Abstract
Systemic sclerosis (SSc)-related pulmonary fibrosis, for which there are few effective therapies, is the most common cause of SSc-related mortality. We examined insulin-like growth factor (IGF)-II expression in explanted lung tissues from control and SSc patients to determine its role in the pathogenesis of fibrosis. IGF-II levels in vivo were detected using immunohistochemistry. Primary lung fibroblasts were cultured from lung tissues, and IGF-II mRNA was measured using reverse transcriptase-polymerase chain reaction. Western blot analysis measured extracellular matrix (ECM) production and phosphorylated signaling molecules. Immunostaining revealed increased IGF-II expression in fibroblastic foci of SSc lungs. Furthermore, primary SSc lung fibroblasts had a fourfold increase in IGF-II mRNA and a twofold increase in IGF-II protein compared with normal lung fibroblasts. IGF-II mRNA in SSc lung fibroblasts was expressed primarily from the P3 promoter of the IGF-II gene, and IGF-II induced both a dose- and time-dependent increase in collagen type I and fibronectin production. IGF-II triggered the activation of both phosphatidylinositol-3 kinase and Jun N-terminal kinase signaling cascades, the inhibition of which diminished IGF-II-induced ECM production. Our study demonstrates increased local IGF-II expression in SSc-associated pulmonary fibrosis both in vitro and in vivo as well as IGF-II-induced ECM production through both phosphatidylinositol-3 kinase- and Jun N-terminal kinase-dependent pathways. Our results provide novel insights into the role of IGF-II in the pathogenesis of SSc-associated pulmonary fibrosis.
Systemic sclerosis (SSc)-related pulmonary fibrosis is the most common cause of SSc-related mortality.1 Fibrosis in SSc is believed to result from the interaction of immune mediators and other growth factors with fibroblasts, which respond by increasing matrix production in the skin and internal organs. Insulin-like growth factors and their binding proteins (IGFBP) have been implicated in the pathogenesis of pulmonary fibrosis. Increased IGF-I has been reported in bronchoalveolar lavage fluid in patients with SSc-related pulmonary fibrosis2 as well as other forms of pulmonary fibrosis such as idiopathic pulmonary fibrosis, sarcoidosis, and coal miner’s pneumoconiosis.3,4 IGF-II levels, on the other hand, have not been examined. We have found IGFBP-3 and -5 to be elevated in lung tissues of patients with pulmonary fibrosis.5 We have also shown that IGFBP-5 can induce fibrosis in vitro and in vivo.6,7 Our findings suggest that the IGF/IGFBP system plays a role in the development and perpetuation of pulmonary fibrosis.
IGF-II is a mitogenic peptide that is required for fetal growth and lung development.8 IGF-II is normally highly expressed in the fetus. Shortly after birth, IGF-II levels dramatically decrease in the systemic circulation, but IGF-II remains locally expressed in a variety of tissues. Elevated IGF-II levels in malignancy have classically been studied in Wilms’ tumor9 and subsequently implicated in other malignancies such as colon adenocarcinoma and breast cancer.10,11 Elevated IGF-II expression has also been implicated in Beckwith-Wiedemann syndrome,12 which is characterized by somatic overgrowth, glossomegaly, and organomegaly. IGF-II has been shown to stimulate human lung fibroblast proliferation.13,14 However, the role of IGF-II in fibrosis and wound healing has not been delineated. No published studies to date have examined the role of IGF-II in human pulmonary fibrosis or in SSc.
IGF-II gene transcription can be initiated from one of four promoter sites (P1 to P4), producing unique mRNA species, which generate the same IGF-II peptide.15,16 Gene expression from each promoter is tissue- and development-specific.15,17 Fetal tissues normally express IGF-II from P2, P3, and P4, whereas the adult liver expresses IGF-II from P1. A variety of malignancies have been found to express IGF-II from P3 and P4.18,19,20,21
We have identified IGF-II as a novel factor involved in the pathogenesis of pulmonary fibrosis in SSc. Using tissues derived from explanted lungs of patients with scleroderma-associated pulmonary fibrosis, we show that IGF-II is aberrantly expressed in lung fibroblasts. We also demonstrate that the IGF-II gene in SSc lung fibroblasts is transcribed primarily from the P3 promoter. Additionally, we show that IGF-II induces extracellular matrix (ECM) deposition in primary lung fibroblasts. We thus conclude that IGF-II local expression in SSc lung is increased and propose that IGF-II contributes to the pathogenesis of pulmonary fibrosis in SSc by inducing ECM deposition in an autocrine or paracrine manner.
Materials and Methods
Tissues and Cells
Lung explants were obtained from SSc patients undergoing lung transplantation at the University of Pittsburgh Medical Center under a protocol approved by the University of Pittsburgh Institutional Review Board. Normal lung tissues were obtained from organ donors whose lungs were not used for transplant surgery. Fibroblasts were cultured from minced tissue in Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), penicillin, streptomycin, and antimycotic agent (Invitrogen). Cells were used in passages two to six. Fibroblasts were stimulated with recombinant human IGF-II (R&D Systems, Minneapolis, MN). Kinase inhibitors LY294002 (Cell Signaling Technology, Danvers, MA), U0126 (Cell Signaling Technology), and Jnk II inhibitor (Calbiochem, San Diego, CA) were added to media of cells 1 hour before IGF-II stimulation.
Immunohistochemistry
Six-μm sections of paraffin-embedded lung tissues were deparaffinized, and endogenous peroxidases were quenched with 3% H2O2. Sections were blocked with 5% serum and incubated with polyclonal anti-IGF-II antibody or IgG isotype controls (R&D Systems). Sections were washed and incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). Bound secondary antibody was detected using the Vectastain ABC kit (Vector Laboratories) and the AEC Red kit (Zymed, San Francisco, CA). A light hematoxylin counterstain was used to identify nuclei. Images were taken on an Eclipse 800 microscope (Nikon Instruments, Huntley, IL) using identical camera settings. Four separate SSc and normal lung tissues were analyzed and representative images were shown.
Immunofluorescence
Double-immunofluorescent staining was performed using polyclonal anti-IGF-II (R&D Systems) and monoclonal anti-α-smooth muscle actin (Sigma-Aldrich) antibodies or goat (R&D Systems) and mouse (Lab Vision Corporation, Fremont, CA) isotype controls. Sections were incubated with biotinylated secondary antibodies and visualized with Texas Red- and AMCA-avidin-conjugated stains (Vector Laboratories). Images were taken on an Olympus Provis microscope (Olympus America, Melville, NY) using identical camera settings.
Immunocytochemistry
Primary lung fibroblasts were fixed in acetone and blocked with 5% serum. Cells were incubated with anti-IGF-II or goat IgG antibodies, washed, and incubated with biotinylated secondary anti-goat antibody. Bound secondary antibody was detected with Texas Red Avidin D stain (Vector Laboratories). Nuclei were stained with 4,6-diamidino-2-phenylindole (Vector Laboratories). Images were taken on an Olympus Fluoview 1000 (Olympus America) under identical settings. Signal intensity was measured using Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Assay
Primary lung fibroblasts were grown in media containing 0.5% fetal bovine serum, and total mRNA was isolated using Trizol reagent (Invitrogen). Normal liver tissue was obtained through the Liver Tissue Procurement and Distribution System, Pittsburgh, Pennsylvania, which is supported by the National Institutes of Health contract no. N01-DK-9-2310. Total RNA from homogenized whole liver tissue was isolated using cesium chloride density-gradient ultracentrifugation as previously described.22 Total RNA from HepG2 hepatocarcinoma cell lines was isolated using Trizol. Complementary DNA was prepared using Superscript II reverse transcriptase and random hexamer primers (Invitrogen). PCR was performed using primers designed specifically for total IGF-II mRNA amplification: forward primer 5′-TCCTCCCTGGACAATCAGAC-3′ and reverse 5′-AGAAGCACCAGCATCGACTT-3′. Primers for promoter-specific IGF-II mRNA species were previously described.18 Promoter-specific forward primers were 5′-CAGTGACTCCCCGGTCCTCTTTAT-3′ (P1, 362 bp), 5′-ACCGGGCATTGCCCCCAGTCTCC-3′ (P2, 249 and 409 bp), 5′-CAGAGCGGCGCTGGCAGAGGAGT-3′ (P3, 151 bp), and 5′-CAGCGAGCCTTCTGCTGAGCTGTA-3′ (P4, 76 bp). Reverse primer p11 used for promoter-specific mRNA was 5′-GAAGCTTAGAAGCACCAGCATCGACTTC-3′. Primers used for β-actin were forward primer 5′-ATGTTTGAGACCTTCAACAC-3′ and reverse primer 5′-CACGTCACACTTCATGATGG-3′. PCR products were separated by electrophoresis on an agarose or polyacrylamide gel and visualized after ethidium bromide staining. Expression levels were measured by scanning densitometry.
Western Blot Analysis
Culture supernatants and cell lysates were obtained as previously reported.5,7 Samples were analyzed by Western blotting with polyclonal anti-type I collagen, monoclonal anti-fibronectin, monoclonal anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-Akt (Thr308), anti-Akt, anti-phospho-GSK-3β, anti-phospho-SAPK/JNK, anti-JNK, and anti-phospho-c-Jun antibodies (Cell Signaling Technology). Signals were detected after incubation with horseradish peroxidase-conjugated secondary antibody and chemiluminescence (Perkin Elmer, Boston, MA).
3H-Thymidine Incorporation Assay
Equal numbers of fibroblasts were plated in triplicate in 96-well plates and cultured in serum-deprived media for 24 hours. Fibroblasts were then stimulated with IGF-II for a total of 48 hours. 3H-thymidine was added (1 μCi/well) for the last 24 hours of incubation. 3H-thymidine counts were measured in a β-emission counter in triplicate for each condition and results were averaged and normalized to counts obtained using unstimulated fibroblasts.
Statistical Analysis
All comparisons were performed using paired t-test.
Results
IGF-II Expression Is Increased in SSc-Related Pulmonary Fibrosis
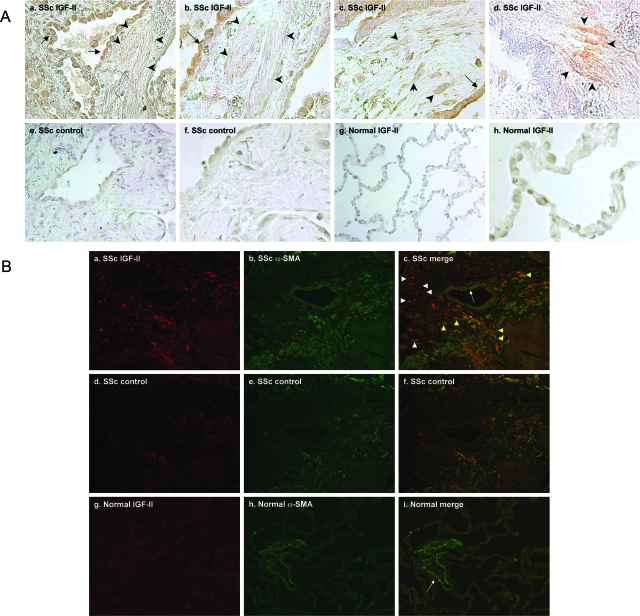
Lung sections were obtained from SSc patients undergoing lung transplantation at the University of Pittsburgh Medical Center between 1999 and 2006. All SSc lung sections were examined by a pulmonary pathologist and were characterized as usual interstitial pneumonia pattern (Table 1). Normal lung sections were obtained from a pool of potential organ donors through the Center for Organ Recovery and Education (Table 2). IGF-II protein expression was analyzed by immunohistochemistry in paraffin-embedded lung sections from patients and compared with lung sections from healthy donors (Figure 1A). Normal lungs did not show any significant IGF-II staining. IGF-II expression was increased in SSc lungs compared to normal lung tissues and localized to spindle-shaped cells in the interstitial space that were suggestive of fibroblastic foci. IGF-II also localized to epithelial cells of alveoli and airway. Immunohistochemical staining of four SSc and four normal lungs showed similar results.
Table 1.
Clinical Characteristics of Patients with SSc
| Age (years)/sex | Lung histology | Smoking (pack years) | Disease type | Autoantibody | |
|---|---|---|---|---|---|
| SSc-5 | 48/F | UIP | 0 | Diffuse | Topoisomerase |
| SSc-8 | 26/F | UIP | 0 | Sine | U11/U12 RNP |
| SSc-24 | 44/M | UIP | 0 | n/a | n/a |
| SSc-26 | 57/M | UIP | 0 | Diffuse | n/a |
| SSc-27 | 41/F | UIP | 5 | Diffuse | U11/U12 RNP |
| SSc-28 | 58/M | UIP | 30 | Diffuse | RNA polymerase |
| SSc-30 | 58/F | UIP | 0 | n/a | n/a |
Diffuse, diffuse cutaneous skin involvement; sine, systemic sclerosis sine scleroderma; n/a, not available.
Table 2.
Clinical Characteristics of Normal Lung Donors
| Age (years)/sex | Cause of death | Smoking | |
|---|---|---|---|
| NL-14 | 43/M | Cerebrovascular accident | Y |
| NL-18 | 26/F | Aspirin overdose | n/a |
| NL-22 | 57/F | Intracranial hemorrhage | N |
| NL-26 | 18/F | Trauma | N |
| NL-32 | 27/n/a | Motor vehicle accident | N |
| NL-41 | 53/F | Subarachnoid hemorrhage | Y |
| NL-42 | 22/F | n/a | n/a |
n/a: not available.
Figure 1.
IGF-II levels are increased in vivo in SSc lung tissues. A: Immunohistochemical staining of SSc was performed using anti-IGF-II antibody (a–d). Goat IgG was used as an antibody control (e, f). Normal lung was also stained with anti-IGF-II antibody (g, h). SSc lungs have increased expression of IGF-II in fibroblastic foci (arrowheads) and epithelial cells (arrows). B: Immunofluorescent staining of SSc lung tissue using IGF-II (red) or α-SMA antibodies (green) (a–c), SSc lung with control antibodies (d–f), and normal lung with IGF-II and α-SMA antibodies (g–i). IGF-II co-localizes with α-SMA in some interstitial cells in SSc lungs (yellow arrowheads). IGF-II is also expressed in non-α-SMA-producing cells (white arrowheads). Pulmonary vessels stain for α-SMA, but not IGF-II (white arrows). Original magnifications: ×200 [A (a, e, g), B); ×400 [A (b–d, f, h)].
To determine whether myofibroblasts are a source of IGF-II, IGF-II and α-smooth muscle actin (α-SMA) were detected in SSc and normal lung tissues using double-immunofluorescence staining (Figure 1B). In SSc lung tissues, IGF-II was detected in both α-SMA-positive and -negative interstitial cells. This suggests that IGF-II is expressed by both activated and nonactivated lung fibroblasts in SSc-associated pulmonary fibrosis. In normal lungs, α-SMA was detected in pulmonary vessels whereas no significant IGF-II was detected, confirming our immunohistochemical findings.
Primary Lung Fibroblasts Express Increased IGF-II
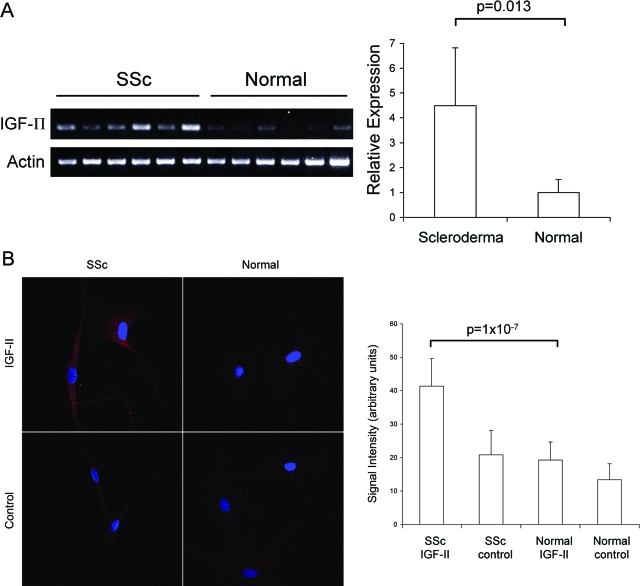
In vivo analysis of IGF-II expression in SSc lungs suggested that fibroblasts are a source of IGF-II expression in SSc lung fibrosis. To examine IGF-II production by pulmonary fibroblasts, we cultured primary lung fibroblasts from the same lung tissues. Equal numbers of fibroblasts from SSc and normal lungs were cultured, and equal amounts of RNA were used in RT-PCR for the detection of IGF-II mRNA levels. Steady-state mRNA levels were 4.5-fold higher in SSc lung fibroblasts compared to normal controls (4.5 ± 2.3 versus 1.0 ± 0.5, respectively; P = 0.013) (Figure 2A). Additionally, immunofluorescent analysis of fibroblasts showed a significant increase in IGF-II protein expression in primary lung fibroblasts from SSc lungs compared to normal fibroblasts (41.3 ± 8.3 versus 19.3 ± 5.4, respectively, in arbitrary units; P = 1 × 10−7) (Figure 2B). The increased IGF-II mRNA and protein levels in SSc lung fibroblasts suggest that IGF-II is aberrantly expressed from lung fibroblasts in SSc-related pulmonary fibrosis.
Figure 2.
Steady-state IGF-II mRNA (A) and protein levels (B) are increased in SSc lung fibroblasts. A: RT-PCR analysis of total IGF-II mRNA levels in primary lung fibroblasts shows significantly increased expression in SSc versus normal fibroblasts (n = 6, 4.5 ± 2.3 versus 1.0 ± 0.5, respectively; P = 0.013). Relative expression was determined by scanning densitometry of PCR products normalized to β-actin mRNA levels. B: IGF-II protein was detected by immunofluorescence using polyclonal anti-IGF-II antibody and compared to goat isotype control antibody. Signal intensity was quantified with Metamorph imaging software (Molecular Devices) and shown in arbitrary units. SSc lung fibroblasts show a twofold increase in IGF-II protein compared to normal lung fibroblasts (n = 4, P = 1 × 10−7). Values and bars represent mean and SD, respectively. Original magnifications, ×400.
Preferential Promoter Usage of IGF-II Gene Expression
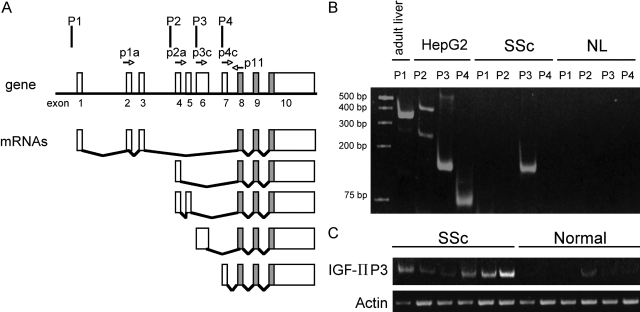
Expression of the IGF-II gene is regulated by four promoters (P1 to P4) and generates a variety of mRNA species that ultimately encode the same IGF-II peptide. Promoter usage has been shown to be tissue- and development-specific.15,17 We performed a semiquantitative analysis of IGF-II promoter usage by using previously described primer pairs designed to amplify specific IGF-II transcripts derived from P1 to P4 (Figure 3A).18 Primary lung fibroblasts from patients with SSc showed an increased expression of IGF-II derived from P3, but no mRNA from P1, P2, and P4 was detected (Figure 3B). Additionally, primary lung fibroblasts derived from six patients with SSc all show IGF-II gene expression from P3 (Figure 3C). Only one of six primary fibroblasts from normal lungs showed any detectable levels of IGF-II mRNA from P3. Overall, normal fibroblasts did not express IGF-II P3 mRNA.
Figure 3.
IGF-II gene expression is derived from P3 in SSc lung fibroblasts. A: RT-PCR of total RNA was performed using primer pairs specific for mRNA species generated from each promoter. As previously described,18 expected PCR product sizes were 363 bp for P1, 249 and 409 bp for P2, 151 bp for P3, and 76 bp for P4. B: IGF-II expression from one representative SSc lung fibroblast shows abundant expression from P3. There is no detectable mRNA from P1, P2, or P4. IGF-II expression from a representative normal lung fibroblast shows no IGF-II expression from any of the four promoters. Adult liver tissue and HepG2 cells served as positive controls for each primer pair. C: Comparison of mRNA from six SSc lung fibroblasts show increased IGF-II expression from P3 compared to six normal lung samples.
IGF-II Induces a Dose- and Time-Dependent Increase in ECM Production
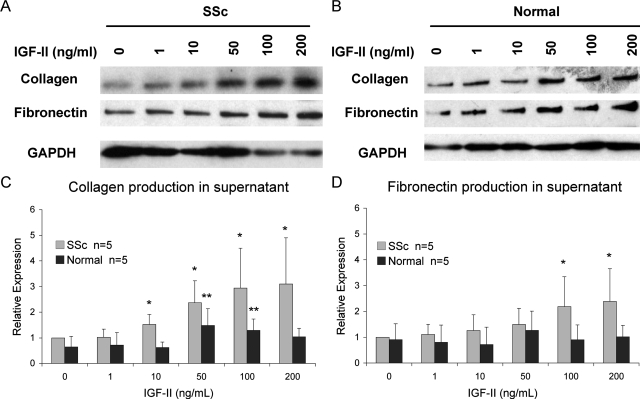
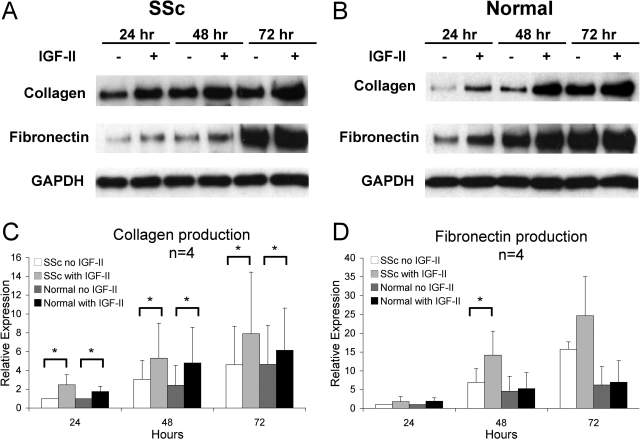
Fibroblasts are the major source of ECM production and deposition in pulmonary fibrosis. To determine whether IGF-II contributes to ECM deposition, we measured expression of fibronectin and type I collagen, the major components of ECM in pulmonary fibrosis. Equal numbers of fibroblasts were cultured with varying concentrations of recombinant IGF-II for 48 hours. IGF-II increased ECM production and secretion in lung fibroblasts from SSc patients in a dose-dependent manner (Figure 4). SSc fibroblasts showed increased collagen and fibronectin production with the addition of 100 and 200 ng/ml of IGF-II compared to vehicle control. Normal fibroblasts showed significantly increased collagen production and a trend toward increased fibronectin production with 50 ng/ml of IGF-II, but these effects were more modest with increasing amounts of IGF-II.
Figure 4.
IGF-II induces collagen type I and fibronectin production in SSc primary lung fibroblasts in a dose-dependent manner (A) compared to normal fibroblasts (B). Equal numbers of fibroblasts were cultured with increasing concentrations of recombinant IGF-II for 48 hours. Collagen (C) and fibronectin (D) expression was quantified using scanning densitometry, and values were normalized to baseline SSc expression levels. SSc fibroblasts show a statistically significant increase in collagen (at 200 ng/ml IGF-II, 3.09 versus 1.0, ±1.8, in arbitrary units; P = 0.016) and fibronectin production (2.39 versus 1.0 ± 1.3; P = 0.019). Normal fibroblasts show peak collagen expression at 50 ng/ml of IGF-II (1.48 versus 0.64 ± 0.64; P = 0.020). Data shown represent experiments using five different SSc and five different normal fibroblasts. Asterisks indicate P < 0.05 compared with no IGF-II stimulation in SSc (one asterisk) and normal fibroblasts (two asterisks).
To determine whether prolonged exposure of fibroblasts affects ECM production, fibroblasts were incubated in media with and without IGF-II for 24 to 72 hours. SSc and normal lung fibroblasts secreted increasing amounts of collagen and fibronectin with prolonged incubation in growth media alone (Figure 5). Both SSc and normal lung fibroblasts showed significantly increased collagen production at 24, 48, and 72 hours with IGF-II compared to control treated cells. A significant increase in fibronectin production was seen in SSc fibroblasts stimulated with IGF-II for 48 hours. Although normal lung fibroblasts showed a trend toward a time-dependent increase in fibronectin production, differences in fibronectin levels did not reach statistical significance. The dose- and time-dependent effects of IGF-II on fibronectin and collagen secretion in SSc lung fibroblasts suggest that IGF-II directly contributes to ECM production and that SSc fibroblasts may be more susceptible to the ECM-inducing effects of IGF-II compared to normal fibroblasts.
Figure 5.
IGF-II induces collagen type I and fibronectin production in a time-dependent manner in primary lung fibroblasts from SSc (A) and normal lungs (B). Equal numbers of SSc and normal fibroblasts were cultured in media with or without IGF-II (200 ng/ml) for 24 to 72 hours. C: Both SSc and normal fibroblasts secreted significantly increased amounts of collagen throughout time compared to media alone. D: Only SSc fibroblasts showed significant fibronectin production at 48 hours. Data represent four different SSc and four different normal fibroblasts. Asterisk denotes P < 0.05.
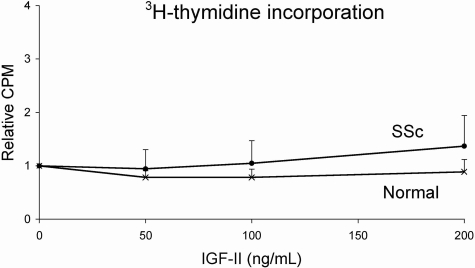
IGF-II Does Not Induce Fibroblast Proliferation
IGF-II is known to exert proliferative effects on a variety of cell types. We treated SSc and normal fibroblasts with different concentrations of IGF-II for 48 hours. IGF-II did not induce a significant difference in 3H-thymidine incorporation in SSc fibroblasts (P = 0.17) (Figure 6). Similarly IGF-II had no significant effect on the proliferation of normal fibroblasts (P = 0.14).
Figure 6.
IGF-II does not induce fibroblast proliferation. SSc and normal lung fibroblasts were treated with IGF-II for a total of 48 hours and proliferation measured using 3H-thymidine incorporation. SSc and normal fibroblasts do not show a statistically significant increase in proliferation using different IGF-II concentrations. Data are shown as a ratio of IGF-II stimulated to unstimulated fibroblast proliferation.
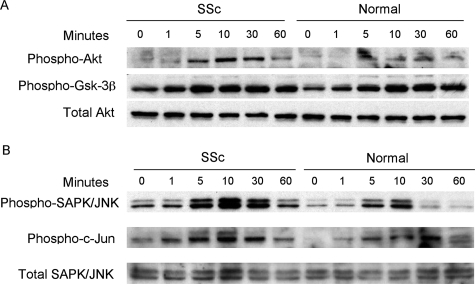
IGF-II-Induced ECM Production Depends on Akt and JNK Activation
IGF-II stimulates cell activity via tyrosine kinase receptors IGF-IR and insulin receptor isoform A (IR-A), which activate a variety of signaling cascades. The PI3 kinase pathway of intracellular signaling was examined by Western blot analysis of activated signaling molecules. Phosphorylation of Akt and GSK-3β increased within 5 minutes of IGF-II stimulation (Figure 7A). Similarly, the SAPK/JNK cascade was examined by detection of phosphorylated kinases. JNK and c-Jun displayed peak activation between 5 to 10 minutes of IGF-II stimulation (Figure 7B). These findings suggest that IGF-II causes activation of PI3 kinase and JNK signaling cascades in primary fibroblasts.
Figure 7.
Activation of Akt (A) and JNK (B) signaling cascades in SSc and normal lung fibroblasts. Fibroblasts were treated with recombinant IGF-II (200 ng/ml) for 1 to 60 minutes or media alone (0 minutes). A: Both SSc and normal fibroblasts show peak activation of Akt and GSK-3β within 5 minutes of IGF-II stimulation. B: SAPK/JNK and c-Jun also show activation within 5 to 10 minutes after IGF-II stimulation.
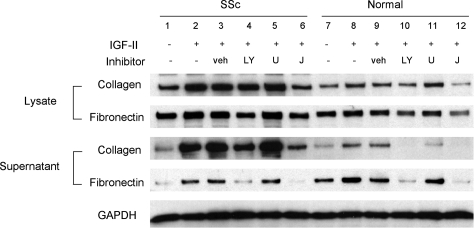
To confirm the role of these signaling cascades in the response of primary fibroblasts to IGF-II, we pretreated SSc and normal lung fibroblasts with specific inhibitors of PI3K (LY294002), MEK (U0126), and JNK. As shown in Figure 8, IGF-II-induced fibronectin and collagen secretion was abrogated by PI3K and JNK inhibition. On the other hand, IGF-II-induced ECM secretion was not blocked by MEK inhibition. These findings further confirm that IGF-II induces ECM production in primary fibroblasts via PI3K- and JNK-dependent pathways.
Figure 8.
IGF-II-induced ECM is inhibited by PI-3 kinase and JNK inhibitors. Primary lung fibroblasts from SSc (lanes 1 to 6) and normal lungs (lanes 7 to 12) were incubated with 10 μmol/L LY294002 (LY), U0126 (U), JNK inhibitor II (J), or vehicle control (veh) for 1 hour before the addition of IGF-II (200 ng/ml). Fibroblasts were stimulated with IGF-II for 48 hours before collection of lysates and supernatants. IGF-II induced collagen and fibronectin production (lanes 2, 3, 8, 9). This effect is blocked with the addition of a PI-3 kinase inhibitor (lanes 4 and 10) and JNK inhibitor (lanes 6 and 12). MEK inhibition did not have any effect on IGF-II-induced ECM production (lanes 5 and 11).
Discussion
Pulmonary fibrosis is the leading cause of death in individuals with SSc, but few therapies are effective. Although the IGF system has been implicated in the pathogenesis of pulmonary fibrosis, no studies to date have examined the role of IGF-II in pulmonary fibrosis. We demonstrate IGF-II expression is increased in vivo in lung tissues of patients with SSc-related pulmonary fibrosis. Additionally, our studies show increased IGF-II expression in vitro in SSc lung fibroblasts compared to normal fibroblasts.
Retrospective studies suggest that a nonspecific interstitial pneumonia pattern is more common than a usual interstitial pneumonia pattern in SSc patients who underwent lung biopsy.23,24 These studies also show that nonspecific interstitial pneumonia carries a better prognosis compared to usual interstitial pneumonia. All of our patients underwent lung transplantation for end-stage pulmonary fibrosis. Not surprisingly, these lungs all had a usual interstitial pneumonia pattern. Normal lung samples were limited to a pool of potential lung donors, which generally are younger than lung transplant recipients. Therefore, one weakness of our study is the age difference between SSc patients and normal donors. Although no studies have examined the relationship of age and local IGF-II expression in the lung, serum IGF-II levels are known to decrease with age in adults.25,26 This suggests that our findings are significant despite the age difference between SSc patients and normal controls.
IGF-I and -II share similar structures, but they exert unique biological effects. Increased IGF-I expression has been detected in bronchoalveolar lavage fluid in patients with SSc.2 Additionally, IGF-I levels were higher in those who had radiographical evidence of pulmonary fibrosis compared to those without fibrosis. However, the role of IGF-I in the pathogenesis of fibrosis is not clear because transgenic mice expressing IGF-I in lung epithelial cells displayed a phenotype with epithelial hyperplasia, not pulmonary fibrosis.27 IGFBPs are known to regulate the activity of IGFs by binding to them and sequestering IGFs within the extracellular matrix. We have previously shown increased expression of IGFBP-3 and IGFBP-5 in lung tissues of patients with idiopathic pulmonary fibrosis5 and the skin of patients with SSc.6,22 Furthermore, IGFBP-5 led to increased ECM deposition in vitro and in vivo in mouse lung and skin.6,7 Increased IGF-II expression may act in conjunction with IGFBPs to induce ECM deposition and fibrosis.
IGF-II is known to have mitogenic effects and alter fibroblast function. Previous studies have analyzed the effects of IGF-II on lung fibroblast proliferation and synthetic function. Hetzel and colleagues13 showed by 3H-thymidine incorporation that IGF-II significantly stimulated proliferation of normal lung fibroblasts, but not fibroblasts derived from patients with idiopathic pulmonary fibrosis. They also showed that IGF-II increased fibronectin production in idiopathic pulmonary fibrosis fibroblasts while conversely decreasing fibronectin production in normal fibroblasts. However, their results did not meet statistical significance, likely because of small sample size. These studies differ from ours in that they used lower concentrations of IGF-II (30 ng/ml compared to 200 ng/ml in our study). Using primary lung fibroblasts from patients with SSc-associated pulmonary fibrosis and normal donors, we have not detected a significant effect of IGF-II on cell proliferation.
Our results demonstrate that IGF-II significantly increases collagen and fibronectin production in SSc fibroblasts, while only minimally altering ECM production in normal fibroblasts. SSc fibroblasts show a dose- and time-dependent increase in collagen and fibronectin production (Figures 5 and 6). SSc fibroblasts appear to secrete more collagen in response to IGF-II compared to normal fibroblasts. Additionally, SSc fibroblasts have significantly increased fibronectin production, but normal fibroblasts do not. This suggests that SSc fibroblasts may be more sensitive to the ECM-inducing effects of IGF-II compared to normal fibroblasts. Additionally, IGF-II was able to induce a stronger response in the activation of the signaling proteins Akt and JNK in SSc fibroblasts compared to normal fibroblasts (Figure 7). This provides further support that SSc fibroblasts are more sensitive to the effects of IGF-II.
The unique fibrogenic properties of IGF-II may be explained by the differential signaling effects on the target cells. IGF-II interacts with several receptors that are shared with IGF-I and insulin. IGF-II is capable of activating signal transduction cascades through IGF-IR, although the binding affinity of IGF-II for IGF-IR is three times less than that of IGF-I.28 Insulin and IGF-II can bind insulin receptor isoform A (IR-A), whereas IGF-I has low affinity for this receptor. Subunits of IGF-IR and IR can also exist in hybrid receptor forms that bind IGF-II. Additionally, IGF-II uniquely binds to IGF-IIR, which unlike IGF-IR and IR, acts as a negative regulator of IGF-II by internalizing and degrading IGF-II.29 IGF-II-induced ECM production in lung fibroblasts may thus depend on the relative abundance of IGF-IR, IR-A, and IGF-IIR. Mechanisms of IGF-IIR interactions in SSc lung fibroblasts are currently under investigation in our laboratory.
We examined the role of signaling cascades in IGF-II-induced ECM production. IGF-II may exert unique biological effects by inducing a milieu of phosphorylated signaling molecules that is distinct from the one activated by IGF-I.30 We show that IGF-II can activate JNK and Akt phosphorylation. Additionally, inhibitors of PI3 kinase and JNK were able to block IGF-II-induced collagen and fibronectin production, providing further support that these signaling molecules are involved in IGF-II-induced collagen and fibronectin production. C-jun is a transcription factor that can bind upstream promoter regions of collagen type I and fibronectin genes and thus activate their transcription.31,32 Whether IGF-II-activated c-jun leads to increased transcriptional activity of collagen and fibronectin or activation of other intracellular processes remains to be determined.
IGF-II expression is normally high during fetal development. Systemic IGF-II levels dramatically decrease shortly after birth. However, IGF-II continues to be expressed in some tissues and may exert effects locally. Immunohistochemical staining of lung sections from patients with SSc shows increased IGF-II expression in fibroblastic foci and epithelial cells. It is conceivable that increased IGF-II expression from epithelial cells, in addition to fibroblast-derived IGF-II, may exert local effects on interstitial fibroblasts to promote fibroblast development and ECM deposition.
No published studies have analyzed IGF-II gene expression in fibrotic disorders. IGF-II gene expression can be initiated at one of four promoter sites. We demonstrate a fourfold increase in IGF-II mRNA levels in lung fibroblasts from patients with SSc-associated pulmonary fibrosis. We also show that this mRNA in SSc fibroblasts is generated primarily from P3. There were no detectable levels of P1, P2, or P4 expression in any of the SSc and normal lung fibroblasts. Some normal lung fibroblasts showed detectable levels of total IGF-II mRNA, but only one normal fibroblast showed detectable levels of P3 IGF-II mRNA. IGF-II mRNA detected in Figure 2, but not with the P1 to P4 promoter-specific primers in Figure 3, may be attributable to very low levels of expression from other promoters or a yet unidentified promoter. Promoter usage has been found to be tissue- and development-specific. IGF-II mRNA derived from P1 is primarily expressed in adult liver tissue.17 On the other hand, IGF-II expression from P3 and P4 has been found in fetal tissues and various malignancies such as Wilms’ tumor, colon carcinoma, hepatocellular carcinoma, and ovarian cancer.15,18,19,20,21 Our results suggest that IGF-II gene expression in SSc lung fibrosis is regulated by mechanisms that are different from those in normal liver tissue. Preferential promoter usage for IGF-II transcription may be a potential site of regulation of IGF-II expression. Studies to identify the mechanism resulting in altered gene regulation and promoter usage of IGF-II in SSc-associated pulmonary fibrosis are currently under way in our laboratory.
IGF-II is an imprinted gene that is normally expressed from the paternal allele whereas the maternal allele remains silent.33 Loss of imprinting leads to IGF-II mRNA expression from both alleles. Previous studies have shown that fetal liver produces IGF-II transcripts through promoters P2 to P4 from one parental allele,34 suggesting that imprinting mechanisms affect transcription from promoters P2 to P4. Conversely, the adult liver expresses IGF-II from P1 and both alleles. Therefore, imprinting mechanisms do not regulate IGF-II gene expression from P1. Some Wilms’ tumors have loss of imprinting of the IGF-II gene, leading to increased IGF-II gene expression. These tumors show biallelic expression from P2 to P4.18 Loss of imprinting has also been found in other tumors associated with increased IGF-II levels.35 Whether increased IGF-II expression in SSc lung fibrosis is related to altered imprinting mechanisms of the IGF-II gene remains to be determined.
In conclusion, we demonstrate that IGF-II expression is increased in vivo and in vitro in lung tissues from patients with SSc-associated pulmonary fibrosis. In primary SSc fibroblasts, IGF-II transcription occurs preferentially via the P3 promoter. IGF-II contributes to the fibrotic phenotype by inducing collagen and fibronectin production from lung fibroblasts in a dose- and time-dependent manner. Increased expression of IGF-II from fibroblasts may act in an autocrine or paracrine manner to increase ECM deposition. Furthermore, PI3K and JNK signaling pathways mediate IGF-II-induced ECM production. Our studies establish IGF-II as a novel factor involved in the pathogenesis of SSc-related pulmonary fibrosis and a new potential target for the development of more effective therapy for pulmonary fibrosis.
Footnotes
Address reprint requests to Carol A. Feghali-Bostwick, University of Pittsburgh, Division of Pulmonary, Allergy, and Critical Care Medicine, 628 NW Montefiore, 3459 Fifth Ave., Pittsburgh, PA, 15213. E-mail: feghalica@upmc.edu.
Supported by the National Institutes of Health (grants R01-AR050840, F32-HL091654, and T32 HL007563-21) and the Simmons Fund for Idiopathic Pulmonary Fibrosis.
References
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NK, Cambrey AD, Myers AR, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci (Lond) 1994;86:141–148. doi: 10.1042/cs0860141. [DOI] [PubMed] [Google Scholar]
- Homma S, Nagaoka I, Abe H, Takahashi K, Seyama K, Nukiwa T, Kira S. Localization of platelet-derived growth factor and insulin-like growth factor I in the fibrotic lung. Am J Respir Crit Care Med. 1995;152:2084–2089. doi: 10.1164/ajrccm.152.6.8520779. [DOI] [PubMed] [Google Scholar]
- Vanhee D, Gosset P, Wallaert B, Voisin C, Tonnel AB. Mechanisms of fibrosis in coal workers’ pneumoconiosis. Increased production of platelet-derived growth factor, insulin-like growth factor type I, and transforming growth factor beta and relationship to disease severity. Am J Respir Crit Care Med. 1994;150:1049–1055. doi: 10.1164/ajrccm.150.4.7921435. [DOI] [PubMed] [Google Scholar]
- Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H, Jukic DM, Zhou Z, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor binding protein 5 induces skin fibrosis: a novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169:1633–1642. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Venihaki M, Guo WH, Lopez MF. Igf2 deficiency results in delayed lung development at the end of gestation. Endocrinology. 2006;147:5584–5591. doi: 10.1210/en.2006-0498. [DOI] [PubMed] [Google Scholar]
- Scott J, Cowell J, Robertson ME, Priestley LM, Wadey R, Hopkins B, Pritchard J, Bell GI, Rall LB, Graham CF, Knott TJ. Insulin-like growth factor-II gene expression in Wilms’ tumour and embryonic tissues. Nature. 1985;317:260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Ghahary A, Tredget EE, Shen Q, Kilani RT, Scott PG, Houle Y. Mannose-6-phosphate/IGF-II receptors mediate the effects of IGF-1-induced latent transforming growth factor beta 1 on expression of type I collagen and collagenase in dermal fibroblasts. Growth Factors. 2000;17:167–176. doi: 10.3109/08977190009001066. [DOI] [PubMed] [Google Scholar]
- Giani C, Pinchera A, Rasmussen A, Fierabracci P, Bonacci R, Campini D, Bevilacqua G, Trock B, Lippman ME, Cullen KJ. Stromal IGF-II messenger RNA in breast cancer: relationship with progesterone receptor expressed by malignant epithelial cells. J Endocrinol Invest. 1998;21:160–165. doi: 10.1007/BF03347295. [DOI] [PubMed] [Google Scholar]
- Morison IM, Becroft DM, Taniguchi T, Woods CG, Reeve AE. Somatic overgrowth associated with overexpression of insulin-like growth factor II. Nat Med. 1996;2:311–316. doi: 10.1038/nm0396-311. [DOI] [PubMed] [Google Scholar]
- Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- Scarpa RC, Carraway RE, Cochrane DE. Insulin-like growth factor (IGF) induced proliferation of human lung fibroblasts is enhanced by neurotensin. Peptides. 2005;26:2201–2210. doi: 10.1016/j.peptides.2005.03.044. [DOI] [PubMed] [Google Scholar]
- de Pagter-Holthuizen P, Jansen M, van der Kammen RA, van Schaik FM, Sussenbach JS. Differential expression of the human insulin-like growth factor II gene. Characterization of the IGF-II mRNAs and an mRNA encoding a putative IGF-II-associated protein. Biochim Biophys Acta. 1988;950:282–295. doi: 10.1016/0167-4781(88)90124-8. [DOI] [PubMed] [Google Scholar]
- Holthuizen P, van der Lee FM, Ikejiri K, Yamamoto M, Sussenbach JS. Identification and initial characterization of a fourth leader exon and promoter of the human IGF-II gene. Biochim Biophys Acta. 1990;1087:341–343. doi: 10.1016/0167-4781(90)90010-y. [DOI] [PubMed] [Google Scholar]
- de Pagter-Holthuizen P, Jansen M, van Schaik FM, van der Kammen R, Oosterwijk C, Van den Brande JL, Sussenbach JS. The human insulin-like growth factor II gene contains two development-specific promoters. FEBS Lett. 1987;214:259–264. doi: 10.1016/0014-5793(87)80066-2. [DOI] [PubMed] [Google Scholar]
- Vu TH, Hoffman A. Alterations in the promoter-specific imprinting of the insulin-like growth factor-II gene in Wilms’ tumor. J Biol Chem. 1996;271:9014–9023. doi: 10.1074/jbc.271.15.9014. [DOI] [PubMed] [Google Scholar]
- Lu L, Katsaros D, Wiley A, Rigault de la Longrais IA, Puopolo M, Schwartz P, Yu H. Promoter-specific transcription of insulin-like growth factor-II in epithelial ovarian cancer. Gynecol Oncol. 2006;103:990–995. doi: 10.1016/j.ygyno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Singh P, Dai B, Given RL, Lu X, Holthuizen PE. Differential activation of IGF-II promoters P3 and P4 in Caco-2 cells during growth and differentiation. Gastroenterology. 1998;114:1221–1229. doi: 10.1016/s0016-5085(98)70428-7. [DOI] [PubMed] [Google Scholar]
- Tang SH, Yang DH, Huang W, Zhou M, Zhou HK, Lu XH, Ye G. Differential promoter usage for insulin-like growth factor-II gene in Chinese hepatocellular carcinoma with hepatitis B virus infection. Cancer Detect Prev. 2006;30:192–203. doi: 10.1016/j.cdp.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis Rheum. 1999;42:1451–1457. doi: 10.1002/1529-0131(199907)42:7<1451::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, Haslam PL, Vassilakis DA, Black CM, du Bois RM. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–1586. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- Kim DS, Yoo B, Lee JS, Kim EK, Lim CM, Lee SD, Koh Y, Kim WS, Kim WD, Colby TV, Kitiaichi M. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:121–127. [PubMed] [Google Scholar]
- Pfeilschifter J, Scheidt-Nave C, Leidig-Bruckner G, Woitge HW, Blum WF, Wuster C, Haack D, Ziegler R. Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50- to 80-year-old men and women. J Clin Endocrinol Metab. 1996;81:2534–2540. doi: 10.1210/jcem.81.7.8675573. [DOI] [PubMed] [Google Scholar]
- Ceda GP, Dall'Aglio E, Magnacavallo A, Vargas N, Fontana V, Maggio M, Valenti G, Lee PD, Hintz RL, Hoffman AR. The insulin-like growth factor axis and plasma lipid levels in the elderly. J Clin Endocrinol Metab. 1998;83:499–502. doi: 10.1210/jcem.83.2.4548. [DOI] [PubMed] [Google Scholar]
- Frankel SK, Moats-Staats BM, Cool CD, Wynes MW, Stiles AD, Riches DW. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol. 2005;288:L805–L812. doi: 10.1152/ajplung.00420.2004. [DOI] [PubMed] [Google Scholar]
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid insulin-like growth factor II receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem. 1985;260:9435–9442. [PubMed] [Google Scholar]
- Rolfe KJ, Cambrey AD, Richardson J, Irvine LM, Grobbelaar AO, Linge C. Dermal fibroblasts derived from fetal and postnatal humans exhibit distinct responses to insulin like growth factors. BMC Dev Biol. 2007;7:124. doi: 10.1186/1471-213X-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Agarwal A, Uitto J, Mauviel A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J Biol Chem. 1996;271:3272–3278. doi: 10.1074/jbc.271.6.3272. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- Vu TH, Hoffman AR. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature. 1994;371:714–717. doi: 10.1038/371714a0. [DOI] [PubMed] [Google Scholar]
- Jelinic P, Shaw P. Loss of imprinting and cancer. J Pathol. 2007;211:261–268. doi: 10.1002/path.2116. [DOI] [PubMed] [Google Scholar]