the background. The biotin anti-human-Fc antibody was diluted 1:200 and after washing, a streptavidin-phycoerythrin labeled antibody (PharMingen) was added.
This procedure was followed by staining with lineage marker antibodies resuspended in normal mouse and rat serum at 1 to 2%. For T cell and B cell specific stains antibodies against CD4, CD8, CD19, and B220 (PharMingen; fluorescein isothiocyanate [FITC[-labeled) were used. Two- and three-color FACS analyses were performed on a FACSCalibur (Becton Dickinson) as described.51 The following anti-mouse antibodies were used (all PharMingen): peridinin chlorophyll-a protein-labeled anti-B220, FITC-labeled anti-CD21, phycoerythrin-labeled anti-CD23, phycoerythrin-anti-CD8, FITC-anti-CD4, phycoerythrin-anti-CD11b, FITC-anti-CD8, Annexin V, and FoxP3. 7-Amino-actinomycin D (7-AAD) analysis was performed using a FACSCalibur using CELLQuest software (Becton Dickinson). Postacquisition analysis was performed using WinMDI 2.8 software and FlowJo (Institute for Neuropathology). FACS analysis and quantification of early T lineage progenitors (ETPs) was performed as described.53
Northern Blot Analysis and in Situ Hybridization
Northern blot analysis and in situ hybridization of frozen spleen sections were performed as previously described.54 The following cDNA probes were used: B-lymphocyte chemoattractant (nucleotides 6 to 762, GenBank AF044196), secondary lymphoid organ chemokine (nucleotides 10 to 784, GenBank U88322), stromal cell-derived factor-1α (nucleotides 34 to 687), macrophage-derived chemokine (nucleotides 13 to 567), EBV-induced molecule 1 ligand CC chemokine (nucleotides 15 to 750), and complete murine GAPDH. Total RNA was prepared using TRIzol reagent (Life Technologies), and multiprobe RNase protection assay using different probe set (BD PharMingen) was performed according to manufacturer’s recommendations.
RT-PCR
Total thymic, splenic, or lymph nodal RNA from wt and tgLTα/β mice were isolated in TRIzol reagent (Life Technologies) and reverse transcribed using the GeneAmp kit (Roche) according to the manufacturer’s instructions. Purified RNA was DNase treated according to the manufacture’s manual (Roche). Reverse transcribed cDNA (GeneAmp kit; Roche) of total thymic, splenic, or lymph nodal RNA from wt and tg mice was used for Taq-Man PCR. Transgene specific probes for LTα and LTβ were generated as follows: Taq-man PCR for LTα: 5′ Prp Taq: 5′-CCAATTTAGGAGAGCCAAGCA-3′ and 3′ LTα Taq: 5′-TGCCAAGCACCCTCAAGAG-3′. LTα specific probe: 5′-CATCATGACACTGCTCGGCCGTCT-3′. Labeling of the probe was conducted as follows (reporter, 6-carboxyfluorescein; quencher, 6-carboxytetramethylrhodamin): Taq-man PCR for LTβ: 5′ Prp Taq: (the same primer as for LTα). 3′ LTβ Taq: 5′-CTGCCACAGCCAGCAAGA-3′. Probe: 5′-CAGGCCCTGCAGTCCCCGTG-3′. As control for Taq-Man PCR procedure and possible DNA contamination DNase treated RNA from wt and tg mice that was not reverse transcribed was used. Taq-Man PCR was performed on an ABI Prism 7700 Sequence detector.
Cytokine Assays
Thymi were placed in 10 vol of Tris-HCl buffer (50 mmol/L, pH7.4) with NaCl (0.6 M), Triton X-100 (0.2%), and bovine serum albumin (0.5%) containing freshly dissolved protease inhibitors: benzamidine (1 mmol/L), benzethonium chloride (0.1 mmol/L), and phenylmethylsulfonyl fluoride (0.1 mmol/L). Samples were homogenized (TissueTearor; BioSpec Products, Bartlesville, OK) for 10 seconds, sonicated (Vibra Cell; Sonics & Materials, Newtown, CT) for 20 seconds at 10 mV, and centrifuged at 12,000 rpm for 20 minutes at 4°C. The supernatants were aliquoted and frozen at −80°C until the cytokine assays were performed. Cytokine protein levels in the serum and thymic homogenates were measured using a multiplexed particle-based flow cytometric cytokine assay (Vignali, 2000). Fluorokine Multi Analyte Profiling mouse kit for TNFα was purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). The procedures closely followed the manufacturer’s instructions. The analysis was conducted using a conventional flow cytometer (LSRII von Becton Dickinson). The detection limit for TNFα was 0.4 pg/ml.
Enzyme-Linked Immunosorbent Assay for LTα3, LTα1β2, and LTα2β1
For enzyme-linked immunosorbent assay (ELISA), a Nunc Immuno Maxi-Sorp plate was coated with 0.1M Na2HPO4, pH9 at room temperature for 2 hours. After removal of 0.1M Na2HPO4, 100 μl/well of the respective 10% organ homogenate was added, and the plate was sealed and incubated over night at 4°C. Next, the plate was washed three times with PBS/0.05% Tween, incubated in blocking buffer with 10% fetal calf serum/PBS (filtered in 0.22 μm) for 2 hours at room temperature, and subsequently washed six times with PBS/0.05% Tween. One hundred μl of the primary antibody (MAB-748; R&D; 1:1000 diluted in blocking buffer/Tween (0.05%) were added and incubated in the sealed plate for 90 minutes at room temperature or overnight at 4°C. After subsequent washing six times with PBS/0.05% Tween, the secondary antibody (Dako #E0468) was diluted 1:2000 in blocking buffer/Tween (0.05%), 100 μl were added/well, and the plate was sealed for 1 hour at room temperature. After subsequent washing six to eight times with PBS/0.05% Tween, a strepavidin-HRP complex (Biosource SNN2004; 0.95 mg/ml) was diluted 1:40,000 fold in blocking buffer/Tween (0.05%) and 100 μl were added/well. The plate was sealed and incubated at room temperature in the dark. After subsequent washing six to eight times, 100 ml of stabilized Chromogen BioSourceSBo1 was added and the plate was incubated at room temperature, wrapped in aluminum-foil, and incubated on a shaker for color development for 5 to 30 minutes. The reaction was stopped by adding 50 μl stop solution (0.5M H2S04)-color of positive wells turns yellow. The optical density of the wells was read at 405 nm on a conventional ELISA reader. For the establishment of a standard curve we used recombinant-murine “generic” LTαβ consisting of both LTα2β1 and LTα3 (kindly provided by Dr. Jeffrey Browning, Biogen Idec, Inc.) or recombinant mouse Ltα (Cat.No.749-TB/CF; R&D) (0.5 μg/100 μl to 7 ng/100 μl). For negative control homogenates derived from LTα−/− mice were used. In our hand the detection limit/background was ∼15 ng/well.
Quantification of Relative Thymus Weight and Absolute Cell Number
For the analysis of relative thymus weight, body weight of the respective mice was weighted before thymi of different tg, wt, and knockout mice were isolated by microsurgery. Afterward, isolated thymi were weighted and thymic lobes were smashed to isolate connective tissue and fat from total thymic cells. Cells were diluted in PBS 1:10, 1:100, and 1:1000 and counted in a modified Neubauer chamber, as well as a CASY cell counter (Schärf Systems). For quantification of absolute thymic cells the average of at least three independent counting rounds was used.
Viruses and Peptides
The LCMV, WE strain, originally obtained from Dr. F. Lehmann-Grube (Hamburg, Germany), was propagated on L929 cells at a low multiplicity of infection and was plaqued as previously described.55 The LCMV-GP peptide KAVYNFATM (gp33) and the LCMV-NP peptide FQPGNGQFI (np396) were purchased from Neosystem (Strasbourg, France). Virus infection was performed with LCMV-WE (200 plaque-forming units) or LCMV-DOCILE (2 × 106 plaque-forming units). For densitometric analysis the Soft Imaging System AnalySISD (Olympus) was used.
Analysis of Insulin-Like Growth Factor-1 and Growth Hormone Serum Levels
Serum insulin-like growth factor (IGF)-I levels were measured by double-antibody IGF binding protein-blocked radioimmunoassay.56 Mouse growth hormone (GH) was measured by using a radioimmunoassay (RPA 551; Amersham) with a detection range of 1.3 to 100 ng/ml.
LCMV-NP Specific ELISA
The LCMV nucleoprotein-specific ELISA has been described previously using LMCV-NP expressed by Spodoptera frugiperda 9 (Sf9) cells after infection with a recombinant baculovirus.
Footpad Swelling Reaction
The indicated amounts of LCMV-WE were injected in a volume of 50 ml in balanced salt solution into both hind footpads in experimental groups of three mice. The footpad thickness was measured at the indicated time points with a spring-loaded caliper.
Construction of Tetrameric Class I-Peptide Complexes and Flow Cytometry
MHC class I (H-2Db) tetramers complexed with gp33 were produced as previously described. Briefly, H2-Db and human b2-microglobulin molecules were recombinantly expressed in E. coli (the plasmids were kindly provided by John Altman, Emory University, Atlanta, GA). Biotinylated H2-Db peptide complexes were purified using and Ákta Explorer 10 chromatography system (Pharmacia, Sweden) and tetramerized by addition of streptavidin-phycoerythrin (Molecular Probes, Eugene, OR). At the indicated time points after immunization, animals were bled and single cell suspensions were prepared of spleen and lymph nodes. Aliquots of 5 × 105 cells or three drops of blood were stained using 50 ml of a solution containing tetrameric class I-peptide complexes at 37°C for 10 minutes followed by staining with anti-CD8-FITC (PharMingen) at 4°C for 20 minutes. Erythrocytes in blood samples were lysed with FACS lysis solution (Becton Dickinson) and the cells were analyzed on a FACScan flow cytometer (Becton Dickinson) after gating on viable leukocytes. For the determination of absolute cell counts, the number of total viable leukocytes was assessed in an improved Neubauer chamber. For blood, the number of total viable leukocytes was automatically determined in an Advia counter (Bayer, Germany).
Results
Homo- and Heterotrimeric LT is Increased in Transgenic Thymi
Transgenic (tg) mice expressing LTα or LTβ under transcriptional control of the distal lck promoter57,58 (Figure 1A) were generated by pronuclear microinjection, and screened by Southern blot analysis (Figure 1B). In addition, double tg mice expressing both LTα and LTβ were generated by co-injecting both constructs. Four tg(Ltα) founder mice out of 35 offspring from microinjected zygotes, 5 tg(Ltβ) out of 53, and 4 tg(Ltαβ) out of 19 were identified by Southern blot and PCR analysis to harbor the respective transgenes (Figure 1B and data not shown). Two independent tg(Ltα), three independent tg(Ltβ), and two independent tg(Ltαβ) lines were obtained that expressed and passed the tg on to their progeny with a Mendelian ratio of approximately 50% (Table 1). For each construct, two lines were further bred for experimentation: tg(Ltαβ)856 and 857, tg(Ltα)54 and 57, as well as tg(Ltβ)19 and 44.
Figure 1.
Generation and characterization of tg mice expressing T cell derived LT. A: Schematic representation of tgLTα and tgLTβ constructs used for pronuclear microinjection on the basis of the 5′HG expression cassette.58 Arrows 1 to 6 indicate various primers used for PCR and RT-PCR analysis (see Material and Methods). B: Genomic Southern blot analysis identified founder mice positive for tgLtα, tgLtβ or DP for tgLtαβ. LTα or LTβ, endogenous (end.) and transgenic (tg) LTα or LTβ EcoRI fragments detected by a radioactively labeled probe. kbp: kilobase pairs C: RPA showing increased LTα and LTβ mRNA expression in spleen, thymus, MLN, and Peyer’s patches of double and single tg mice (lines: tg(Ltαβ)856 and tg(Ltα)54). TNF, IL6, IFNγ, IFNβ TGFβ1, TGFβ2, and MIF mRNA expression levels remained unchanged. tgLtαβ thymi mice displayed a three- to fivefold up-regulation of TGFβ3 mRNA. D: Histogram of FACS analysis for LTα1β2 expression on T (CD4+ and CD8+) and B cells (B220+/CD19+) in spleen, thymus, and MLN. Green lines depict tg(Ltαβ)856, purple fill Ltα−/− and pink line wt mice. B220+/CD19+ cells positive for LTα1β2 were detected in tg thymi. E: Analysis of LTα2β1, LTα1β2, and LTα3 in organ homogenates (thymus, spleen, MLN) of various transgenic lines, wt mice, and Ltα−/− mice by ELISA, reveals the presence of LTα3 in tgLtαβ and tgLtα lines but not in Ltα−/− mice.
Table 1.
List of tg Lines and Transgene Copy Numbers
| tg construct(s) | tg line | tg copy number |
|---|---|---|
| LckLtα | tg(Ltα) 6 | tgLtα 19 |
| tg(Ltα) 8 | tgLtα 1 | |
| tg(Ltα) 54 | tgLtα 1 | |
| tg(Ltα) 57 | tgLtα 4 | |
| LckLtβ | tg(Ltβ) 19 | tgLtβ 1 |
| tg(Ltβ) 20 | tgLtβ 14 | |
| tg(Ltβ) 31 | tgLtβ 7 | |
| tg(Ltβ) 44 | tgLtβ 1 | |
| tg(Ltβ) 47 | tgLtβ 7 | |
| LckLtα and LckLtβ | tg(Ltαβ) 855 | tgLtα 4/tgLtβ 5 |
| tg(Ltαβ) 856 | tgLtα 2/tgLtβ 2 | |
| tg(Ltαβ) 857 | tgLtα 6/tgLtβ 4 |
Lines marked in bold gave rise to tg-positive offspring, which expressed the tg and transmitted the tg in a Mendelian ratio. Number of tg copies was evaluated by phosphor-imaging and was quantified in relation to the signal that was the result of both endogenous alleles.
RNase protection assays showed increased overall LTα and/or LTβ mRNA expression in thymus, MLN, Peyer’s patches, and to a lesser extent in spleen of tgLtαβ and LT single tg mice (Figure 1C and data not shown). Statistical analysis of three independent RPA experiments revealed a significant up-regulation of LTα and LTβ mRNA in thymus (LTα: P < 0.02 and LTβ: P < 0.03), MLN (LTα: P < 0.01 and LTβ: P < 0.03) and spleen (LTα: P < 0.05 and LTβ: P < 0.04) as evaluated for lines tg(Ltαβ)856 and 857. Magnetic activated cell sorting was performed to analyze LTα and LTβ mRNA expression in enriched CD4+ or CD8+ thymic T cells of tg and wt mice. An increase in mRNA expression of LTα (16- to 32-fold) and LTβ (7- to 16-fold) was detected in tgCD4+ T cells. In tgCD8+ T cells a less pronounced increase was detected (LTα: 5- to 16-fold; LTβ: 4- to 10-fold) (see Supplemental Figure S1A at http://ajp.amjpathol.org). We also investigated the expression of additional TNF family members and cytokines in various organs. TGFβ3 mRNA expression was increased three- to fivefold in thymus and MLN of tgLtαβ mice. Importantly, TNFα, TNFR1, and TNFR2 mRNA expression were unchanged (Figure 1C and see Supplemental Figure S1B at http://ajp.amjpathol.org). Expression of membrane bound LTα1β2 protein was analyzed by flow cytometry of B and T cells from 6-week-old tg(Ltαβ)856 (green), wt (dark purple), and Ltα−/− (pink) naïve mice. Tg CD4+ and CD8+ cells expressed 5- to 40-fold more LTα1β2 in thymus, MLN, and spleen, when compared with wt T cells (Figure 1D, upper and middle row). B220+/CD19+ cells gated from MLN or spleen of wt and tg mice did not express increased LTα1β2 levels (Figure 1D, lower panels). In contrast to wt thymi, tg thymi contained B220+/CD19+ DP populations, which were positive for LTα1β2 (Figure 1D). Therefore, tg LTαβ expression was restricted to T cells with LTα1β2 expressing B cells in tg thymi.
With the currently available tools we analyzed homo- and heterotrimeric LT expression by ELISA in organ homogenates of 6 to 8-week-old mice. Thymi, MLNs, and spleens of lines tg(Ltαβ)856, 857 and tg(Ltα)54 and 57 showed detectable LT expression. In contrast, we could hardly detect homo- and heterotrimeric LT expression in wt mice and lines tg(Ltβ)19 and 44 (Figure 1 E and data not shown), and spleens and thymi of Ltα−/− lacked detectable LT expression. In the following we will mainly focus on line tg(Ltαβ)856. Lines tg(Ltαβ)857, tg(Ltα)54 and 57 displayed identical or very similar phenotypes. tg(Ltβ)19 and 44 lacked an overt phenotype and behaved like wt mice in all experiments described below.
Enhanced Expression of LT on T Cells Induced Accelerated Thymic Involution
We analyzed the time course of the expression of LT and other TNF family members in wt and tg(Ltαβ)856 thymi by RPA followed by quantitative fluoroscopy (Figure 2A). LTα and LTβ transcripts were up-regulated ∼10-fold in tgLtαβ thymi at postnatal day 1 (P1). At P7 LTα and LTβ were overexpressed ∼15-fold and ∼3-fold, respectively, and remained increased two- to fourfold over wt mice at all time points thereafter (Figure 2A).
Figure 2.
Macroscopic and cellular characterization of thymic involution in tgLtαβ mice. A: RNA expression of LT and various cytokines using RPA during postnatal development (P1 to P42). One representative experiment out of three for each time point is shown. LTα and LTβ were up-regulated in tg thymi during early postnatal development and in adult mice. B: Macroscopic analysis of postnatal thymus development in tgLtαβ mice and negative littermates. Thymic involution of tg mice can be detected between P7 and 42 (scale bar: 1 mm). C: Time course of alterations in relative thymus weight (ie, thymus weight with respect to body weight). Each time point is the mean of at least 3 mice analyzed. Onset of thymic involution in male C57BL/6 mice is earlier than in C57BL/6 female mice.7 tg thymi start involuting between postnatal day 7 and 14. D: FACS analysis of tg and wt thymi at P42. Numbers indicate the percentage of the respective cell populations. Pooled tgLtαβ thymi (n = 3 to 4) (lower row) show a reduction in DP T cells and an inverted CD4:CD8 ratio. A representative experiment from four is shown. E: FACS analysis of Lin− DN thymocytes of tgLtαβ and wt thymi (n = 4).
Tg thymi were normal in size at P1 (Figure 2B, females: P < 0.059; males: P < 0.70), confirming the absence of a primary anlage defect. However, the thymic weight—both in absolute terms and as ratio to body weight—was drastically reduced at P7 and P14 (P < 0.008, Figure 2C and see Supplemental Figure S2A at http://ajp.amjpathol.org), and reduced to rudiments at P42 and at all time points thereafter until P336 (P < 0.001).
In view of the above results we evaluated LTα and β mRNA expression by Taq-Man analysis in wt thymi of older age (3, 6, and 18 months). For control, thymi of Ltα−/− and Ltβ−/− mice were used (n = 3; data not shown). We found a modest increase in LTα and LTβ mRNA expression in thymi of wt mice over time ranging from two- to eightfold (see Supplemental Figure S2B at http://ajp.amjpathol.org), which was significant at 6 and 18 months of age (6 months of age: LTα, P < 0.02; LTβ, P < 0.03; 18 months of age: LTα, P < 0.01; LTβ, P < 0.03).
Because cortisol, IGF-I, and GH may contribute to thymic involution,5 we assessed the serum levels of these hormones in tgLtαβ and wt littermates at the age of 6 weeks (n = 3 to 4 each). No differences were detected in serum levels of cortisol (tgLtαβ: 17 ± 3 nmol/L; wt: 18 ± 4 nmol/L), IGF-I (tgLtαβ: 312 ± 20 ng/ml; wt: 324 ± 31 ng/ml; n = 4 each), and GH (female tgLtαβ: 44 ± 17 ng/ml; male tgLtαβ: 49 ± 21 ng/ml; female wt: 41 ± 14 ng/ml; male wt: 46 ± 28 ng/ml; three males and three females each).
Abnormal T Cell Development in tg Thymi
Flow cytometry of tgLtαβ thymocytes derived from pooled thymi (P42) revealed a decrease of double-positive (DP) CD4+CD8+ T cells (wt: 83 to 86%; tgLtαβ: 11 to 29%). The relative prevalence of CD8+ T cells was higher in tgLtαβ (7 to 12%) than in wt mice (3 to 5%), yet their absolute number was significantly reduced (P < 0.01, Figure 2D and see Supplemental Figure S2C at http://ajp.amjpathol.org). This was confirmed by quantification of total thymocytes (see Supplemental Figure S2C at http://ajp.amjpathol.org). A relative increase was also detected in CD4−CD8− double-negative (DN) cells (wt: 5 to 9%; tgLtαβ: 68 to 81%; Figure 2D). Absolute numbers of DN T cells were reduced in tgLtαβ (n = 4) (0.45 × 107 ± 0.1 × 107 cells/thymus) when compared with wt thymi (n = 4) (0.3 × 108 ± 0.15 × 108 cells/thymus; P = 0.001M and see Supplemental Figure S2C at http://ajp.amjpathol.org).
Tg thymi contained relatively increased CD44highCD4+ and CD44highCD8+ T cell subsets (Figure 2D, right panels, and see Supplemental Figure S2D and S2E at http://ajp.amjpathol.org). Also, DN CD25−CD44+ thymocytes were hyperprevalent, whereas DN CD25+CD44− thymocytes were decreased in tgLTαβ thymi (Figure 2D and see Supplemental Figure S2E at http://ajp.amjpathol.org, middle column).
To distinguish the various DN T cell subsets (DN1-DN4) from other populations in the thymus (eg, B cells, NK cells) we have excluded Lin+ thymocytes by magnetic activated cell sorting and analyzed Lin− thymocytes of tgLtαβ and wt thymi (n = 3 each) by flow cytometry analysis (Figure 2E). A significant reduction of Lin− cells within tgLTαβ thymi was observed (tgLTαβ: 0.25 × 107 ± 0.1 × 107 cells/thymus; wt: 0.45 × 108 ± 0.1 × 108 cells/thymus; P < 0.001). There was a slight decrease in the absolute number of tgLtαβ DN1 T cells (n = 3; 0.73 × 106 ± 0.11 × 106 cells/thymus) when compared to wt mice (n = 4; 0.85 × 106 ± 0.1 × 106 cells/thymus; P < 0.5), and a highly significant reduction of DN2, DN3, and DN4 T cells (P < 0.001; >2 log-fold reduction in all cases). FACS analysis for LTα1β2 revealed transgene expression in the DN3 and DN4 populations of tgLTαβ mice (data not shown).
Thymic early T lineage progenitors (Lin−CD25−CD44+c-kit+ ETPs) undergo depletion in aged mice.53 We found significant depletion of ETPs already in 6-week-old tgLTαβ thymi (n = 4; tgLtαβ: 0.15 × 105 ± 0.1 × 105; n = 3; wt: 0.5 × 105 ± 0.2 × 105; n = 3; P < 0.02).
The peripheral blood of tgLtαβ mice contained fewer total leukocytes (P < 0.03), CD4+ T cells (P < 0.001), and CD8+ T cells (P < 0.002; see Supplemental Figure S3A and B at http://ajp.amjpathol.org). This was accompanied by a relative increase of CD44high lymphocytes (P < 0.02) (see Supplemental Figure S3D and C at http://ajp.amjpathol.org).
Histological examination of the tgLtαβ thymic micro-architecture revealed a conspicuous reduction of cortical areas already at P1 (Figure 3), well before the onset of discernible involution, with concomitant enlargement of the medullary compartment (Figure 3 and 4A) as assessed with antibody anti-MTS10. The latter was even more pronounced at P42 (MTS10+area mm2/20 mm2 thymic tissue: P1: tgLtαβ: 6 ± 1.5, wt: 3 ± 0.4, P < 0.03; P42: tgLtαβ: 13 ± 2, wt: 3.5 ± 0.8, P < 0.001).
Figure 3.
Analysis of thymic microenvironment in tg mice. Immunohistochemical analysis of tg and wt thymi at P1. Even though no obvious macroscopic differences were observed at P1, tg thymic cortex was already reduced and thymic medulla enlarged (asterisks). Scale bar: 200 μm.
Figure 4.
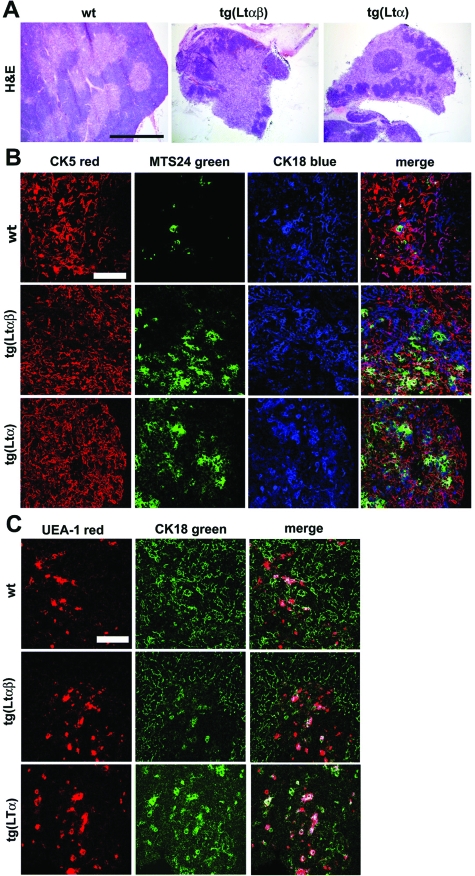
Increase in MTS24+ staining in tgLtαβ mice. A: H&E staining of wt, tgLtαβ, or tgLtα mice showing the relative enlargement of the thymic medullary compartment in tg mice when compared to wt mice. Scale bar: 400 μm. B: Confocal microscopy of thymi cryosections stained for CK5, CK18, and MTS24 were performed to investigate medullary and cortical micro-architecture by confocal microscopy. No apparent differences were observed by CK5 and CK18 staining. However, an increase in MTS24+ staining was observed in tg mice. Scale bar: 50 μm. C: Confocal microscopy of tg and wt thymi stained for UEA-1 and CK18 were performed. No differences in the distribution pattern or UEA-1/CK18 double positivity were observed between wt and tgLtαβ or tgLtα mice. Scale bar: 50 μm.
We further investigated whether the MTS24+ precursors of medullary and cortical stromal cells were changed in tgLtαβ mice.50 MTS24+ staining was ∼10-fold increased in tgLtαβ thymi at 6 weeks of age (Figure 4B). A similar increase was detected in lines tg(Ltαβ)857, tg(Ltα)54, and tg(Ltα)57, thereby excluding any integrational artifacts (Figure 4B). The strong increase in MTS24+ staining was not detected at P14 in any tg mouse lines (data not shown) and must therefore must have developed between P14 and P42. No significant difference in the number of UEA-1+ medullary epithelial cells was detected between tgLtαβ (P < 0.6) or tgLtα (P < 0.7) and wt thymi (Figure 4C). Normal differentiation of thymic medullary epithelial cells into medullary cells in thymi of tgLtαβ mice was observed (see Supplemental Figure 4 at http://ajp.amjpathol.org) and confocal microscopy of the cortico-medullary junction failed to reveal differences in the organization of CK5+ thymic medullary epithelial cells and CK18+ thymic cortical cells (see Supplemental Figure S5A at http://ajp.amjpathol.org).
The autoimmune regulatory element (Aire), expressed by the thymic stroma was reported to be regulated by the lymphotoxin pathway.59 We investigated the impact of T cell derived LTαβ on the Aire mRNA expression in the thymic stroma by RT-PCR analysis of tgLtαβ and wt thymi at various time points. Aire mRNA expression was similar in tgLtαβ and wt mice at 3, 6, and 9 weeks of age (see Supplemental Figure S5B at http:// ajp.amjpathol.org).
LT Induced Thymic Involution in tgLtαβ Mice Depends on LTβR and TNFR1 Signaling
LTα3 and LTα1β2 signal mainly through TNFR1 and TNFR2, and through LTβR, respectively.14,26 Hence thymic involution may result from signaling through any of these receptors. We therefore crossed tgLtαβ mice to receptor-deficient mice (Figure 5). Additionally, we tested whether B cells participate in thymic involution by crossing tgLtαβ and B-cell deficient JH−/− mice.19,20,34,35,60,61,62 Thymic pathology was prevented in Tnfr1−/− and Ltβr−/−, but not in Tnfr2−/−, Ltα−/−, Ltβ−/−, and JH−/− mice (all 6 to 7 weeks old; n = 4 each; Figure 5A and see Supplemental Figure S6A at http://ajp.amjpathol.org), as assessed by thymus weight (tgLtαβ vs. Tnfr1−/− x tgLtαβ or Ltβr−/− x tgLtαβ, P < 0.001), absolute number of thymocytes (Figure 5B-C), and distribution of DP, DN, and single-positive (SP) T cells (Figure 5D). These data indicate that LTα3 is expressed in tg mice and that it participates in the induction of the thymic phenotype.
Figure 5.
T cell triggered thymic involution is induced via TNFR1 and LTβR signaling. A: Macroscopic analysis of thymi from various knockout mice crossed to tgLtαβ mice (6 to 8 weeks old). Backcrossing to Tnfr1−/− and Ltβr−/− mice rescued the thymic phenotype due to LTαβ overexpression on T cells. Scale bar: 2 mm. B and C: Quantitative analysis of relative thymic weight and absolute number of thymocytes from these crossing experiments confirmed this observation. D: FACS analysis of tgLtαβ x Tnfr1−/− or tgLtαβ x Ltβr−/− mice reveals an amount of SP CD4+ and CD8+, DP CD4+CD8+ and DN CD4−CD8− T cell populations in thymus comparable to wt mice. A representative of three independent experiments is shown.
Increased Annexin V/7-AAD DP Cells in tg Thymi
We investigated whether accelerated thymic involution in tgLtαβ mice results from apoptosis (n = 4). Flow cytometry for Annexin V and 7-AAD revealed a strong increase of apoptotic 7-AAD/Annexin V positive cells in tg thymi (P28–42) (Figure 6A): Annexin V SP cells, indicative of apoptotic cells, increased from 1.4 ± 0.3% in wt thymi to 18 ± 8% in tgLtαβ thymi (P < 0.002). Annexin V/7-AAD DP cells increased from 1.3 ± 0.2% in wt to 5.5 ± 2% in tgLtαβ thymi (P < 0.01). Increased apoptosis of tgLtαβ thymi was also confirmed by positive terminal deoxynucleotidyl transferase-mediated dUTP staining of histological cryosections from wt and tgLtαβ thymi, revealing a highly significant increase in the number of positive cells, mainly in the cortical areas of the thymus (data not shown). We densitometrically analyzed the area of positivity mm2/10 mm2 of various thymus sections from individual mice (n = 3) at 3 and 6 weeks of age (2 weeks: tgLtαβ: 0.78 ± 0.2 mm2/10 mm2; wt: 0.13 ± 0.04 mm2/10 mm2, P < 0.005 and 6 weeks: tgLtαβ: 0.38 ± 0.3 mm2/10 mm2; wt: 0.15 ± 0.09 mm2/10 mm2, P < 0.01).
Figure 6.
Stromal TNFR1 and LTβR signaling triggered by tg T cells is responsible for thymic involution in tgLtαβ mice. A: Flow cytometric analysis of total thymus cells for Annexin V and 7-AAD was performed to investigate the magnitude of apoptosis in pooled tg (n = 3) and wt thymi (n = 3). A representative of three independent experiments is shown. B: Macroscopic analysis of thymi from mice reconstituted by BM transplantation from several genotypes: wt BM into wt, tg BM into wt, Tnfr1−/−, or Ltβr−/− mice. Transfer of tgLtαβ BM cells into wt mice induced thymic involution. In contrast, tgLtαβ BM failed to induce thymic involution in Tnfr1−/− and Ltβr−/− mice. C and D: Quantitative analysis of relative thymic weight and absolute number of thymocytes from the respective crossing experiments revealed prevention of the thymic, hypoplastic phenotype, particularly in Tnfr1−/− mice. E: Analysis of thymic micro-architecture confirmed normal distribution of CD4+ and CD8+ T cells in Tnfr1−/− or Ltβr−/− mice crossed to tgLtαβ mice. Scale bars: 50 μm (left 2 columns) and 200 μm (right 2 columns).
Stromal TNFR1 and LTβR Signaling Induce Thymic Involution
Thymic involution may be caused by autocrine effects of LTs onto T cells, or by destruction of the thymic TNFR1+ and LTβR+ stroma via tgLtαβ T cells.44,45 To address this question, we generated BM chimaeras whose LT signaling defects (either in TNFR1 or LTβR signaling) were confined to the stromal compartment.
Wt→wt, tgLtαβ→wt; wt→tgLtαβ; tgLtαβ→Tnfr1−/− and tgLtαβ→Ltβr−/− chimeric mice were generated (n = 4 each; Figure 6B). These mice were either competent or deficient for TNFR1 or LTβR-mediated signaling in thymic stromal cells, whereas their hematopoietic compartment was either tgLtαβ or wt. Reconstitution experiments were controlled by transplanting BM of β-ActGFP mice63 into lethally irradiated wt mice, which led to 95 to 98% reconstitution, as assessed by flow cytometry of peripheral blood cells (n = 3; data not shown).
Reconstitution of wt mice with tgLtαβ BM led to thymic rudimentalization and micro-architectural pathologies similar to those observed in tgLtαβ mice (Figure 6B and E), whereas reciprocal reconstitution (wt→tgLtαβ) did not revert macroscopic thymic involution, absolute number of thymocytes, or relative thymic weight (Figure 6B-D). In contrast, the absolute numbers of thymocytes and relative thymus weights of wt→wt chimaeras were similar to those of aged-matched (12 to 14 weeks) wt mice (Figures 6C-D and see Supplemental Figure S6B at http://ajp.amjpathol.org).
Tnfr1−/− mice reconstituted with tgLtαβ BM developed a normally sized thymus with number of thymocytes comparable to unreconstituted wt or Tnfr1−/− mice, in contrast to wt mice reconstituted with tgLtαβ BM (tgLtαβ→wt vs. tgLtαβ→Tnfr1−/−: P < 0.002) (Figure 6B-E and (see Supplemental Figure S7 at http://ajp.amjpathol.org). Therefore, stromal TNFR1-mediated signaling in non-hematopoietic thymic stromal cells induces thymic involution in the presence of tgLtαβ BM cells (Figure 6B). Similar results were observed in tgLtαβ BM→Ltβr−/− chimeras. We could observe an unaltered thymus size, relative thymic weight (tgLtαβ→wt vs. tgLtαβ→Ltβr−/−:P < 0.007), absolute number of thymocytes (Figure 6C-D), and undisturbed thymic micro-architecture (Figure 6E), when compared with wt mice that were reconstituted with tgLtαβ BM. These data suggest that thymic stromal TNFR1 and LTβR signaling pathways synergistically cause precocious thymic involution. Interestingly, crossings of tgLtαβ with Tnfr1+/− and Ltβr+/− mice revealed that thymic involution can be partially prevented with one allele of LTβR but not with one allele of TNFR1 in tgLtαβ mice (see Supplemental Figure S7 at http://ajp.amjpathol.org).
T Cell-Derived LT Modulates T Cell-Dependent Immune Response in vivo
TgLtαβ mice showed hypoplastic thymi with reduced numbers of thymic and peripheral CD4+ and CD8+ T cells. To study the functional significance of this phenotype, we investigated the immune response of tg mice on viral infection. We intravenously challenged tgLtαβ, tgLtβ, and wt mice (n = 4 each) with 200 plaque-forming units of LCMV-WE. Organs were taken 9 days post-infection and the efficiency of viral clearance was assessed by a plaque assay for LCMV-WE. In primary and secondary lymphoid organs (thymic rudiment, MLN, and spleen) the clearance of LCMV-WE was not as efficient as in wt controls (see Supplemental Figure S8A at http://ajp.amjpathol.org). Injection of LCMV-WE into footpads of mice led to markedly reduced footpad swelling in tgLtαβ mice than in tgLtβ or wt mice, indicating inefficient local CD8+ T cell response (see Supplemental Figure S8B at http://ajp.amjpathol.org). Despite this mitigated inflammatory response, the immunoglobulin class switch for antibodies specific for the LCMV nuclear protein (np396) remained unaltered (see Supplemental Figure S8C at http://ajp.amjpathol.org). Reduced activity of LCMV-specific T cells and reduced LCMV-WE specific CD8+ T cells were found by direct ex vivo killer assays in MLN, spleen, and blood (see Supplemental Figures S8D and 9 at http://ajp.amjpathol.org).
LCMV Infection Induces Acute Thymic Involution
Thymic involution of tgLtαβ mice with concomitant increase of CD44 expression on CD4+ and CD8+ T cells resembled a T cell activation phenotype. We therefore asked whether LCMV infection per se may induce thymic involution. Wt mice (n = 4 each) were infected with LCMV-WE or LCMV-DOCILE. Given the stronger virulence of LCMV-DOCILE when compared to LCMV-WE64 we anticipated a more pronounced effect in LCMV-DOCILE infected animals.
Age matched, untreated wt mice (n = 4) served as controls. Mice were sacrificed at 9 days post-infection, and thymi were investigated for size, weight, thymocyte cellularity, and micro-architecture. Macroscopically thymus size was reduced in LCMV-WE infected wt mice and even more drastically in LCMV-DOCILE infected wt mice when compared to untreated, age-matched controls (8 weeks old). (Figure 7A). Thymic involution was confirmed by quantification of the relative thymus weight and absolute cell number of thymocytes (Figure 7A and 7B). Histological analysis revealed disturbance of the thymic micro-architecture of LCMV infected wt mice with a reduction of the thymic cortex and a relative enlargement of the thymic medulla similar to what was observed in tgLtαβ mice (Figure 7C). The relative enlargement of the thymic medulla was densitometrically analyzed in LCMV-WE and LCMV-DOCILE infected animals (n = 4 each; 4 step cuts/mouse). Similar to tgLTαβ mice at P42 we observed a significant relative enlargement of the thymic medulla after infection with both, LCMV-WE and LCMV-DOCILE (MTS10+area mm2/20 mm2 thymic tissue: LCMV-WE: 8.5 ± 2.5, wt not-infected: 4.5 ± 1.5, P < 0.02; LCMV-DOCILE: 12.5 ± 3.5, wt not-infected: 4.5 ± 1.5, P < 0.003).
Figure 7.
Viral infection induces thymic involution and LT expression on T cells that is partially rescued in Tnfr1−/− mice. A and B: Reduction in relative thymic weight and absolute number of thymocytes in wt mice infected LCMV-WE, detected to greater extent in wt mice infected with LCMV-DOCILE at day 9 post-infection. C: Immunohistochemistry analysis of thymi from LCMV-DOCILE infected wt mice and controls revealed a reduction of the thymic cortex. Scale bar = 200 μm. D: Increased levels of TNFα in serum and thymus protein homogenate of LCMV-DOCILE infected wt and Tnfr1−/− mice. E and F: Partial rescue of thymic involution in Tnfr1−/− female mice and to a lesser degree in Tnfr1−/− male mice, as compared with uninfected wt mice or wt mice infected with LCMV-DOCILE. G: FACS analysis of thymi of LCMV-WE infected and non-infected wt mice. Thymus DP cells are reduced in LCMV-WE infected wt mice, whereas SP CD4+ and CD8+ T cells are increased. Histograms gated on CD8+ T cells indicate a strong up-regulation of LTα1β2 in 2 out 3 of thymi from LCMV-WE infected wt mice, and weak up-regulation in the third one. Interestingly, a similar but reduced effect was also detected on DN CD4−CD8− thymocytes.
We noted that TNFα levels were increased in sera and thymi of LCMV-DOCILE- and LCMV-WE-infected mice (Figure 7D and data not shown). We further investigated whether blocking of TNFR1 signaling could prevent thymic involution after LCMV-DOCILE infection. Tnfr1−/− mice were infected with LCMV-DOCILE and compared to infected and uninfected wt mice (n = 4 each, female and male). Infected Tnfr1−/− mice displayed increased TNFα levels similarly or slightly higher to infected wt mice (n = 3) (Figure 7D). Infected wt mice displayed a drastic reduction of relative thymic weight and absolute thymocytes regardless of the sex of the infected mice (Figure 7E and F). Female Tnfr1−/− mice infected with LCMV-DOCILE displayed significantly less thymic involution than infected wt mice (P < 0.01), whereas rescue was marginal in male Tnfr1−/− mice (P = 0.12).
Following LCMV-WE or LCMV-DOCILE infection, LTα1β2 expression was increased on the surface of CD8+ thymocytes and to lesser degree on DN CD4−CD8− T cells as investigated by flow cytometry analysis (Figure 7G and data not shown). In addition, we investigated homo- and heterotrimeric LT protein expression in thymic homogenates of LCMV-WE or LCMV-DOCILE infected animals at 9 days post-infection. We could observe an increase in LT expression in thymic homogenates of LMCV-WE or LCMV-DOCILE infected mice but not in control C57BL/6 mice (see Supplemental Figure S10 at http://ajp.amjpathol.org).
This was accompanied by a reduction in the absolute number of DP thymic T cells of wt mice infected with LCMV-WE or LCMV-DOCILE (42 to 71%; P < 0.01) when compared to uninfected wt mice (81 to 84%). In contrast SP CD4+ and CD8+ T cells were increased in relative and absolute numbers in LCMV-WE infected mice (infected CD4+ T cells: 13 to 34%, P < 0.01; CD8+ T cells: 6 to 13%, P < 0.03; uninfected CD4+ T cells: 6 to 9%; CD8 T cells: 3 to 5%). Moreover, primed, tetramer (gp33-reactive) positive CD8+ T cells, indicative of viral specific T cells that re-entered, were detected at 9 days post-infection in thymi of LCMV-WE-infected wt mice (see Supplemental Figure S10A and B at http://ajp.amjpathol.org).
The prevalence of gp33-specific CD8+ T cells in thymus (1.5 to 3% of all thymic gated CD8+ T cells) was lower than in spleen (9 to 12% of all splenic gated CD8+ T cells) (see Supplemental Figure S10A at http://ajp.amjpathol.org). In LCMV-DOCILE infected mice the amount of tetramer positive CD8+ T cells was less pronounced in spleen and thymus (data not shown). Accordingly, the absolute and relative number of splenic CD8+ T cells was only slightly, not significantly increased when compared to uninfected wt mice (data not shown). As reported earlier at that time point exhaustion of T cells in spleen and thymus of LCMV-DOCILE infected mice could already be detected (64 and data not shown).
Discussion
Here we investigated the functional consequences of chronic overexpression of LT by T cells on thymus development and homeostasis. Multiple lines of tgLtαβ and tgLtα mice showed accelerated thymic involution, aberrant T cell development, and changes in thymic micro-architecture. None of these pathologies were observed on overexpression of LTβ alone, suggesting that LTα, whose expression is tightly controlled in T cells, is crucial to the phenotype described.65 Overexpression of T cell-derived LT did not lead to an increase of cortisol, IGF-I, or GH serum levels,66,67 indicating that the pathologies were not mediated by these hormones.
SP CD4+ and CD8+ thymic tgLtαβ T cells, as well as CD4+CD8+ cells, were significantly reduced. Despite their relative increase within tgLtαβ thymic rudiments, the absolute numbers of DN T cells were also decreased. A breakdown of Lin− DN T cell subsets in tgLtαβ mice revealed a slight reduction of DN1 and a strong reduction of DN2, DN3, and DN4 T cell subsets.
ETPs, a subset of the DN1 T cell subset, are thought to reflect the developmental potential in the thymus53 and decrease over time in parallel with the progressively reduced thymic regenerative potential.53 We found that tgLtαβ thymi hosted fewer ETPs, similarly to aged wt thymi.53 Thymic atrophy of tgLtαβ went along with a strong reduction of the thymic cortex and a relative enlargement of the thymic medullary compartment, again reminiscent of age-related thymic involution.
MTS24+ cells, which are precursors of medullary and cortical stromal cells, were increased in thymi of tgLtαβ and tgLtα mice. This increase was observed at the peak of involution (P42) but not at birth, suggesting that it represents a compensatory mechanism.
Thymic involution was rescued by removal of either TNFR1 or LTβR, but not of TNFR2, LTα, LTβ, or B cells (as in JH−/− mice). This indicates that pathology is mediated by two non-redundant pathways: LTα3 signaling through TNFR1, and LTα1β2 signaling through LTβR. No up-regulation of TNFα mRNA was observed in tgLtαβ thymus and MLN.
What is the mechanism of thymic pathology? LT overexpression may conceivably lead to the cell-autonomous premature demise of thymocytes, thereby depleting the thymus of immature T cells. Alternatively, LT-expressing T cells may induce thymic involution by engaging pro-apoptotic receptors on thymic stromal cells. This question was clarified with reciprocal BM transplants. Thymic involution occurred in tgLtαβ→wt mice, but was prevented in tgLtαβ→Tnfr1−/− chimeric mice. Therefore tgLtαβ T cells primarily targeted the thymic stromal compartment, presumably via LTα3. In view of phenotype of tgLtαβ × Tnfr2−/− mice, LTα3→TNFR2 signaling seems irrelevant in this context.
Membrane-bound LTα1β2 mainly signals via LTβR. Thymic involution was rescued in tgLtαβ→ Ltβr−/− chimeric mice, but to a somewhat lesser extent than in Tnfr1−/− recipient mice. Therefore thymic damage is executed through TNFR1 and LTβR. Finally, administration of tgLtαβ BM to wt mice resulted in thymic destruction within 6 weeks after BM transplantation, ruling out intrinsic homing defects of tgLtαβ thymocytes as the cause of thymic involution. Interestingly, hemizygous crosses revealed that thymic involution was partially prevented by removing one allele of LTβR but not one allele of TNFR1, establishing a hierarchy in the signaling events leading to thymic pathology. These results help interpreting the previously reported finding41 that T cell-specific expression of a ligand of LTβR and herpesvirus entry mediator, induced thymic involution with some similarities to the phenotypes described here.
Efficient selection and release of CD4+ and CD8+ T cells is important for both the innate and adaptive immune system, suggesting that the immune competence of tgLtαβ mice might be compromised. Indeed, we found a reduced capacity of the host immune system to cope with LCMV-WE infection.
The above results raised the possibility that the acute thymic involution induced by viral infections might be caused by LT signaling and might be preventable by interfering with TNFR1 signaling. Infection with either of two LCMV strains raised LTα1β2 expression by thymocytes and TNFα levels in sera and TNFα and homo- as well as heterotrimeric LT levels in wt thymi. This coincided with thymic involution and a relative reduction of the cortical and a concomitant increase of the medullary thymic compartment. Moreover, we observed a reduction of the thymus/body weight ratio and of thymocyte numbers. Crucially, LCMV-induced thymic involution was greatly reduced in Tnfr1−/− female mice. However, this rescue was much less pronounced in Tnfr1−/− males, suggesting the contribution of gender-dependent factors most likely including steroid hormones.4,5,6,7,12
Tnfr1−/− mice were found to host an increased number of thymocytes, 42 suggesting that TNF signaling may participate both positively and negatively to the regulation of thymocyte production. Alternatively, the expression of ligands to TNFR family members by T cells appears to exert a negative-trophic effect on thymic stroma, and the resulting stromal atrophy may lead to reduced thymocyte output from the thymus.
Conditions that increase the expression of agonistic TNFR1/LTβR ligands by T cells may dramatically accelerate stromal atrophy and thymic involution. LCMV infection is an acute paradigm for such conditions. As subpopulations of T cells resident in the thymus are likely to experience activation at any time, it seems likely that the phenomena outlined above may contribute to physiological, age-dependent thymic involution.
Supplementary Material
Acknowledgments
We thank Prof. Rolf Zinkernagel for critically reading the manuscript and intellectual input; Dr. Fabio Montrasio for support during the construction of tgLt mice; Ahmed Hegazy for support with magnetic activated cell sorting; and Prof. Odermatt, Sylvia Behnke, Petra Schwarz, Mirzet Delic and Ilan Margalith for technical assistance. LTβR-Fc reagent for FACS analysis and “generic LTα2β1” was kindly provided by Dr. Jeffrey Browning, Biogen Idec, Inc.
Footnotes
Address reprint requests to Adriano Aguzzi, Institute of Neuropathology, University Hospital of Zürich, Schmelzbergstrasse 12, CH-8091 Zürich, Switzerland. E-mail: adriano.aguzzi@usz.ch.
Supported by grants from the Bundesamt für Bildung und Wissenschaft, the Swiss National Foundation (SNF), and the National Center of Competence in Research on neural plasticity (to A.A.), an EMBO fellowship (to J.P.), the foundation for Research at the Medical Faculty, the University of Zurich, the Verein zur Förderung des akademischen Nachwuchses (FAN), the Schweizer-MS foundation, the Bonizzi-Theler foundation and the Prof. Dr. Max-Cloëtta and Bonizzi-Theler foundation (to M.H.). S.A.N. is an International Research Scholar of the Howard Hughes Medical Institute. G.A.H. was supported by a grant (FP6) of the European Union, the SNF (grant 3100-68310.02), and the NIH (grant ROI-A1057477–01).
M.H. and M.P. contributed equally to this work.
Supplemental material for this article can be found on http://ajpamjpathol.org.
Current address of M.P.: Department of Neuropathology, University of Freiburg, Freiburg, Germany. Current address of A.T.: Department of Pathology, University of Chicago, Chicago, IL. Current address of T.J.: Novartis Institutes for BioMedical Research GmbH&Co, Vienna, Austria.
References
- von Boehmer H, Aifantis I, Gounari F, Azogui O, Haughn L, Apostolou I, Jaeckel E, Grassi F, Klein L. Thymic selection revisited: how essential is it? Immunol Rev. 2003;191:62–78. doi: 10.1034/j.1600-065x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- Thoman ML. The pattern of T lymphocyte differentiation is altered during thymic involution. Mech Ageing Dev. 1995;82:155–170. doi: 10.1016/0047-6374(95)01597-s. [DOI] [PubMed] [Google Scholar]
- Goya RG, Bolognani F. Homeostasis, thymic hormones and aging. Gerontology. 1999;45:174–178. doi: 10.1159/000022082. [DOI] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. Evolution of the thymus size in response to physiological and random events throughout life. Microsc Res Tech. 2003;62:464–476. doi: 10.1002/jemt.10408. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. Age-related changes in primary and secondary immune organs of the mouse. Immunol Invest. 1998;27:153–165. doi: 10.3109/08820139809089453. [DOI] [PubMed] [Google Scholar]
- Imami N, Aspinall R, Gotch F. Role of the thymus in T lymphocyte reconstitution. Transplantation. 2000;69:2238–2239. doi: 10.1097/00007890-200006150-00002. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D, Pido-Lopez J. Age-associated changes in thymopoiesis. Springer Semin Immunopathol. 2002;24:87–101. doi: 10.1007/s00281-001-0098-z. [DOI] [PubMed] [Google Scholar]
- Brown OA, Sosa YE, Dardenne M, Pleau J, Goya RG. Growth hormone-releasing activity of thymulin on pituitary somatotropes is age dependent. Neuroendocrinology. 1999;69:20–27. doi: 10.1159/000054399. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D. Thymic atrophy in the mouse is a soluble problem of the thymic environment. Vaccine. 2000;18:1629–1637. doi: 10.1016/s0264-410x(99)00498-3. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins LIGHT and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21:655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. Lymphotoxin-alpha-dependent spleen microenvironment supports the generation of memory B cells and is required for their subsequent antigen-induced activation. J Immunol. 2000;164:2508–2514. doi: 10.4049/jimmunol.164.5.2508. [DOI] [PubMed] [Google Scholar]
- Mackay F, Majeau GR, Lawton P, Hochman PS, Browning JL. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur J Immunol. 1997;27:2033–2042. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Browning JL. Turning off follicular dendritic cells. Nature. 1998;395:26–27. doi: 10.1038/25630. [DOI] [PubMed] [Google Scholar]
- Browning JL, Dougas I, Ngam-ek A, Bourdon PR, Ehrenfels BN, Miatkowski K, Zafari M, Yampaglia AM, Lawton P, Meier W. Characterization of surface lymphotoxin forms. Use of specific monoclonal antibodies and soluble receptors, J Immunol. 1995;154:33–46. [PubMed] [Google Scholar]
- Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL, Sizing ID, Lawton P, Bourdon PR, Rennert PD, Majeau GR, Ambrose CM, Hession C, Miatkowski K, Griffiths DA, Ngam-ek A, Meier W, Benjamin CD, Hochman PS. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Worm M, Geha RS. CD40 ligation induces lymphotoxin alpha gene expression in human B cells. Int Immunol. 1994;6:1883–1890. doi: 10.1093/intimm/6.12.1883. [DOI] [PubMed] [Google Scholar]
- Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. Journal of Experimental Medicine. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikenwalder M, Zeller N, Seeger H, Prinz M, Klohn PC, Schwarz P, Ruddle NH, Weissmann C, Aguzzi A. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science. 2005;307:1107–1110. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- Grech AP, Riminton DS, Gabor MJ, Hardy CL, Sedgwick JD, Godfrey DI. Increased thymic B cells but maintenance of thymic structure. T cell differentiation and negative selection in lymphotoxin-alpha and TNF gene-targeted mice, Dev Immunol. 2000;8:61–74. doi: 10.1155/2000/13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R, Mebius R, Browning JL, Michie SA, van Tuijl S, Kraal G, van Ewijk W, McDevitt HO. Effects of tumor necrosis factor and lymphotoxin on peripheral lymphoid tissue development. Int Immunol. 1998;10:727–741. doi: 10.1093/intimm/10.6.727. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX. Lymphotoxin receptor is required for the migration and selection of autoreactive T cells in thymic medulla. J Immunol. 2007;179:8069–8075. doi: 10.4049/jimmunol.179.12.8069. [DOI] [PubMed] [Google Scholar]
- Hirahara H, Ogawa M, Kimura M, Iiai T, Tsuchida M, Hanawa H, Watanabe H, Abo T. Glucocorticoid independence of acute thymic involution induced by lymphotoxin and estrogen. Cell Immunol. 1994;153:401–411. doi: 10.1006/cimm.1994.1038. [DOI] [PubMed] [Google Scholar]
- Fayad R, Sennello JA, Kim SH, Pini M, Dinarello CA, Fantuzzi G. Induction of thymocyte apoptosis by systemic administration of concanavalin A in mice: role of TNF-alpha. IFN-gamma and glucocorticoids, Eur J Immunol. 2005;35:2304–2312. doi: 10.1002/eji.200526062. [DOI] [PubMed] [Google Scholar]
- Wang J, Chun T, Lo JC, Wu Q, Wang Y, Foster A, Roca K, Chen M, Tamada K, Chen L, Wang CR, Fu YX. The critical role of LIGHT, a TNF family member, in T cell development. J Immunol. 2001;167:5099–5105. doi: 10.4049/jimmunol.167.9.5099. [DOI] [PubMed] [Google Scholar]
- Baseta JG, Stutman O. TNF regulates thymocyte production by apoptosis and proliferation of the triple negative (CD3-CD4-CD8-) subset. J Immunol. 2000;165:5621–5630. doi: 10.4049/jimmunol.165.10.5621. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force WR, Walter BN, Hession C, Tizard R, Kozak CA, Browning JL, Ware CF. Mouse lymphotoxin-beta receptor. Molecular genetics, ligand binding, and expression, J Immunol. 1995;155:5280–5288. [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- Bluethmann H, Rothe J, Schultze N, Tkachuk M, Koebel P. Establishment of the role of IL-6 and TNF receptor 1 using gene knockout mice. J Leukoc Biol. 1994;56:565–570. doi: 10.1002/jlb.56.5.565. [DOI] [PubMed] [Google Scholar]
- Raeber AJ, Klein MA, Frigg R, Flechsig E, Aguzzi A, Weissmann C. PrP-dependent association of prions with splenic but not circulating lymphocytes of scrapie-infected mice. EMBO J. 1999;18:2702–2706. doi: 10.1093/emboj/18.10.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA: 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure, Proc Natl Acad Sci USA: 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nat Immunol. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- Prinz M, Heikenwalder M, Junt T, Schwarz P, Glatzel M, Heppner FL, Fu YX, Lipp M, Aguzzi A. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425:957–962. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- Saparov A, Wagner FH, Zheng R, Oliver JR, Maeda H, Hockett RD, Weaver CT. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 1999;11:271–280. doi: 10.1016/s1074-7613(00)80102-8. [DOI] [PubMed] [Google Scholar]
- Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- Shakhov AN, Lyakhov IG, Tumanov AV, Rubtsov AV, Drutskaya LN, Marino MW, Nedospasov SA. Gene profiling approach in the analysis of lymphotoxin and TNF deficiencies. J Leukoc Biol. 2000;68:151–157. [PubMed] [Google Scholar]
- Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs, Growth Regul. 1994;4:11–19. [PubMed] [Google Scholar]
- Chaffin KE, Beals CR, Wilkie TM, Forbush KA, Simon MI, Perlmutter RM. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber AJ, Sailer A, Hegyi I, Klein MA, Rülicke T, Fischer M, Brandner S, Aguzzi A, Weissmann C. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication, Proc Natl Acad Sci USA: 1999;96:3987–3992. doi: 10.1073/pnas.96.7.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- Neumann B, Luz A, Pfeffer K, Holzmann B. Defective Peyer’s patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Boitchenko VE, Yarovinsky FO, Rice NR, Nordheim A, Ruhlmann A, Nedospasov SA. Cyclosporin A blocks the expression of lymphotoxin alpha, but not lymphotoxin beta, in human peripheral blood mononuclear cells. Blood. 2002;100:1721–1727. [PubMed] [Google Scholar]
- Cole TJ, Liddicoat DR, Godfrey DI. Intrathymic glucocorticoid production and thymocyte survival: another piece in the puzzle. Endocrinology. 2005;146:2499–2500. doi: 10.1210/en.2005-0255. [DOI] [PubMed] [Google Scholar]
- Purton JF, Zhan Y, Liddicoat DR, Hardy CL, Lew AM, Cole TJ, Godfrey DI. Glucocorticoid receptor deficient thymic and peripheral T cells develop normally in adult mice. Eur J Immunol. 2002;32:3546–3555. doi: 10.1002/1521-4141(200212)32:12<3546::AID-IMMU3546>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.