Abstract
Preeclampsia, a common pregnancy disorder associated with an increase in systemic inflammation, is the leading cause of maternal and fetal morbidity and mortality throughout the world. It is associated with shallow extravillous trophoblast invasion of the decidua, leading to uteroplacental blood flow that is inadequate for the developing fetal-placental unit. In preeclamptic women, interleukin-6 (IL-6) levels in plasma, but not placenta, are elevated, prompting evaluation of the decidua as a potential source of this excess, circulating IL-6. The current study found significantly higher immunohistochemical staining for IL-6 in decidual cells from preeclamptic versus preterm, gestational age-matched control placentas. Pro-inflammatory cytokines associated with the genesis of preeclampsia (i.e., tumor necrosis factor-α and interleukin-1β) enhanced IL-6 mRNA levels and increased secreted IL-6 levels in first trimester leukocyte-free decidual cell incubations, as measured by real time quantitative RT-PCR, ELISA, and Western blotting. Therefore, decidual cell-derived IL-6 may contribute to excess circulating IL-6 levels that can promote both endothelial cell dysfunction (and subsequent vascular dysfunction) and the pathogenesis of preeclampsia whereas locally elevated IL-6 levels may contribute to an excess of decidual macrophages implicated in shallow extravillous trophoblast invasion of the decidua.
Maternal-fetal interactions create a mild systemic inflammatory state exemplified by activation of both vascular endothelium and leukocytes that is most apparent in the third trimester of uncomplicated human pregnancies.1 Preeclampsia is a common disorder of human pregnancy that is associated with a further increase in systemic inflammation.1 It is a leading cause of maternal and fetal morbidity and mortality throughout the world and complicates an estimated 6 to 8% of pregnancies in the United States.2 A comprehensive model of preeclampsia2 indicates that immune maladaption at the implantation site contributes to impaired trophoblast invasion and subsequent incomplete transformation of the decidual spiral arteries and arterioles.3,4 The resulting reduced utero-placental blood flow produces placental ischemia and hypoxia.5,6 The latter is associated with a maternal circulation containing reduced levels of angiogenic factors7,8,9 and elevated levels of anti-angiogenic factors,9,10,11 placental debris,12,13 reactive oxygen species,14,15,16 and proinflammatory cytokines.16,17,18,19,20,21,22,23,24,25,26 Altered levels of these circulating factors promote endothelial cell activation and vascular damage leading to proteinuria and hypertension, the primary symptoms of the maternal syndrome of preeclampsia.2,27
Numerous reports indicate that the plasma of preeclamptic patients contains elevated levels of interleukin-6 (IL-6),16,17,18,19,20,21,22,23,24,25,26 a multifunctional cytokine that regulates hematopoiesis as well as the acute phase reaction28 and modulates both pro- and anti-inflammatory events.29 That IL-6 also interferes with endothelial cell function30,31 suggests that preeclampsia-associated elevated plasma IL-6 levels contribute to the systemic endothelial activation and vascular damage that culminates in the maternal syndrome. Several studies examined the placenta as a source of excess circulating IL-6, but found that IL-6 expression was either unchanged18,32,33,34 or reduced35 in preeclamptic versus control placentas.
The current study evaluated the implantation site as a potential source of IL-6 expression in preeclampsia. Immunohistochemical comparisons were made between IL-6 levels in decidual cells and in adjacent interstitial cytotrophoblasts in preeclamptic versus gestational age-matched control placentas. Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) are associated with several aspects of the pathogenesis of preeclampsia.36,37,38,39 A significant subset of cases of preeclampsia is associated with underlying maternal infections that are a source of these proinflammatory cytokines.2 Moreover, TNF-α, derived from an excess of macrophages in the preeclamptic decidua,40 is implicated in inhibition of trophoblast invasion.38,39 Therefore, regulation of IL-6 mRNA and protein expression was assessed in cultured decidual cells, the predominant cell type encountered by invading cytotrophoblasts.41 Specifically, leukocyte-free first trimester human decidual cells were incubated with each proinflammatory cytokine together with estradiol (E2) as the control incubation, or with E2 plus medroxyprogesterone acetate (MPA) to mimic the steroid milieu of pregnancy.
Materials and Methods
Patients and Tissue
Biopsies of decidua basalis used for immunohistochemistry obtained from placentas of both preeclamptic and gestational age-matched controls, were formalin-fixed and paraffin-embedded under approval of the Yale University School of Medicine Human Investigation Committee. Table 1 provides relevant clinical details of the patients and controls. Patients with preeclampsia (n = 10) were diagnosed according to standard criteria,42 with nine of the patients meeting the criteria for severe preeclampsia with either a systolic blood pressure >160 mmHg or a diastolic blood pressure >110 mmHg on two occasions 6 hours apart, with the presence of significant proteinuria >5 g in a 24-hour urine collection. Placental specimens were obtained from cesarean delivery without labor in all preeclamptic patients or gestational age-matched controls with idiopathic preterm labor without signs of preeclampsia (n = 10). Control placental specimens were obtained after either cesarean or vaginal delivery and none displayed any clinical or histological evidence of chorioamnionitis or chronic villitis.
Table 1.
Characteristics of the Women Who Provided Placental Samples
| Variable | Preeclampsia (n = 10) | Preterm control (n = 10) | P value |
|---|---|---|---|
| Age, years* | 31 ± 3 | 24 ± 3 | 0.065 |
| Nulliparity† | 5 (50) | 5 (50) | 1.000 |
| Gestational age, weeks* | 29.7 ± 1.2 | 30.5 ± 1.0 | 0.605 |
| Systolic blood pressure, mmHg* | 172 ± 7 | 117 ± 6 | <0.001 |
| Diastolic blood pressure, mmHg* | 102 ± 2 | 62 ± 3 | <0.001 |
| Dipstick protein‡ | 4 [2 to 4] | 0 [0 to 0] | <0.001 |
| 24-hour protein, g | 5 [0.7 to 7.2] | NA | NA |
| IUGR† | 3 (30) | 0 (0) | 0.211 |
| HELLP syndrome† | 3 (30) | 0 (0) | 0.211 |
| PPROM† | 0 (0) | 5 (50) | 0.033 |
| Birth weight, g* | 1077 ± 139 | 1532 ± 202 | 0.085 |
| Cesarean delivery† | 8 (80) | 3 (30) | 0.070 |
| Histological chorioamnionitis stages II to III† | 0 (0) | 0 (0) | 1.000 |
Data presented as mean ± SEM and analyzed by Student’s t-test.
Data presented as n (%) and analyzed by Fisher’s exact test.
Data presented as median [range] and analyzed by Mann-Whitney test.
PPROM, preterm premature rupture of the membranes; IUGR, intrauterine growth restriction; HELLP, syndrome of hemolysis, elevated liver enzymes, and low platelets.
First-trimester decidual specimens were obtained from nine elective terminations between 8 and 12 weeks of gestation under Institutional Review Board approval at Bellevue Hospital, New York, NY. After separating the decidua from the amnio-chorion, a small portion of each specimen was formalin-fixed and paraffin-embedded then histologically examined for signs of underlying acute and chronic inflammation. The remainder was used for decidual cell isolation.
Immunohistochemistry
Five-μm sections of paraffin-embedded samples were deparaffinized in xylene and rehydrated in a graded ethanol series. The slides were then boiled in citrate buffer (10 mmol/L, pH 6.0) for 15 minutes for antigen retrieval. Sections were immersed in 3% hydrogen peroxide (in 50% methanol/50% distilled water) for 15 minutes to block endogenous peroxidase then incubated in a humidified chamber with 5% blocking horse serum in Tris-buffered saline (TBS) (Lab-Vision, Fremont, CA) for 30 minutes at room temperature. After removing excess serum, the serial sections were incubated with either mouse monoclonal anti-human IL-6 antibody (10 μg/ml) in 1% blocking horse serum in TBS (R&D Systems, Minneapolis, MN) or mouse monoclonal anti-vimentin antibody or anti-cytokeratin antibody (1:100 dilution in TBS) (DakoCytomation, Carpinteria, CA) overnight at 4°C in a humidified chamber. For negative controls, normal mouse IgG isotypes were used at the same concentrations as the primary antibodies. The sections were washed three times for 5 minutes in TBS then treated with biotinylated horse anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) at a 1:400 dilution for 30 minutes at room temperature. The antigen-antibody complex was detected with an avidin-biotin-peroxidase kit (Vector Laboratories). Diaminobenzidine (3,3-diaminobenzidine tetrahydrochloride dehydrate) (Vector Laboratories) was used as the chromogen, and sections were counterstained with hematoxylin and mounted with Permount (Fisher Chemicals, Springfield, NJ) on glass slides.
The intensity of IL-6 immunostaining was evaluated semiquantitatively using the following categories: 0 (no staining), 1+ (weak, but detectable, staining), 2+ (moderate or distinct staining), and 3+ (intense staining). For each specimen an HSCORE value was derived by calculating the sum of the percentage of cells that stained in each intensity category and multiplying that value by the weighted intensity of the staining, using the formula HSCORE = Pi (i + l), where i represents the intensity score, and Pi is the corresponding percentage of cells.43 In each slide, five different areas and 100 cells per area were evaluated microscopically with a ×40 objective magnification. The percentage of cells at each intensity within these areas was determined at different times by two investigators blinded to the source of the samples, and the average score was used.
Isolation of Decidual Cells
Digestion of minced tissues with 0.1% collagenase type IV and 0.01% DNase in RPMI containing 20 μg/ml of penicillin/streptomycin and 1 μl/ml of fungizone) (Invitrogen, Carlsbad, CA) in a 37°C shaking water bath for 30 minutes was followed by washing with sterile phosphate-buffered saline (PBS) for three times and then subjected to consecutive filtration through 100-μm, 70-μm, and 40-μm Millipore filters (Millipore Corp., Bedford, MA). Cells were resuspended in RPMI, grown to confluence on polystyrene tissue culture dishes, harvested using trypsin/ethylenediaminetetraacetic acid, and analyzed by flow cytometric analysis with anti-CD45 and anti-CD14 monoclonal antibodies (BD Pharmingen, San Diego, CA) to monitor the presence of leukocytes after each passage. After three to four passages, cell cultures were found to be leukocyte-free (<1%). Cultured decidual cells were vimentin-positive and cytokeratin-negative and displayed decidualization-related morphological and biochemical changes during incubation with a progestin. Decidualization-related biochemical changes included enhanced expression of prolactin and plasminogen activator inhibitor-1 (PAI-1) and inhibited expression of interstitial collagenase and stromelysin-1 (data not shown). Cell aliquots were frozen in fetal calf serum/dimethyl sulfoxide (9:1) (Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen.
Experimental Incubations
Thawed cells were incubated in basal medium [a phenol red-free 1:1 v/v mix of Dulbecco’s modified Eagle’s medium (Invitrogen) and Ham’s F-12 (Flow Labs, Rockville, MD), with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone] supplemented with 10% charcoal-stripped calf serum. After two additional passages, confluent cultures were incubated in parallel in BMS containing either 0.1% ethanol, as vehicle control or 10−8 mol/L estradiol (E2) with or without 10−7 mol/L MPA (Sigma-Aldrich), which was used in place of progesterone because of its greater stability in culture.44 The use of E2 as the control incubation for E2 plus MPA was prompted by elevated circulating E2 and progesterone levels during gestation. After 7 days, the cultures were washed twice with Hanks’ balanced salt solution to remove residual serum elements. The cultures were switched to a defined medium (DM) consisting of BM plus ITS+ (Collaborative Research, Waltham, MA), 5 μmol/L FeSO4, 50 μmol/L ZnSO4, 1 nmol/L CuSO4, 20 nmol/L Na2SeO3, trace elements (Invitrogen), 50 μg/ml ascorbic acid (Sigma-Aldrich), and 50 ng/ml epidermal growth factor (Becton-Dickinson, Bedford, MA) with either vehicle or steroids or 0.01 to 10 ng/ml of IL-1β or TNF-α (R&D Systems). After the test period, cells were harvested by scraping into ice-cold PBS, pelleted, and extracted in ice-cold lysis buffer. Conditioned medium supernatants and cell lysates were stored at −70°C. Total RNA was extracted from parallel incubations with Tri Reagent (Sigma-Aldrich).
Enzyme-Linked Immunosorbent Assay (ELISA)
Total cell protein levels were measured by a modified Lowry assay (Bio-Rad Laboratories, Inc., Hercules, CA). An ELISA kit from R&D Systems measured IL-6 levels in the cell-conditioned DM according to the manufacturer’s instructions. The sensitivity of the ELISA is 0.7 pg/ml with intra-assay and interassay coefficients of variation of 3.1% and 2.7%, respectively.
Western Blotting
Western blot analysis was performed on concentrated conditioned DM supernatants, which were diluted 1:1 in Laemmli sample buffer and then boiled for 3 minutes. The samples were subjected to electrophoresis on a 10 to 20% sodium dodecyl sulfate polyacrylamide linear gradient gel (Bio-Rad) with subsequent electroblotting transfer onto a 0.2-μm nitrocellulose membrane (Bio-Rad). After transfer, the membrane was blocked overnight in Tris-buffered saline (Fisher, Fairlawn, NJ) with 4% nonfat dry milk and then incubated for 2 hours with 0.2 μg/ml of a mouse anti-human IL-6 monoclonal antibody (R&D Systems). Membranes were rinsed in PBS and 0.2% Tween 20 before and after incubation with horseradish peroxidase-conjugated anti-mouse IgG (ICN Biomedicals, Aurora, OH). Chemiluminescence was detected with enhanced chemiluminescence reagents (Perkin Elmer Life Sciences, Boston, MA) and audioradiography film (Amersham Pharmacia, Buckinghamshire, UK) according to the manufacturers’ instructions.
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To verify that the IL-6 and β-actin probes yielded the correct bands, extracted RNA from experimental cell incubations were subjected to semiquantitative RT-PCR using a kit from Invitrogen, performing 35 cycles with the Eppendorf Mastercycler (Eppendorf, Westbury, NY). To perform quantitative real-time RT-PCR, reverse transcription was initially performed with AMV reverse transcriptase (Invitrogen). A quantitative standard curve was created between 40 pg to 2.5 ng of cDNA with a Roche Light Cycler (Roche, Indianapolis, IN) by monitoring increasing fluorescence of PCR products during amplification. On establishing the standard curve, quantitation of the unknowns was determined with the Roche Light Cycler and adjusted to the quantitative expression of β-actin from the corresponding unknowns. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation. All products obtained yielded correct melting temperatures. Products were then run on a 1.2% agarose gel along with a 100-bp DNA ladder and then stained with ethidium bromide for visualization. The following primers were synthesized and gel-purified at the Yale DNA Synthesis Laboratory, Critical Technologies: β-actin sense, 5′-CGTACCACTGGCATCGTGAT-3′; antisense 5′-GTGTTGGCGTACAGGTCTTTG-3′; 452-bp product; IL-6 sense, 5′-ATGCAATAACCACCCCT-3′; antisense, 5′-AGTGTCCTAACGCTCATAC-3′; 277-bp product.
Statistical Analysis
Control and treatment groups were compared by the Kruskal-Wallis analysis of variance on ranks test followed by the Student-Newman-Keuls post hoc test with a P value <0.05 representing statistical significance. The intensity of immunostaining in control versus preeclamptic cases was compared by the same tests, with significance set at a probability of <0.05.
Results
Immunostaining of IL-6 in Decidual Specimens
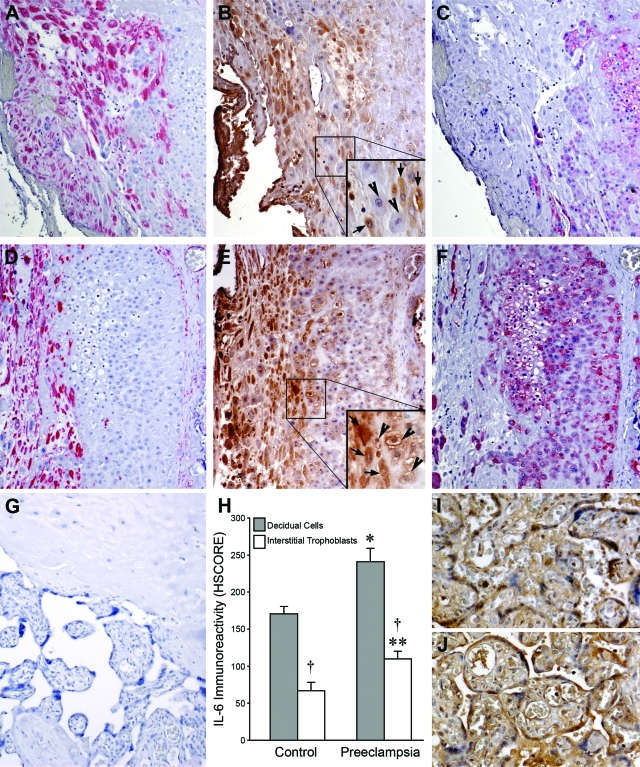
Control and preeclamptic decidua were immunostained for IL-6, vimentin, and cytokeratin (Figure 1). Decidual cells are identified by the positive (red) vimentin staining in control (Figure 1A) and preeclamptic (Figure 1D) specimens. Interstitial cytotrophoblasts are identified by positive (red) cytokeratin staining in control (Figure 1C) and preeclamptic (Figure 1F) specimens. Immunostaining for IL-6 was significantly greater in the cytoplasm of decidual cells from preeclamptic specimens (Figure 1E) compared with control specimens (Figure 1B). Immunostaining for IL-6 was also greater in interstitial cytotrophoblasts from preeclamptic decidua (Figure 1E) versus control decidua (Figure 1B). However, in contrast to the homogeneous cytoplasmic IL-6 immunostaining observed in the vimentin-positive decidual cells, IL-6 immunostaining in the cytokeratin-positive interstitial cytotrophoblasts was perimembranous and most prominent in cytotrophoblasts adjacent to the decidual cells. As evident by differences in HSCORE values, immunostaining for IL-6 was greater in both decidual cells and interstitial trophoblasts of preeclamptic specimens (Figure 1E, inset; ×200) compared to control specimens (Figure 1B, inset; ×200). Specifically, in the decidual cells, the HSCORE values for IL-6 staining in the preeclamptic specimens (241 ± 18, mean ± SEM) were significantly higher than that for the controls (171 ± 10, P < 0.05; Figure 1H). The HSCORE values for IL-6 in interstitial trophoblasts were also significantly higher in preeclamptic specimens (110 ± 10) than in control specimens, (67 ± 11, P < 0.05; Figure 1H). Moreover, the immunostaining intensity for IL-6 was more prominent in decidual cells compared to interstitial cytotrophoblasts, in both cases and controls (P < 0.05). By contrast, IL-6 staining in placental villi was not significantly different between control (Figure 1I) and preeclamptic (Figure 1J) villi. Negative control IgG revealed no staining (Figure 1G).
Figure 1.
Immunohistochemical analysis of IL-6 expression in preeclamptic decidua. Control specimens are shown in A–C and I, and preeclamptic specimens are shown in D–G and J. Vimentin staining (red) identifies decidual cells in control (A) and preeclamptic specimens (D), whereas cytokeratin staining (red) identifies cytotrophoblasts in control (C) and preeclamptic specimens (F). More intense IL-6 staining (brown) is seen in the cytoplasm of preeclamptic (E) versus control decidual cells (B). IL-6 immunostaining is also greater in preeclamptic (E) versus control interstitial cytotrophoblasts (B). The increased IL-6 immunostaining in preeclamptic interstitial cytotrophoblasts is mostly perimembranous and most prominent in cytotrophoblasts (E, inset, arrowheads; enlarged from the boxed area) adjacent to decidual cells (E, inset, arrows), whereas in control cytotrophoblasts, perimembranous IL-6 staining is weak or absent (B, inset, arrowheads), even adjacent to decidual cells (B, inset, arrows). G: Staining with a mouse isotype was used as a negative control for the IL-6 monoclonal antibody, shown in a preeclamptic specimen. H: IL-6 staining HSCOREs (mean ± SEM) between normal (n = 10) and preeclamptic specimens (n = 10); *versus control decidual cells; **versus control interstitial cytotrophoblasts; †interstitial cytotrophoblasts versus corresponding decidual cells; P < 0.05. Notably, IL-6 immunoreactivity was similar in control (I) and placental villi (J). Original magnifications: ×100 (A–G); ×150 (I, J); ×200 (B and E, insets).
IL-6 Protein Expression in Cultured Decidual Cells
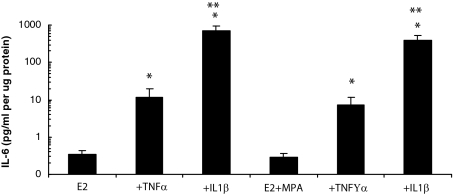
Figure 2 displays the separate and interactive effects of MPA and cytokines on immunoreactive levels of IL-6 in conditioned DM during incubation with leukocyte-free, first trimester decidual cells. It indicates that secreted levels of IL-6 were similar in incubations with E2 or with E2 + MPA, whereas the cytokines elicited a marked, differential up-regulation in IL-6 output. Specifically, basal IL-6 output in incubations with E2 alone was 0.35 ± 0.09 pg/ml/μg protein and increased with the addition of 1.0 ng/ml of TNF-α or IL-1β to 11.4 ± 8.1 and 713.3 ± 254.8 pg/ml/μg protein, respectively. These increases resulted in corresponding fold changes from baseline in IL-6 output of 25.7 ± 10.0 and 2170 ± 625 (P < 0.05) for TNF-α or IL-1β, respectively. Similarly, in incubations with E2 + MPA alone, IL-6 output was 0.28 ± 0.09 pg/ml/μg protein and the addition of 1.0 ng/ml of TNF-α or IL-1β enhanced IL-6 output to 7.3 ± 4.4 and 382 ± 155 pg/ml/μg protein, respectively (P < 0.05). The corresponding fold changes were 26.0 ± 13.4 and 1539 ± 514, respectively (P < 0.05). The specific cytokine-induced increases in the E2 + MPA group were not significantly different from those in the E2 group.
Figure 2.
Effects of E2, MPA, TNF-α, and IL-1β on IL-6 output by decidual cell monolayers. Confluent, leukocyte-free, first trimester decidual cells were incubated for 7 days in 10−8 mol/L E2 or E2 + 10−7 mol/L MPA then switched to DM with corresponding steroid(s) ± 1 ng/ml of TNF-α or IL-1β for 24 hours. IL-6 levels were measured by ELISA in conditioned DM and normalized to cell protein (n = 9 separate patients’ decidual specimens, mean ± SEM). Ordinate represents log scale of IL-6 pg/ml/μg cell protein. *Versus E2 or E2 + MPA (P < 0.05); **versus + corresponding TNF-α (P < 0.05).
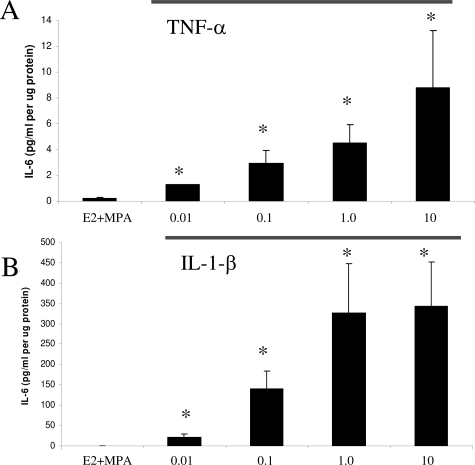
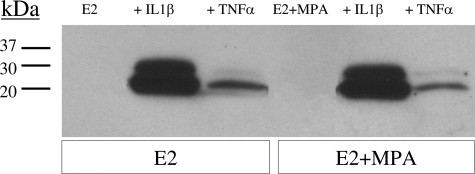
In view of the absence of progestin effects on basal and cytokine-induced IL-6 output, dose-response studies of TNF-α (Figure 3A) and IL-1β ((Figure 3B) were performed on cultured decidual cells that were primed with, and then maintained in E2 + MPA. The results show that both cytokines elicited clear concentration-dependent effects. Western blotting was performed to confirm the ELISA results of Figure 2. The Western blot depicted in Figure 4 indicates that the conditioned DM from decidual cells incubated with TNF-α or IL-1β contains a doublet that arises from differences in glycosylation as described by Gross and colleagues.45 Figure 4 also confirms the differential effects elicited by TNF-α and IL-1β revealed by the ELISA results.
Figure 3.
Concentration-dependent effects of TNF-α and IL-1β on IL-6 output by decidual cell monolayers maintained in E2 + MPA. Confluent, leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 mol/L E2 + 10−7 mol/L MPA, then switched to DM with the steroids ± the indicated amount of TNF-α (A) (0.01 to 10.0 ng/ml) or IL-1β (B) (0.01 to 10.0 ng/ml). The IL-6 levels were measured by ELISA in conditioned DM and normalized to cell protein (n = 3 separate patients’ decidual specimens, mean ± SEM). *Versus E2 + MPA, P < 0.05.
Figure 4.
Western blot analysis of TNF-α and IL-1β effects on IL-6 output by decidual cell monolayers maintained in E2 or E2 + MPA. Confluent, leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 mol/L E2 or E2 + 10−7 mol/L MPA, then switched to a DM with corresponding steroid(s) ± 1 ng/ml of TNF-α or IL-1β for 24 hours. Conditioned DM was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (see Materials and Methods).
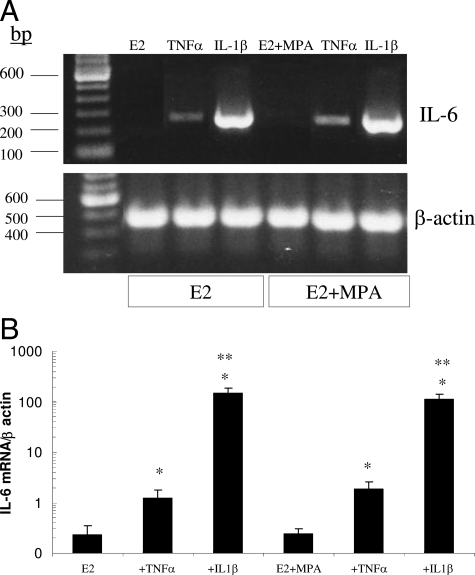
IL-6 mRNA Expression in Cultured Decidual Cells
Figure 5 depicts IL-6 mRNA expression in the first trimester decidual cell monolayers by end point RT-PCR run on an agarose gel (Figure 5A) and by quantitative real-time RT-PCR (Figure 5B). Consistent with the IL-6 protein results presented above, IL-1β was more effective than TNF-α in up-regulating steady state IL-6 mRNA levels and inclusion of MPA during incubation of the decidual cells affected neither basal nor inflammatory cytokine-enhanced IL-6 output. Inspection of the gel in Figure 5A indicates that unlike the marked up-regulation of IL-6 mRNA levels by TNF-α and IL-1β, β-actin levels were unchanged. These conclusions were confirmed by quantitative real-time RT-PCR. Specifically, Figure 5B indicates that TNF-α and IL-1β enhanced IL-6 mRNA levels by 6.7 ± 2.3-fold and 933 ± 205-fold, (mean ± SEM, P < 0.05), respectively, compared with incubations with E2 alone. By contrast, no differences in β-actin levels were observed (results not shown). Similar changes were found in incubations with E2 + MPA, which did not significantly differ from E2 incubations.
Figure 5.
Effects of E2, MPA, TNF-α, and IL-1β on IL-6 mRNA levels in decidual cell monolayers. Confluent leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 mol/L E2 or 10−8 mol/L E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 1 ng/ml of TNF-α or IL-1β for 6 hours. Aliquots of extracted total RNA were used to measure IL-6 mRNA levels by A: agarose gel stained with ethidium bromide for endpoint RT-PCR products for IL-6 mRNA (top) and β-actin mRNA (bottom). B: Quantitative real-time RT-PCR; ordinate, IL-6 mRNA/β-actin mRNA levels plotted on a log scale (n = 4 separate patient decidual specimens, mean ± SEM). *Versus E2 or E2 + MPA; **versus + corresponding TNF-α; P < 0.05.
Discussion
Members of the IL-6 cytokine family bind to the α chain of specific surface receptors to form complexes with at least one subunit of the gp130 signal transduction protein.28,29 Studies with transgenic mice indicate roles for three family members: IL-6, IL-11, and leukemia inhibitory factor in implantation-associated events.46 In the case of IL-6, gene knockouts experienced reduced fertility and a significant decrease in numbers of viable implantation sites.47 In human endometrium, maximal IL-6 mRNA and protein levels were observed in the mid-luteal, peri-implantational phase.48
The current study compared IL-6 immunostaining in the decidua of preeclamptic and gestational age-matched control preterm women. In the decidual cells, IL-6 was localized primarily in the cytoplasm. As reflected in differences in HSCOREs a marked increase in staining intensity and in numbers of IL-6-positive decidual cells was observed in decidual cells of preeclamptic versus control patients. Although IL-6 immunostaining in adjacent interstitial cytotrophoblasts was also significantly greater in preeclamptic versus control specimens, the staining was much less prominent than that observed in the decidual cells. Moreover, unlike the decidual cells, IL-6 immunostaining was localized primarily around the cell membranes in adjacent interstitial cytotrophoblasts. Previous publications observed that IL-6 expression was either unchanged18,32,33,34 or even reduced,35 in preeclamptic versus control placentas, but did not specifically examine IL-6 expression in interstitial cytotrophoblasts. Therefore, this differential localization of IL-6 suggests a potential, previously undisclosed, paracrine interaction in which IL-6 is synthesized and secreted by decidual cells and then targets neighboring interstitial trophoblasts.
Consistent with the decidua as a source of new IL-6 protein synthesis, Dudley and colleagues49 demonstrated that TNF-α and IL-1 enhanced expression of IL-6 in term decidual cell cultures. The current results extend this observation by demonstrating that TNF-α and IL-1β also markedly up-regulated IL-6 mRNA and protein expression in leukocyte-free first trimester decidual cells. Taken together, the results presented in this study suggest that decidual cells, the major cell type encountered by invading extravillous trophoblasts at the implantation site,41 are a likely source of circulating IL-6 during preeclampsia.
Binding of IL-6 to target cell-expressed surface receptors promotes dimerization with GP-130 to initiate intracellular signaling.28,29 That endothelial cells are such targets is indicated by reports that IL-6 promotes vascular dysfunction. Specifically, IL-6 enhances endothelial cell permeability likely by altering the ultrastructural distribution of tight junctions30 and IL-6 inhibits vascular prostacyclin production by down-regulating cyclooxygenase expression.31 In addition, IL-6 can induce aberrant angiogenesis.50 Compared with plasma from control women, the plasma of preeclamptic patients is also reported to contain elevated levels of placental debris12,13 and reactive oxygen species,14,15,16 as well as augmented levels of proinflammatory cytokines such as TNF-α,16,17,18,19,25 soluble forms of the TNF receptor,19,24,26 and interleukin-8.16,20,21 These inflammatory mediators likely synergize with elevated plasma IL-6 levels to promote systemic vascular damage, particularly in the kidney, that results in the characteristic proteinuria and hypertension of the maternal syndrome of preeclampsia.2,27
Previously, we51 and others38,52 found that the preeclamptic deciduas contained a marked excess of macrophages. In several diseases, IL-6 mediates the transition from acute to chronic inflammation in part by promoting monocyte/macrophage chemoattraction and activation while reciprocally inhibiting neutrophil chemoattraction and activation.29 Although monocytes can give rise to either dendritic cells or macrophages, which comprise an estimated 1 to 4% and 20 to 25%, respectively, of the immune cells at the implantation site.41,53 IL-6 has been shown to switch the differentiation away from dendritic cells to macrophages;54 however, we found that treatment of first trimester decidual cells with proinflammatory cytokines markedly up-regulated the expression of several monocyte/macrophage chemoattracting and activating chemokines including CCL2, CCL5, CXCL2, CXCL3, and CXCL8.51,55 These chemokines and IL-6 stimulate cells via different surface receptors suggesting that they synergize to contribute to the excess of macrophages described in the preeclamptic decidua. The results of in vitro studies suggest that macrophage-derived TNF-α inhibits cytotrophoblast invasion of the decidua by causing the cytotrophoblasts to undergo apoptosis38 and/or by reciprocally inhibiting expression of urokinase type-plasminogen activator and enhancing expression of plasminogen activator-1, which then inactivates urokinase bound to its receptor at the leading edge of trophoblast invasion.39,56
In summary, the current study provides the first evidence that the decidua is a potential source of the characteristic elevation in plasma IL-6 levels observed in preeclamptic patients and demonstrates that inflammatory cytokines associated with preeclampsia, IL-1β and TNF-α, markedly up-regulate IL-6 mRNA and protein expression by the resident decidual cells. This augmented expression of decidual IL-6 may play roles in the pathogenesis of preeclampsia by: serving as a source of a key circulating factor that promotes systemic maternal endothelial cell dysfunction; and indirectly impeding cytotrophoblast invasion by contributing to excess macrophage infiltration of the decidua.
Footnotes
Address reprint requests to Frederick Schatz, Ph.D., Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale University School of Medicine, 333 Cedar St., Room 810 LCI, P.O. Box 208063, New Haven, CT 06520-8063. E-mail: frederick.schatz@yale.edu.
Supported by the National Institutes of Health (grants 2 R01HD33937-05 and 1 R01 HL070004-04 to C.J.L.).
References
- Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24 (Suppl A):S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Dekker G, Kupferminic M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunell NO, Lewander R, Mamoun I, Nylund L, Sarby S, Thornstrom S. Uteroplacental blood flow in pregnancy induced hypertension. Scand J Clin Lab Invest Suppl. 1984;169:S28–S35. doi: 10.3109/00365518409085374. [DOI] [PubMed] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- Nadar SK, Karalis I, Al Yemeni E, Blann AD, Lip GY. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb Haemost. 2005;94:1071–1076. doi: 10.1160/TH05-03-0167. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Qian C, Leshane ES, Yu KF, England LJ, Schisterman EF, Wataganara T, Romero R, Bianchi DW. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004;190:707–713. doi: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Barden A, Ritchie J, Walters B, Michael C, Rivera J, Mori T, Croft K, Beilin L. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension. 2001;38:803–808. doi: 10.1161/hy1101.092969. [DOI] [PubMed] [Google Scholar]
- Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol. 1996;174:975–982. doi: 10.1016/s0002-9378(96)70336-8. [DOI] [PubMed] [Google Scholar]
- Walker JJ. Antioxidants and inflammatory cell response in preeclampsia. Semin Reprod Endocrinol. 1998;16:47–55. doi: 10.1055/s-2007-1016252. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Ellis J, Wennerholm UB, Bengtsson A, Lilja H, Pettersson A, Sultan B, Wennergren M, Hagberg H. Levels of dimethylarginines and cytokines in mild and severe preeclampsia. Acta Obstet Gynecol Scand. 2001;80:602–608. [PubMed] [Google Scholar]
- Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, Ernerudh J, Ekerfelt C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84:937–940. [PubMed] [Google Scholar]
- Takacs P, Green KL, Nikaeo A, Kauma SW. Increased vascular endothelial cell production of interleukin-6 in severe preeclampsia. Am J Obstet Gynecol. 2003;188:740–744. doi: 10.1067/mob.2003.134. [DOI] [PubMed] [Google Scholar]
- Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82:797–802. doi: 10.1034/j.1600-0412.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstet Gynecol. 1996;88:420–427. doi: 10.1016/0029-7844(96)00179-2. [DOI] [PubMed] [Google Scholar]
- Gammill HS, Roberts JM. Emerging concepts in preeclampsia investigation. Front Biosci. 2007;12:2403–2411. doi: 10.2741/2242. [DOI] [PubMed] [Google Scholar]
- Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signaling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–329. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res. 2002;104:118–123. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- Maruo N, Morita I, Ishizaki Y, Murota S. Inhibitory effects of interleukin 6 on prostaglandin I2 production in cultured bovine vascular endothelial cells. Arch Biochem Biophys. 1992;292:600–604. doi: 10.1016/0003-9861(92)90037-w. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ueda Y, Ohkura T, Inaba N. Interleukin-6 concentrations in the placenta and blood in normal pregnancies and preeclampsia. Horm Metab Res. 2005;37:419–424. doi: 10.1055/s-2005-870231. [DOI] [PubMed] [Google Scholar]
- Al-Othman S, Omu AE, Diejomaoh FM, Al-Yatama M, Al-Qattan F. Differential levels of interleukin 6 in maternal and cord sera and placenta in women with pre-eclampsia. Gynecol Obstet Invest. 2001;52:60–65. doi: 10.1159/000052943. [DOI] [PubMed] [Google Scholar]
- Opsjøn SL, Austgulen R, Waage A. Interleukin-1, interleukin-6 and tumor necrosis factor at delivery in preeclamptic disorders. Acta Obstet Gynecol Scand. 1995;74:19–26. doi: 10.3109/00016349509009937. [DOI] [PubMed] [Google Scholar]
- Kauma SW, Wang Y, Walsh SW. Preeclampsia is associated with decreased placental interleukin-6 production. J Soc Gynecol Investig. 1995;2:614–617. doi: 10.1016/1071-5576(95)00007-2. [DOI] [PubMed] [Google Scholar]
- Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Tempfer CB, Gregg AR. Polymorphisms within the interleukin-1 beta gene cluster and preeclampsia. Obstet Gynecol. 2001;97:664–648. doi: 10.1016/s0029-7844(01)01128-0. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Obstetric Practice ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- Kayisli O, Kayisli UA, Luleci G, Arici A. In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol Reprod. 2004;71:714–721. doi: 10.1095/biolreprod.104.027235. [DOI] [PubMed] [Google Scholar]
- Arici A, Marshburn PB, MacDonald PC, Dombrowski RA. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Gross V, Andus T, Castell J, Vom Berg D, Heinrich PC, Gerok W. O- and N-glycosylation lead to different molecular mass forms of human monocyte interleukin-6. FEBS Lett. 1989;247:323–326. doi: 10.1016/0014-5793(89)81361-4. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- Robertson SA, O'Connell A, Ramsay A. The effect of interleukin-6 deficiency on implantation, fetal development and parturition in mice. Proc Aust Soc Reprod Biol. 2000;31:97. [Google Scholar]
- Vandermolen DT, Gu Y. Human endometrial interleukin-6 (IL-6): in vivo messenger ribonucleic acid expression, in vitro protein production, and stimulation thereof by IL-1 beta. Fertil Steril. 1996;66:741–747. [PubMed] [Google Scholar]
- Dudley DJ, Trautman MS, Araneo BA, Edwin SS, Mitchell MD. Decidual cell biosynthesis of interleukin-6: regulation by inflammatory cytokines. J Clin Endocrinol Metab. 1992;74:884–889. doi: 10.1210/jcem.74.4.1548355. [DOI] [PubMed] [Google Scholar]
- Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005;73:237–243. doi: 10.1095/biolreprod.104.038000. [DOI] [PubMed] [Google Scholar]