Abstract
Epidemiological studies have demonstrated that the use of methamphetamine (meth), a sympathomimetic stimulant, is particularly common among patients infected with HIV. However, there is a lack of direct evidence that meth promotes HIV infection of target cells. This study examined whether meth is able to enhance HIV infection of macrophages, the primary target site for the virus. Meth treatment resulted in a significant and dose-dependent increase of HIV reverse transcriptase activity in human blood monocyte-derived macrophages. Dopamine D1 receptor antagonists (SCH23390 and SKF83566) blocked this meth-mediated increase in the HIV infectivity of macrophages. Investigation of the underlying mechanisms of meth action showed that meth up-regulated the expression of the HIV entry co-receptor CCR5 on macrophages. Additionally, meth inhibited the expression of endogenous interferon-α and signal transducer and activator of transcription-1 in macrophages. These findings provide direct in vitro evidence to support the possibility that meth may function as a cofactor in the immunopathogenesis of HIV infection and may lead to the future development of innate immunity-based intervention for meth users with HIV infection.
Methamphetamine (meth) and related amphetamine compounds are among the most commonly used illicit drugs, with more than 35 million users worldwide. In the United States, approximately 1.5 million individuals regularly use/abuse meth.1,2 An estimated 11 million Americans at the age of 12 and older reported trying meth at least once during their lifetime. Meth use and HIV type 1 infection frequently coexist because of the association of meth use with engagement of high-risk behaviors.3,4,5,6 The risk for HIV infection attributable to meth use continues to increase.7,8,9 Several studies have shown that there is a high prevalence of HIV infection among meth users10,11,12 and that among men who sell sex to men, those who use meth have a higher HIV risk than nonusers.13 Active meth users displayed higher levels of HIV loads than nonusers,14 which may be attributable to increased viral replication, as was shown in an animal study.15 However, the direct effects of meth on HIV infection and HIV disease progression are still poorly understood.16 In particular, the deleterious effect of meth on the host’s immune response and its role in the immunopathogenesis of HIV infection remain to be elucidated. Therefore, study of the interactions between meth and HIV has become a greater research priority.17
The microenvironment in which the interactions between HIV and target cells take place has a crucial role in modulating HIV infectivity. Besides CD4+ T lymphocytes, cells from the mononuclear phagocyte system are the primary targets for HIV infection. Monocytes and macrophages as the primary sites of HIV replication are among the first cells infected by HIV and later function as reservoirs for the virus.18,19 Although abuse of drug such as opioids have been implicated in modulation of functions of monocytes/macrophages20 and microglia,21 there is limited information about the impact of meth on functions of monocytes/macrophages. Meth inhibited polyinosinic:polycytidylic acid-induced antiviral activity in murine peritoneal macrophages.22 Meth also modulated the patterns of gene expression in monocyte-derived immature and mature dendritic cell.23,24 Although these findings suggest that meth is immunosuppressive, there is a lack of direct evidence at cellular and molecular levels to demonstrate that meth has the ability to enhance HIV infection of macrophages, the primary target for the virus. In the present study, we investigated the impact of meth on HIV infection of human blood monocyte-derived macrophages and explored the mechanisms underlying the meth action on HIV infection.
Materials and Methods
Monocyte Isolation and Culture
Peripheral blood samples from healthy adult donors were provided by the University of Pennsylvania Center for AIDS Research, which has Institutional Review Board review and approval for the sample collection. These blood samples were screened for all normal viral blood-borne pathogens and certified to be pathogen free. Monocytes were purified according to a previously described technique.25 In brief, heparinized blood was separated by centrifugation over lymphocyte separation medium (Organon Teknika, Durham, NC) at 400 to 500 × g for 45 minutes. The mononuclear cell layer was collected and incubated with Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) in a 2% gelatin-coated flask for 45 minutes at 37°C, followed by removal of the nonadherent cells with Dulbecco’s modified Eagle’s medium. Adherent monocytes were detached with 10 mmol/L EDTA. After the initial purification, greater than 97% of the cells were monocytes, as determined by nonspecific esterase staining and flow cytometry analysis using monoclonal antibody against CD14, the marker specific for monocytes and macrophages. Isolated monocytes were plated in 24- or 48-well culture plates at a density of 5 or 2.5 × 105 cells/well in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. Whereas monocytes refer to freshly isolated (within 24 hours) monocytes, macrophages refer to 7-day-cultured monocytes in vitro. Monocyte and macrophage viability was monitored by trypan blue exclusion and maintenance of cell adherence.
Reagents
Methamphetamine and the dopamine D1 receptor (D1R) antagonists (SCH23390 and SKF83566) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Fluorescein isothiocyanate-conjugated antibodies against CD14, CD4, and CCR5 and the control IgGs (IgG1, IgG2a, and IgG2b) were obtained from PharMingen (San Diego, CA). Enzyme-linked immunosorbent assay kit for interferon-α (IFN-α) protein was purchased from PBL Biomedical Laboratories (Piscataway, NJ). Rabit polyclonal antibodies against actin and signal transducer and activator of transcription1 (STAT1) were obtained from Sigma-Aldrich. Rabbit polyclonal antibody against Dopamine D1 receptor was purchased from Calbiochem (La Jolla, CA). Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antibody was purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL).
Meth and/or D1R Antagonist Treatment
Seven-day-cultured macrophages (2.5 × 105 cells/well) were treated with different concentrations (1, 10, 100, and 250 μmol/L) of meth for 3, 6, and 24 hours. These concentrations of meth are comparable with the levels found in the blood, urine, or tissue samples of meth-using subjects.23,26,27,28,29 To investigate whether D1 receptor antagonists block meth-induced up-regulation of HIV infection, 10 μmol/L D1 receptor antagonist (SCH23390 or SKF83566) was added to the macrophages cultures 1 hour before meth treatment for 24 hours. The cell cultures were re-fed with fresh media containing meth and/or SCH23390 or SKF83566 every 4 days. There were no cytotoxic effects of meth, SCH23390, and SKF83566 treatment on macrophages as demonstrated by trypan blue dye staining (data not shown).
Infection of Macrophages with HIV Bal Strain
The macrophage-tropic R5 strain (Bal) was obtained from the AIDS Research and Reference Reagent Program (NIH, Bethesda, MD). Macrophages were infected with equal amounts of cell-free HIV Bal (p24 20 ng/106 cells) for 2 hours at 37°C after 24 hours of treatment with or without meth. The cells were then washed three times with Dulbecco’s modified Eagle’s medium to remove unabsorbed virus, and fresh media containing meth and/or SCH23390 or SKF83566 were added to the cell cultures. The final wash was tested for HIV reverse transcriptase (RT) activity and shown to be free of residual inocula. Untreated cells served as a control. Culture supernatants were collected for HIV RT activity assay at days 4, 8, and 12 after infection.
HIV RT Assay
HIV RT activity was determined based on the technique of Guo et al20 and Ho et al30 with modifications. In brief, 10 μl of culture supernatants from macrophages infected with or without HIV was added to a cocktail containing poly(A), oligo(dT) (Amersham Biosciences, Inc., Piscataway, NJ), MgCl2, and [32P]dTTP (Amersham Biosciences, Inc.) and incubated for 20 hours at 37°C. Then, 30 μl of the cocktail was spotted onto DE81 paper, dried, and washed five times with 2× saline-sodium citrate buffer and once with 95% ethanol. The filter paper was then air-dried. Radioactivity was counted in a liquid scintillation counter (PerkinElmer Life Sciences, Boston, MA).
Flow Cytometry
To determine whether meth affects the expression of CD14, CD4, and CCR5 receptors on macrophages, the cells were incubated with or without 100 μmol/L meth for 24 hours and then removed from the culture plate and resuspended in 100 μl of PBS. After incubation with 20 μl of fluorescein isothiocyanate-conjugated antibodies against CD14, CD4, and CCR5 for 45 minutes at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. Fluorescein isothiocyanate-conjugated control IgG was used as a control antibody. Fluorescence-positive cells were analyzed on an EPICS-elite flow cytometer (Beckman Coulter, Inc., Hialeah, FL).
Real-Time RT-PCR
Total RNA was extracted from macrophages using Tri-Reagent (Molecular Research Center, Cincinnati, OH) as previously described.31 Total cellular RNA (1 μg) was subjected to reverse transcription using the reverse transcription system from Promega (Madison, WI). The real time RT-PCR for the quantification of D1R, IFN-α, and glyceraldehyde-3-phosphate dehydrogenase mRNA was performed with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as previously described.32 The amplified products were visualized and analyzed using the software MyiQ provided with the thermocycler (iCycler iQ real time PCR detection system; Bio-Rad Laboratories). The levels of glyceraldehyde-3-phosphate dehydrogenase mRNA were used as an endogenous reference to normalize the quantities of targets mRNA. The special oligonucleotide primers used in this study were listed as follows: D1R, 5′-AAACCCACAAGCCCCTCTGA-3′ (sense) and 5′-GATGAATTAGCCCACCCAAAC-3′ (antisense)33; IFN-α, 5′-TTTCTCCTGCCTGAAGGACAG-3′ (sense) and 5′-GCTCATGATTTCTGCTCTGACA-3′ (antisense); and glyceraldehyde-3-phosphate dehydrogenase, 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (anti-sense). The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Immunofluorescence Assay
The macrophages were cultured on glass coverslips at a density of 0.25 × 106/well in 24-well plates. The macrophages were washed with 1 × cold PBS (with Ca2+ and Mg2+) twice. Cells were fixed at 4°C in 4% paraformaldehyde-4% sucrose in PBS for 20 minutes and then permeated in cold methanol (100%) for additional 10 minutes followed by 0.2% Triton X-100 for additional 10 minutes. Cells were blocked in Block Solution (Pierce, Rockford, IL) for 1 hour at room temperature. To examine expression of D1R, rabbit polyclonal antibody (1:500) against D1R was used as the primary antibody. After washing five times with 1× PBS, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antibody (green, 1:100) for 1 hour. After washing five times with 1× PBS, the cells were mounted on glass coverslips in mounting media (Biomeda, Foster City, CA) and viewed with a fluorescence microscopy (Zeiss, Jena, Germany). Hoechst 33342 was used for nuclei morphology.
Western Blot
Total cell lysates of macrophages were prepared using a radioimmune precipitation assay buffer (Promega) with 1% protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined by the protein assay kit (Bio-Rad Laboratories). Western blot assay was carried out as previously described.31 STAT1 and actin proteins were detected using rabbit anti-STAT1 (1:1000; Sigma-Aldrich) and anti-actin (1:3000; Sigma-Aldrich) polyclonal antibodies, respectively. Peroxidase-conjugated goat anti-rabbit antibody (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA) was used as the second antibody. The bound antibodies were recognized by using SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce) according to the manufacturer’s instruction. Prestained molecular markers (Bio-Rad Laboratories) were used to determine molecular weight of immunoreactive bands.
Enzyme-Linked Immunosorbent Assay
Total cell lysates from the cultured macrophages were prepared using a radioimmune precipitation assay buffer (Promega). Enzyme-linked immunosorbent assay for IFN-α was performed according to the protocol provided by the manufacturer (PBL Biomedical Laboratories).
Statistical Analysis
Student’s t-test was used to evaluate the significance of difference between groups, and multiple comparisons were performed by regression analysis and one-way analysis of variance. P values of less than 0.05 were considered significant. All data are presented as mean ± SD. Statistical analyses were performed with SPSS 11.5 for Windows. Statistical significance was defined as P < 0.05.
Results
Meth Enhances HIV Infection of Macrophages
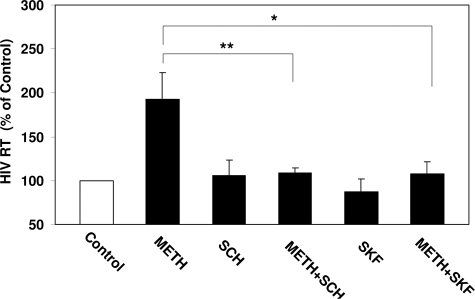
We first determined the effect of meth on HIV infection of macrophages. As shown in Figure 1, meth treatment resulted in increase of HIV RT activity. This meth-mediated increase of HIV RT activities is statistically significant. In addition, the increase of HIV RT activity was dose- and time-dependent (Figure 1, A and B). The highest enhancement of HIV by meth was observed with a dose of 250 μmol/L (Figure 1A) at day 8 after infection (Figure 1B).
Figure 1.
Dose-dependent (A) and time-course (B) effect of meth on HIV Bal replication in macrophages. A: Seven-day-cultured macrophages were incubated with or without meth at indicated concentrations for 24 hours and then incubated with HIV Bal strain for 2 hours in the presence or absence of meth. Day-8 culture supernatants were collected for HIV RT assay. B: Seven-day-cultured macrophages were incubated with or without 100 μmol/L meth for 24 hours before infection with HIV Bal strain for 2 hours and then cultured for 12 days. HIV RT activity was determined in cultured supernatants at indicated time points after infection. Data are expressed as HIV RT activity in meth-treated cells (percentage of control) compared with those in untreated cells, which are defined as 100%. The results represent the mean ± SD of three independent experiments using macrophages from three different donors. Statistical analysis was performed by one-way analysis of variance (A) or Student’s t-test (B), and significance is shown with *P < 0.05 and **P < 0.01 (meth versus control).
D1R Is Involved in Meth-Mediated Up-Regulation of HIV Infection
D1R has been implicated in the pathological effect of meth on the target cells. Thus, it is of importance to determine whether macrophages express D1R that is involved in the meth action on HIV replication. We performed a conventional RT-PCR assay using the primary pair specific for D1R detection. Ethidium bromide staining of RT-PCR-amplified products from macrophages showed a visible 471-bp band that is identical to that from human neuronal cells (NT2-N) (Figure 2A). We also observed that there is expression of D1R in macrophages at protein level (Figure 2B). We then examined whether the enhancing effect of meth on the HIV Bal infection was mediated through the D1R. The D1R antagonists (SCH23390 and SKF83566) completely abrogated the enhancing effect of meth on HIV RT activity (Figure 3), whereas the antagonists alone had little effect on HIV infection of macrophages (Figure 3).
Figure 2.
Dopamine 1 receptor (D1R) expression in macrophages. A: Total mRNA was extracted from 7-day-cultured macrophages and subjected to the real time RT-PCR for D1R. Sizes are estimated from DNA ladder (100-bp fragments) co-electrophoresed as markers. Lane 1, markers; lanes 2 to 4, macrophages from three different donors, respectively; lane 5, human neuronal cells (NT2-N) as a positive control (+); lane 6, negative control (−; the same cells as used in the positive control but processed without reverse transcriptase). B: The macrophages plated on coverslips were fixed, permeabilized, and stained with (top panel) or without (bottom panel) antibody to D1R (green) and with nuclear staining dye (blue). The specimens were examined by a fluorescence microscopy with magnification of ×200. One representative result of three independent experiments is shown.
Figure 3.
Effect of the D1R antagonists on meth-mediated up-regulation of HIV Bal infection. Seven-day-cultured macrophages were incubated with or without the D1R antagonist, 10 μmol/L SCH23390, or 10 μmol/L SKF83566 for 1 hour before treatment with or without 100 μmol/L meth for 24 hours and then infected with HIV Bal strain for 2 hours in the presence or absence of meth and/or SCH23390 or SKF83566. Culture supernatants were collected at day 8 after infection for HIV RT assay. Data are expressed as HIV RT activity in meth-treated cells (percentage of control) to those in untreated cells, which is defined as 100%. The results represent the mean ± SD of three independent experiments. Statistical analysis was performed using one-way analysis of variance, and significance is shown with *P < 0.05 and **P < 0.01 (METH + SCH or METH + SKF versus METH).
Meth Induces HIV Entry Receptor CCR5 Expression
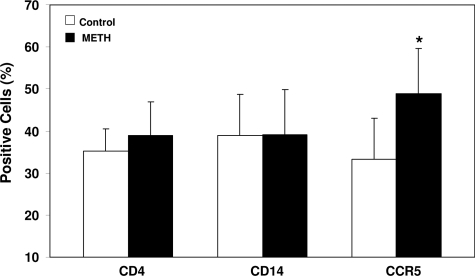
Because CCR5 receptor is a primary co-receptor for HIV M-tropic strain entry into macrophages,34 we examined whether meth has the ability to modulate the expression of CCR5 receptor. Meth treatment (100 μmol/L) significantly up-regulated CCR5 expression on macrophages as determined by flow cytometry (Figure 4). This meth action on CCR5 expression, however, was not mediated by D1R, because the D1R antagonist (SCH23390 and SKF 83566) did not block the meth effect (data not shown). To determine the specificity of the effect of meth on CCR5 expression, we also examined whether macrophage marker (CD14) and CD4 receptor are affected by meth. Meth, when added to macrophage cultures, had little effect on the expression of CD14 and CD4 receptors (Figure 4).
Figure 4.
Effect of meth on the expression of CD4, CD14, and CCR5 receptors. Seven-day-cultured macrophages were treated with or without 100 μmol/L meth for 24 hours. Expression of CD4, CD14, and CCR5 on macrophages was determined by flow cytometry assay. The data shown are the percentage of positive cells for the indicated receptors and represent the mean ± SD of three independent experiments. Statistical analysis was performed using Student’s t-test, and significance is shown with *P < 0.05 (meth versus control).
Meth Suppresses Endogenous IFN-α and STAT1 Expression
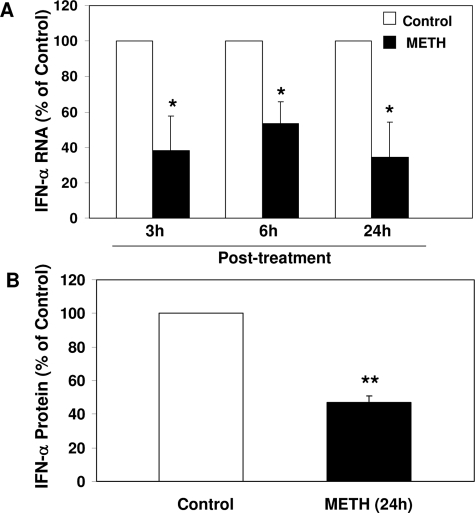
To look for potential mediators involved in meth-mediated enhancement of HIV replication, we examined the impact of meth on IFN-α expression by macrophages. IFN-α is a potent antiviral cytokine that impedes HIV infection of macrophages.35,36,37 We hypothesized that meth inhibits endogenous IFN-α expression in macrophages, which could be a mechanism involved in meth-mediated up-regulation of HIV. Meth treatment of macrophages resulted in a significant decrease of endogenous IFN-α at both mRNA and protein levels (Figure 5, A and B, respectively). This meth-mediated down-regulation of IFN-α in macrophage was completely blocked by the D1R antagonists (SCH23390 and SKF83566) (Figure 6). To further determine whether meth, through suppressing IFN signaling pathway, enhances HIV replication, we investigated the effect of meth on the expression of STAT1, the major component of the IFN signaling cascade.38,39 Macrophages treated with meth expressed lower levels of STAT1 proteins than untreated macrophages (Figure 7).
Figure 5.
Effect of meth on endogenous IFN-α mRNA (A) and protein (B) expression in macrophages. A: Seven-day-cultured macrophages were treated with or without 100 μmol/L meth for the indicated time points, and then cellular RNA were subjected to the real-time RT-PCR for IFN-α mRNA. B: Cell lysates from seven-day-cultured macrophages treated with or without 100 μmol/L METH for 24 hours were subjected to enzyme-linked immunosorbent assay for IFN-α protein. Data are expressed as IFN-α mRNA (A) or protein (B) levels in meth-treated cells (percentage of control) to those in untreated cells, which are defined as 100%. The results represent the mean ± SD of three independent experiments. Statistical analysis was performed by Student’s t-test and significance is shown with *P < 0.05 and **P < 0.01 (meth versus control).
Figure 6.
Effect of the D1R antagonists on meth-mediated down-regulation of IFN-α. Seven-day-cultured macrophages were incubated with or without the D1R antagonist, 10 μmol/L SCH23390, or 10 μmol/L SKF83566 for 1 hour before treatment with or without 100 μmol/L meth for 3 hours. Cellular RNA was subjected to the real-time RT-PCR for IFN-α mRNA. Data are expressed as IFN-α mRNA levels in meth-treated cells (percentage of control) to those in untreated cells, which are defined as 100%. The results represent the mean ± SD of three independent experiments. Statistical analysis was performed using one-way analysis of variance, and significance is shown with *P < 0.05 and **P < 0.01 (METH + SCH or METH + SKF versus METH).
Figure 7.
Effect of meth on STAT1 expression. Seven-day-cultured macrophages were incubated with or without 100 μmol/L meth for 24 hours. Equal amount of proteins extracted from the cells was subjected to the Western blot assay using rabbit antibodies against STAT1 and actin. The numbers in the right panel are the signal intensities expressed as densitometry scanning units (DSU) of protein bands of Western blot shown in the left panel. One representative experiment is shown.
Discussion
Epidemiological studies have demonstrated that meth use is particularly common among HIV-infected patients.10,40 However, very little is known about the deleterious effect of meth on the host’s immune response and the role of meth in the immunopathogenesis of HIV infection. It has been proposed that the modulatory effects of meth on the immune functions related to response to HIV infection may increase the susceptibility of an individual to initial HIV infection and promote the development of HIV infection toward AIDS.14 One of the ways to address the complex interactions between meth use and HIV is to use a permissive cell system such as macrophages. Macrophages play a central role in the immunopathogenesis of HIV disease. Monocytes/macrophages are involved in HIV infection during all stages of disease in which they serve as major target cells, reservoirs, vehicle to other tissues, and transmitters of the virus to CD4+ T cell. Thus, it is necessary to determine whether meth has the ability to enhance HIV infection of macrophages. Our findings that meth increased HIV replication in macrophages (Figure 1) provide direct in vitro evidence to support the clinical study14 showing that plasma virus loads were higher in meth users. In addition, our study is in agreement with the investigation showing that meth exposure can accelerate feline immunodeficiency virus replication.15
Direct action of meth on cellular functions may require its interaction with the dopamine receptors on the target cells. Because D1R has long been implicated in mediating the persistent dopaminergic deficits caused by meth,41,42 we postulated that the D1R expressed on macrophages is involved in immunoregulating macrophage functions. We showed that human blood monocyte-derived macrophages expressed D1R (Figure 2). Most importantly, we demonstrated that D1R expressed on macrophages is functional, because the D1R antagonists blocked the action of meth on HIV infection of macrophages (Figure 3). This finding is supported by a recent report43 showing that meth-mediated up-regulation of DC-SIGN on monocyte-derived dendritic cells was reversed by the D1R antagonist SCH23390. Because both dendritic cells and macrophages are antigen-presenting cells, it is possible that D1R is involved in the meth action in the both cell systems. We also examined the hypothesis that meth has the ability to stimulate D1R expression by macrophages. It has been reported that D1R protein is elevated in nucleus accumbens of human, chronic methamphetamine users.44
However, our experiments examining the impact of meth on D1R failed to reveal the evidence that meth stimulates D1R expression (data not shown), suggesting that up-regulation of D1R is not a mechanism responsible for the meth action on HIV. In addition to dopamine receptors, dopamine was also shown to be involved in the regulation of HIV gene expression in both neuronal cells and cells of the immune system.45 Dopamine activates HIV expression in chronically infected T cells.46 Thus, it is possible that meth, through the induction of endogenous dopamine in macrophages, enhanced HIV replication. We, however, were unable to detect the expression of endogenous dopamine by primary macrophages. This finding is in disagreement with the report that a macrophage cell line produced endogenous dopamine.47 This discrepancy could be due to the difference in the cell types used. In our study, we used primary monocyte-derived macrophages. Nevertheless, the in vivo interpretations of these observations remain to be determined.
In the present study, we have demonstrated two potential mechanisms by which meth may enhance HIV replication. We first showed that meth enhanced HIV infection and replication by inducing the expression of CCR5 receptor on macrophages. This finding supports the report23 that meth up-regulates CCR5 receptor expression in monocyte-derived mature dendritic cells. Our data also are in agreement with the observations of Gavrilin et al15 showing that meth influences the first step of virus (feline immunodeficiency virus) interaction during cell-to-cell transmission of virus. The effect of meth on CCR5, however, is not mediated by D1R, because the D1R antagonists failed to block the meth action (data not shown), suggesting that meth modulates CCR5 expression by a different mechanism. As a key HIV entry co-receptor, CCR5 plays an important role in macrophage tropic or nonsyncytium-inducing HIV strain infection of macrophages.34,48,49 Thus, our finding that meth up-regulated CCR5 expression (Figure 4) provides a plausible mechanism involved in meth-mediated enhancement of HIV infection of macrophages.
In addition, we investigated the impact of meth on the expression on endogenous IFN-α, a potent antiviral cytokine, in macrophages. The role of meth in modulating cytokine expression has been examined by others. For example, meth exposure significantly inhibits Th1 cytokine (interleukin-2 and IFN-γ) production in splenocytes.50,51 Meth is also able to significantly increase the expression of tumor necrosis factor-α and interleukin-6,51 the cytokines that have potential to enhance HIV replication.52 However, it is unclear whether meth can modulate IFN-α expression. Our study for the first time demonstrates that meth has the ability to suppress the expression of intracellular IFN-α in macrophages (Figure 5). This meth action on IFN-α is mediated by D1R, because the D1R antagonists completely blocked the effect of meth on IFN-α expression by macrophages (Figure 6). IFN-α is a key element of the innate defense mechanism against viral infections. It has been demonstrated that IFN-α is a potent inhibitor of HIV infection of CCR5+CD4+ macrophages.36,37 Thus, the suppression of intracellular IFN-α expression by meth would provide a favorable microenvironment for HIV replication in macrophages. To further determine whether meth, through suppressing the IFN signaling pathway, inhibits IFN-α expression in macrophages, we examined the hypothesis that meth inhibits the expression of STAT1, a crucial factor in mediating IFN-dependent biological responses, including the activation of the antiviral state and the induction of type I IFN expression.53,54,55 The finding that meth suppressed STAT1 expression (Figure 7) supports our hypothesis and provides a mechanism for the meth-mediated down-regulation of endogenous IFN-α in macrophages. These data also support the studies by others22,23 showing that meth is immunosuppressive.
Taken together, meth, through the enhancement of CCR5 expression and the suppression of intracellular IFN-α/STAT1 expression, promotes HIV infection of macrophage. These findings provide direct evidence at cellular and molecular levels to support the concept that meth has a cofactor role in enhancing HIV infection and replication. However, further clinical studies are required to validate our in vitro observations and to delineate the mechanisms of meth-mediated enhancement of HIV infection in vivo. These studies will be critical not only for our basic understanding of meth-mediated HIV immunopathogenesis but also for the design and development of innate immunity-based intervention and treatment strategies for meth users with HIV infection.
Acknowledgments
We thank Eric Riedel at the Flow Cytometry Core of Joseph Stokes Jr. Research Institute for the analysis of HIV entry receptor expression.
Footnotes
Address reprint requests to Wen-Zhe Ho, M.D., M.P.H., Division of Allergy and Immunology, The Children’s Hospital of Philadelphia, Department of Pediatrics, University of Pennsylvania School of Medicine, 34th St. and Civic Center Blvd., Philadelphia, PA 19104. E-mail: ho@email.chop.edu.
See related Commentary on page 1467
Supported by the grants from the NIH (NIDA12815 and NIDA22177 to W.-Z.H.)
References
- Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2:194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Anglin MD, Ling W. Will the methamphetamine problem go away? J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Sorensen JL. Differences between methamphetamine users and cocaine users in treatment. Drug Alcohol Depend. 2001;62:91–95. doi: 10.1016/s0376-8716(00)00164-2. [DOI] [PubMed] [Google Scholar]
- Crofts N, Hopper JL, Milner R, Breschkin AM, Bowden DS, Locarnini SA. Blood-borne virus infections among Australian injecting drug users: implications for spread of HIV. Eur J Epidemiol. 1994;10:687–694. doi: 10.1007/BF01719282. [DOI] [PubMed] [Google Scholar]
- Harris NV, Thiede H, McGough JP, Gordon D. Risk factors for HIV infection among injection drug users: results of blinded surveys in drug treatment centers, King County, Washington 1988–1991. J Acquir Immune Defic Syndr. 1993;6:1275–1282. [PubMed] [Google Scholar]
- HIV & drugs: meth use develops stronger link to HIV risk. AIDS Policy Law. 2005;20:5. [PubMed] [Google Scholar]
- Boddiger D. Metamphetamine use linked to rising HIV transmission. Lancet. 2005;365:1217–1218. doi: 10.1016/S0140-6736(05)74794-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Jayne M, Wong C. Stimulant-induced enhanced sexual desire as a potential contributing factor in HIV transmission. Am J Psychiatry. 2007;164:157–160. doi: 10.1176/ajp.2007.164.1.157. [DOI] [PubMed] [Google Scholar]
- Frosch D, Shoptaw S, Huber A, Rawson RA, Ling W. Sexual HIV risk among gay and bisexual male methamphetamine abusers. J Subst Abuse Treat. 1996;13:483–486. doi: 10.1016/s0740-5472(96)00098-0. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Fischgrund BN, Parsons JT. Explanations for methamphetamine use among gay and bisexual men in New York City. Subst Use Misuse. 2005;40:1331–1345. doi: 10.1081/JA-200066900. [DOI] [PubMed] [Google Scholar]
- Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Freese TE. Patient characteristics, HIV serostatus, and risk behaviors among gay and bisexual males seeking treatment for methamphetamine abuse and dependence in Los Angeles. J Addict Dis. 2002;21:91–105. doi: 10.1300/j069v21n01_08. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8:240–249. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Bao J, Lin YW. Neurobiology of HIV, psychiatric and substance abuse comorbidity research: workshop report. Brain Behav Immun. 2007;21:428–441. doi: 10.1016/j.bbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Berman JW, Berman MJ, Masliah E, Chang L, Cox BW, Fox H, Gonzalez RG, Hanson GR, Hauser KF, Ho WZ, Hong JS, Major EO, Maragos W, Masliah E, McArthur JC, Miller DB, Nath A, O'Callaghan JP, Persidsky Y, Power C, Rogers TJ, Royal W., III NeuroAIDS, drug abuse, and inflammation: building collaborative research activities. J Neuroimmune Pharmacol. 2006;1:351–399. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]
- Embretson J, Zupancic M, Beneke J, Till M, Wolinsky S, Ribas JL, Burke A, Haase AT. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci USA. 1993;90:357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao CC, Hegg CC, Thayer S, Peterson PK. Morphine inhibits human microglial cell production of, and migration towards RANTES. J Psychopharmacol. 2000;14:238–243. doi: 10.1177/026988110001400307. [DOI] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Modulation of murine macrophage function by methamphetamine. J Toxicol Environ Health A. 2004;67:1923–1937. doi: 10.1080/15287390490514589. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MP. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther. 2006;10:257–269. doi: 10.1007/BF03256465. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Sykes DE, Schwartz SA, Nair MP. Proteomic analyses of methamphetamine (METH)-induced differential protein expression by immature dendritic cells (IDC). Biochim Biophys Acta. 2007;1774:433–442. doi: 10.1016/j.bbapap.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Gjerde H, Hasvold I, Pettersen G, Christophersen AS. Determination of amphetamine and methamphetamine in blood by derivatization with perfluorooctanoyl chloride and gas chromatography/mass spectrometry. J Anal Toxicol. 1993;17:65–68. doi: 10.1093/jat/17.2.65. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Oyler JM, Joseph RE, Jr, Cone EJ, Moolchan ET, Huestis MA. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49:121–132. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- Takayasu T, Ohshima T, Nishigami J, Kondo T, Nagano T. Screening and determination of methamphetamine and amphetamine in the blood, urine and stomach contents in emergency medical care and autopsy cases. J Clin Forensic Med. 1995;2:25–33. doi: 10.1016/1353-1131(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992;66:573–579. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, Ho WZ. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–1175. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Lin RT, Li Y, Douglas SD, Maxcey C, Ho C, Lai JP, Wang YJ, Wan Q, Ho WZ. Hepatitis C virus inhibits intracellular interferon alpha expression in human hepatic cell lines. Hepatology. 2005;42:819–827. doi: 10.1002/hep.20854. [DOI] [PubMed] [Google Scholar]
- Ostadali MR, Ahangari G, Eslami MB, Razavi A, Zarrindast MR, Ahmadkhaniha HR, Boulhari J. The detection of dopamine gene receptors (DRD1-DRD5) expression on human peripheral blood lymphocytes by real time PCR. Iran J Allergy Asthma Immunol. 2004;3:169–174. [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca-Regen L, Heinzinger N, Stevenson M, Gendelman HE. Alpha interferon-induced antiretroviral activities: restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J Virol. 1994;68:7559–7565. doi: 10.1128/jvi.68.11.7559-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan PR, Guatelli JC, Munis JR, Richman DD, Kornbluth RS. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology. 1993;193:138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Garofalo R, Mustanski BS, McKirnan DJ, Herrick A, Donenberg GR. Methamphetamine and young men who have sex with men: understanding patterns and correlates of use and the association with HIV-related sexual risk. Arch Pediatr Adolesc Med. 2007;161:591–596. doi: 10.1001/archpedi.161.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Suzuki K, Sugiura H, Kawashima N, Okuyama S. Activation of an effector immediate-early gene arc by methamphetamine. Ann NY Acad Sci. 2000;914:22–32. doi: 10.1111/j.1749-6632.2000.tb05180.x. [DOI] [PubMed] [Google Scholar]
- Nair MPN, Mahajan SD, Syes D, Bapardekar MV, Reynolds JL. Methamphetamine modulates DC-SIGN expression by mature dendritic cells. J Neuroimmune Pharmacol. 2006;1:296–304. doi: 10.1007/s11481-006-9027-1. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, Furukawa Y, Ang L, Adams V, Reiber G, Anthony RA, Wickham D, Kish SJ. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E. Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic Acids Res. 1999;27:3291–3299. doi: 10.1093/nar/27.16.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E. Dopamine activates HIV in chronically infected T lymphoblasts. J Neural Transm. 2000;107:1483–1489. doi: 10.1007/s007020070012. [DOI] [PubMed] [Google Scholar]
- Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- House RV, Thomas PT, Bhargava HN. Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacol Immunotoxicol. 1994;16:1–21. doi: 10.3109/08923979409029897. [DOI] [PubMed] [Google Scholar]
- Yu Q, Zhang D, Walston M, Zhang J, Liu Y, Watson RR. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2:951–962. doi: 10.1016/s1567-5769(02)00047-4. [DOI] [PubMed] [Google Scholar]
- Poli G, Vicenzi E, Ghezzi S, Lazzarin A. Cytokines in the acquired immunodeficiency syndrome and other infectious diseases. Int J Clin Lab Res. 1995;25:128–134. doi: 10.1007/BF02592553. [DOI] [PubMed] [Google Scholar]
- Mbow ML, Sarisky RT. What is disrupting IFN-alpha’s antiviral activity? Trends Biotechnol. 2004;22:395–399. doi: 10.1016/j.tibtech.2004.06.002. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]