Abstract
Adenosine is a potent modulator of inflammation and tissue repair. We have recently reported that activation of adenosine A2A receptors promotes collagen synthesis by human dermal fibroblasts and that blockade or deletion of this receptor in mice protects against bleomycin-induced dermal fibrosis, a murine model of scleroderma. Adenosine deaminase (ADA) is the principal catabolic enzyme for adenosine in vivo, and its deficiency leads to the spontaneous development of pulmonary fibrosis in mice. The aim of this study was to characterize further the contributions of endogenous adenosine and adenosine A2A receptors to skin fibrosis. Taking advantage of genetically modified ADA-deficient mice, we herein report a direct fibrogenic effect of adenosine on the skin, in which increased collagen deposition is accompanied by increased levels of key mediators of fibrosis, including transforming growth factor β1, connective tissue growth factor, and interleukin-13. Pharmacological treatment of ADA-deficient mice with the A2A receptor antagonist ZM-241385 prevented the development of dermal fibrosis in this model of elevated tissue adenosine, by reducing dermal collagen content and expression of profibrotic cytokines and growth factors. These data confirm a fibrogenic role for adenosine in the skin and reveal A2A receptor antagonists as novel therapeutic agents for the modulation of dermal fibrotic disorders.
Dermal fibrosis is a pathological hallmark of several disorders such as scleroderma or hypertrophic scar formation. Despite increasing efforts at investigating the mechanisms underlying fibrogenic processes in the skin, no effective antifibrotic therapy exists to date.
Adenosine is present in most biological fluids and is elevated during tissue stress, when it acts as a potent endogenous modulator of inflammation and tissue repair.1,2 The effects of adenosine are mediated through interaction with four G protein-coupled receptors, A1, A2A, A2B, and A3, which are expressed in a cell- and tissue-specific manner.3,4 We have recently reported that activation of the adenosine A2A receptor (A2AR) promotes collagen synthesis by human dermal fibroblasts, and blockade or deletion of this receptor in mice protects against bleomycin-induced dermal fibrosis, a murine model of scleroderma.5
Adenosine deaminase (ADA) is the main catabolic enzyme for adenosine in vivo. Its deficiency leads to marked increases in endogenous adenosine levels, which have been shown to be associated with the spontaneous development of pulmonary fibrosis in mice.6 The pulmonary fibrosis observed in these animals is accompanied by an increase in the number of myofibroblasts, expression of profibrotic cytokines, and deposition of collagen.6,7
To characterize further the contribution of endogenous adenosine and A2ARs to skin fibrosis, we tested the hypothesis that chronic elevation of adenosine due to ADA deficiency leads to dermal fibrosis and that pharmacological blockade of these receptors could prevent the development of this fibrogenic process. We found that endogenously released adenosine plays an important role in the pathogenesis of skin fibrosis, promoting dermal fibrosis associated with elevated expression of profibrotic mediators including connective tissue growth factor (CTGF), transforming growth factor (TGF) β, and interleukin (IL) 13. Pharmacological blockade of the adenosine A2AR prevented the development of dermal fibrosis in this mouse model of elevated tissue adenosine. These data confirm that the adenosine A2AR is a key player in dermal fibrogenesis, and adenosine A2AR antagonism may be a novel therapeutic target in the treatment of dermal fibrosing disorders.
Materials and Methods
Reagents
ZM-241385 was purchased from Tocris (Ballwin, MO). Tissue protein extraction reagent (T-PER) and bicinchoninic acid (BCA) protein assay kit were from Pierce Biotechnology (Rockford, IL). Mouse IL-13, IL-6, and TGF-β1 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D systems (Minneapolis, MN). Polyclonal antibodies for CTGF and α-smooth muscle actin (SMA) were obtained from Abcam (Cambridge, MA). Polyclonal antibody for platelet-derived growth factor (PDGF)-A was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit immunoglobulins and Fast Red substrate system were from DAKO (Carpinteria, CA). Dulbecco’s modified Eagle’s medium, penicillin-streptomycin, and fungizone were purchased from Invitrogen (Grand Island, NY) and all other reagents were from Sigma (Saint Louis, MO).
Mice
ADA-deficient mice were generated and genotyped as described previously.8 Mice homozygous for the null Ada allele were designated ADA-deficient (ADA-KO), whereas mice heterozygous for the null Ada allele were designated as ADA control (wild-type) mice (ADA-WT). ADA-KO mice exhibit a total loss of ADA enzymatic activity,9 whereas ADA-WT mice heterozygous for the null Ada allele have no phenotypic or metabolic differences from mice without the allele because one Ada allele or 50% ADA activity is sufficient to prevent any accumulation of adenosine or deoxyadenosine.8,9 All mice were on a 129sv/C57BL/6J mixed background, and all phenotype comparisons were performed among littermates. Animal care was performed in accordance with Institutional Animal Care and Use Committee of NYU School of Medicine and National Institutes of Health guidelines. All experiments were performed in male mice, since we have previously observed that breaking tension and hydroxyproline content were greater in the skin of male C57/BL6 mice than their female counterparts, in agreement with findings by other investigators.10
Experimental Design: ADA Therapy and Administration of Adenosine A2A Receptor Antagonist to ADA-Deficient Mice
Polyethylene glycol-conjugated ADA (PEG-ADA) was prepared as described previously.11 ADA-KO mice received i.p. injections of PEG-ADA on postnatal days 1, 4, 8, 12, 16, and 20 (0.625, 1.25, 2.5, 2.5, 2.5, and 5 U, respectively; 1 U is defined as the amount necessary to convert 1 μmol/L of adenosine to inosine/min at 25°C). After the last injection, ADA-KO mice were maintained without PEG-ADA for 14 days and then sacrificed. ADA-WT mice were sacrificed at the end of the experimental period (34 days old). To determine the role of the adenosine A2AR, ADA-KO mice were treated with the A2AR antagonist, ZM-241385 (50 mg/kg b.i.d. administered in vehicle i.p.: 15% dimethyl sulfoxide, 15% Cremophor EL, and 70% water, in a total injection volume of 0.1 ml) for the last 8 days before sacrifice, and were compared to ADA-KO male mice.
Morphometric Dermal Measurements
Mice were sacrificed at the end of the experimental period. The backs of the animals were shaved before morphometric measurements. Skin-fold (pinch) thickness was measured using skin calipers on four different areas over the backs of mice. Skin thickness was measured on 6-mm punch biopsies obtained from the back. Breaking strength of the skin was measured on the 6-mm punch biopsies using a tensiometer (Mark-10 Series EG Digital Force Gauge, Mark-10 Corporation Copiague, NY), and the point of maximal stress before tearing of the biopsy was recorded, as we have previously reported.5 These studies were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine.
Immunohistochemistry
After deparaffination and rehydration of 5-μm thick tissue sections, antigen retrieval was performed for 15 minutes at 98°C with 0.01M citrate buffer, pH 6.0. To block nonspecific binding, the slides were incubated for 30 minutes with 5% normal goat serum in Tween 20 Tris buffered saline (TTBS: 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl and 0.1% Tween 20). Primary antibody (anti-CTGF 1/100 or anti-α-SMA 1/100) in TTBS containing 1.5% normal goat serum was incubated overnight at 4°C. After washing, sections were incubated with an alkaline phosphatase-conjugated goat anti-rabbit IgG (1/200) in TTBS containing 1.5% normal goat serum, for 60 minutes at room temperature. Fast Red substrate system was used to detect positive staining. Counterstaining was performed with Gill’s hematoxylin. Negative staining control experiments were performed according to the above-described protocol, with omission of the primary antibody. Photographs were taken with a Qimaging Retiga digital camera mounted on an Olympus BX51 microscope (Olympus America Inc., Center Valley, PA). Quantitation of positive staining (amount of red intensity divided by total skin area) was determined by analyzing six photographs of each animal by using SigmaScan Pro 5 software (version 5.0.0, build 3981).
ELISA Measurements
Skin biopsies were lysed in T-PER tissue protein extraction reagent. Total protein was determined spectrophotometrically by BCA assay kit, using bovine serum albumin as standard protein. Mouse IL-13, IL-6, and total and active TGF-β1 levels in skin lysates were determined by quantitative sandwich enzyme immunoassay technique following manufacturer instructions. Results were expressed as picograms per mg of protein.
Western Blotting
Skin homogenates (15 μg protein/lane) were electrophoresed (4 to 20% SDS Tris-Glycine) and transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked for 2 hours at 4°C in blocking solution (3% BSA in 1× TTBS. After blocking, the membranes were incubated with primary antibody (1:1000 dilution for CTGF, 1:1000 for α-SMA, 1:1000 for PDGF-A, and 1:5000 for β-actin) and incubated for 2 hours at 37°C with gentle shaking on a platform shaker. After incubation with secondary antibody, proteins were visualized using the enhanced chemifluorescence kit (Amersham Biosciences, UK). Band intensities were analyzed by Adobe Photoshop Software program (version 7.0.1) and normalized to β-actin level.
Quantification of Dermal Hydroxyproline Content
Hydroxyproline content in tissue specimens was measured colorimetrically as described previously, with modifications.12 Tissue specimens were dried and hydrolyzed in 6 N HCl at 110°C for 24 hours. Hydrolysates were filtered and neutralized to pH 7 with NaOH. 200 μl of each sample were mixed with 500 μl of chloramine-T solution (1.4% chloramine-T, 10% N-propanol, and 80% citrate-acetate buffer). The mixture was incubated for 20 minutes at room temperature. 500 μl of Ehrlich’s solution was added and the samples were incubated at 65°C for 18 minutes. Absorbance was measured at 560 nm. Standard curves (0 to 10 μg) were generated for each experiment using reagent hydroxyproline as a standard. Results were expressed as μg of hydroxyproline per mg of tissue.
Quantification of Adenosine Levels by High-Pressure Liquid Chromatography
Skin biopsies were washed in PBS containing antibiotics (penicillin 200 U/L, streptomycin 200 μg/L, and fungizone 50 μg/L), cut into small pieces, and incubated in DMEM (containing same antibiotics concentration as before) at 37°C, 5% CO2. After 4 hours of incubation, supernatants were collected and adenosine was extracted and quantitated by high-pressure liquid chromatography as previously described.13 Results were expressed as picomoles of adenosine per mg of tissue.
Statistics
Results are represented as mean ± SEM Data were analyzed by one-way analysis of variance and post hoc analyses of significance of differences between groups was determined by Bonferroni’s multiple comparison tests. All statistical analyses were performed with GraphPad Prism software v. 4.02 (GraphPad Software Inc., San Diego, CA).
Results
Skin Adenosine Levels Are Increased in ADA-Deficient Mice
It has been previously shown that ADA-deficient mice have increased adenosine levels in lung that are associated with spontaneous development of pulmonary fibrosis. To determine whether dermal levels of adenosine are increased in ADA-KO mice, skin was collected after the experimental period and incubated for 4 hours. Supernatants from ADA-KO mice skin, as well as from their WT littermates were analyzed by high-pressure liquid chromatography. As expected, adenosine concentrations were significantly higher (12.4 ± 1.2-fold increase, P < 0.01, n = 5) in ADA-KO mice as compared with ADA-WT mice (Figure 1).
Figure 1.
Adenosine levels are increased in skin of ADA-deficient mice. Skin biopsies were cut and incubated for 4 hours at 37°C, 5%CO2. Culture supernatants were concentrated, and adenosine levels were assessed by high-pressure liquid chromatography. Shown here are differences in adenosine levels between ADA-deficient mice (ADA-KO) and their wild-type littermates (ADA-WT). P < 0.01, n = 5 per group.
ADA-Deficient Mice Exhibit Dermal Fibrosis with Increased Collagen Content
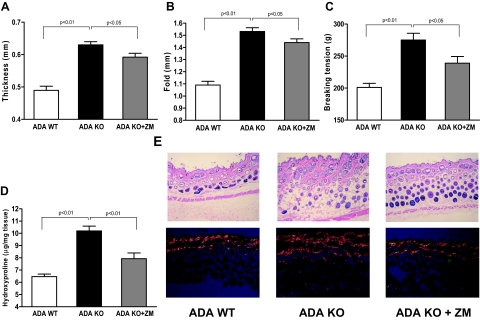
To characterize the skin phenotype of ADA-KO mice, we performed morphometric analyses at the end of the experimental period. Interestingly, ADA-KO mice showed significant increases in dermal thickness, skin-fold thickness, and breaking tension (128.7 ± 2.0%, 140.6 ± 2.8%, and 136.8 ± 5.3% of control, respectively, n = 5, P < 0.001 for all) as compared with ADA-WT mice (Figure 2,A–C). Hematoxylin & eosin staining of paraffin-embedded skin sections also corroborates the increase in ADA-KO dermal thickness, as shown in Figure 2E. There is greater collagen accumulation in the dermis of ADA-KO mice compared with the WT mice, as shown by picrosirius red staining (Figure 2E). Finally, dermal hydroxyproline content, another marker of collagen content, was increased by 57.8 ± 2.8% (n = 5, P < 0.001) in ADA-KO mice (Figure 2D).
Figure 2.
ADA-deficient mice develop dermal fibrosis, which is prevented by A2A receptor antagonist treatment. Shown here are differences in (A) thickness, (B) skin-fold thickness, (C) breaking tension, and (D) hydroxyproline content among ADA-deficient (ADA-KO), wild-type control (ADA-WT) mice, and ADA-deficient mice treated with the adenosine A2AR antagonist ZM-241385 (ADA KO + ZM). E: Histological sections of the different groups were stained with hematoxylin & eosin and picrosirius red viewed under polarized microscopy. P < 0.05, P < 0.01, n = 5 per group.
Profibrotic Mediators Are Elevated in ADA-Deficient Mice
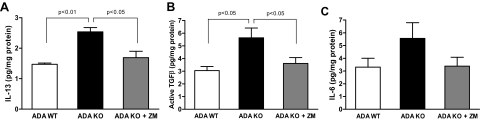
TGF-β1 plays a major role in connective tissue remodeling, scarring, and fibrosis.14 In addition to TGF-β1, other pro-fibrogenic cytokines such IL-13 stimulate fibroblast collagen production by different signaling mechanisms and are involved in the development of many fibrotic disorders.15 To investigate if these profibrotic cytokines were involved in adenosine-mediated dermal fibrotic development, we measured levels of TGF-β1 and IL-13 in skin homogenates by ELISA. Interestingly, IL-13 levels were significantly augmented in skin homogenates from ADA-KO mice (73 ± 15% increase over ADA-WT, P < 0.01, n = 5) (Figure 3A). TGF-β1 levels were also increased in ADA-KO mice skin homogenates as compared with ADA-WT mice (1.9 ± 0.3-fold increase, P < 0.05, n = 5) (Figure 3B). Various studies have also described a role for IL-6 in tissue remodeling and fibrogenesis, showing a direct correlation between IL-6 expression and fibrosis in skin16 and other tissues.17 Furthermore, improvement in skin fibrosis in systemic sclerosis is associated with decreases in IL-6 production.18,19 We have determined IL-6 expression in skin homogenates by ELISA and found increased cytokine levels in the ADA-KO mice as compared with the ADA-WT mice, although the difference was not statistically significant (Figure 3C).
Figure 3.
Effects of adenosine on skin IL-13, TGFβ1, and IL-6 levels. IL-13 (A) and active TGFβ1 (B) levels were significantly increased in skin homogenates of ADA-deficient mice when compared with their wild-type control mice and treatment of ADA-deficient mice with the adenosine A2AR antagonist ZM-241385 significantly reduced levels of these profibrotic cytokines. A similar pattern was found with IL-6 levels (C) although none of the observed changes reached statistical significance. Skin biopsies (6 mm) were homogenated and protein was measured by BCA method. Cytokine levels were determined by ELISA. P < 0.05, P < 0.01, n = 5 per group.
CTGF is another key mediator of fibrosis because it promotes matrix deposition and fibroblast proliferation and is overexpressed in fibrotic lesions of scleroderma patients.20 It has been shown that CTGF is induced by TGF-β1 and is considered a downstream mediator of some effects of TGF-β1 on fibroblasts.20 As ADA-KO mice overexpressed TGF-β1 in the skin, we investigated a potential correlation with CTGF levels. Immunohistochemistry revealed a significant sixfold increase in CTGF expression in ADA-KO skin as compared with ADA-WT skin (7.1 ± 0.8% vs. 1.2 ± 0.3% CTGF staining per total skin area, ADA-KO versus ADA-WT, n = 5, P < 0.01) (Figure 4,A–B). Abundant distribution of CTGF in the skin of ADA-KO mice was observed particularly in the epidermis, dermal cells, and along hair follicles. Western blot analysis corroborated this pattern, as shown in Figure 4C (190.1 ± 15.3% increase in ADA-KO vs. ADA-WT mice, n = 4, P < 0.05).
Figure 4.
Effects of adenosine on skin CTGF expression. A: Immunohistochemistry was performed to determine CTGF expression in skin sections and photomicrographs were taken at original magnification ×400. Arrows indicate positive staining B: Computerized image analysis was used to quantify CTGF staining in sections among the different groups, P < 0.01, n = 5 per group. C: Western blot of skin homogenates was performed to corroborate differences on CTGF expression and a representative immunoblot is shown.
α-SMA-expressing myofibroblasts are considered to be a major cellular component of pathological fibrosis, and an elevated number of these cells is present in fibrotic lesions.14 Consistent with the described dermal fibrotic manifestations, α-SMA-positive cells were more abundant in ADA-KO skin (0.96 ± 0.08% vs. 0.61 ± 0.08% staining per skin area, ADA-KO versus ADA-WT, n = 5, P < 0.01), as determined by immunohistochemistry (Figure 5,A–B). This result was further confirmed by Western blot analysis (Figure 5C), where α-SMA expression was increased in ADA-KO as compared with ADA-WT mice (177.5 ± 31.6%, n = 4, P < 0.05).
Figure 5.
α smooth muscle actin positive cells are increased in skin sections of ADA-deficient mice and normalized by A2AR antagonist treatment. A: Immunohistochemistry was performed to identify α-SMA-positive cells on skin sections, and photomicrographs were taken at original magnification ×400. B: Computerized image analysis was used to quantify α-SMA staining in sections among the different groups. (P < 0.01, n = 5 per group). C: Western blot analysis of skin homogenates was performed to corroborate differences on α-SMA expression and a representative immunoblot is shown.
It has been reported that the expression of PDGF and its receptors are increased in scleroderma patients.21 In particular, PDGF-AA has been implicated in fibroblast proliferation and its expression is up-regulated by IL-13.22,23 As shown in Figure 6, we analyzed the expression of PDGF-AA on skin homogenates by Western blot and found a significant increase in ADA-KO mice as compared with ADA-WT mice (145.2 ± 15.3% increase, P < 0.05, n = 4).
Figure 6.
Effects of adenosine on skin PDGF expression. Western blot was performed to determine PDGF-A expression in skin homogenates. PDGF-A dimers were immunodetected and band intensities were quantified and normalized to β-actin level. A representative immunoblot is shown.
Adenosine A2AR Antagonism Diminishes Dermal Fibrosis and Production of Profibrotic Mediators in ADA-Deficient Mice
We have previously shown that pharmacological blockade or genetic deletion of the A2AR protects against bleomycin-induced dermal fibrosis. To determine whether the adenosine A2AR was involved in the development of the dermal fibrosis and the production of profibrotic markers observed in the ADA-KO mice, we treated ADA-KO mice with the A2AR antagonist ZM241385 (ZM, 50 mg/kg/day, i.p.) for 8 days before sacrifice. Dermal thickness, skin-fold thickness, and dermal breaking strength were reduced in ZM-treated ADA-KO mice as compared with ADA-KO mice (27.2 ± 8.3%, 21.7 ± 7.4%, and 48.9 ± 14.1% decrease, respectively, n = 5, P < 0.05) (Figure 2,A–C). Furthermore, dermal hydroxyproline content was also decreased (61.4 ± 13.5%, n = 5, P < 0.001) (Figure 2D). These results correlate with histological sections that showed decreased dermal thickness as well as reduced collagen density when compared with ADA-KO mice (Figure 2E). ZM-treatment of ADA-WT mice showed no statistically significant difference on morphometric parameters (dermal thickness, skin-fold thickness, and breaking tension) or in hydroxyproline content as compared with ADA-WT mice (Table 1).
Table 1.
Effects of A2AR Antagonist Treatment on ADA-WT Mice
| ADA-WT | ADA-WT + ZM | |
|---|---|---|
| Dermal thickness (mm) | 0.490 ± 0.013 | 0.533 ± 0.036 |
| Fold thickness (mm) | 1.090 ± 0.031 | 1.156 ± 0.074 |
| Breaking tension (g) | 201.1 ± 6.4 | 211.0 ± 11.8 |
| Hydroxyproline (μg/mg tissue) | 6.461 ± 0.207 | 6.735 ± 0.589 |
A2AR antagonist ZM-241385 was administered to ADA-WT for 8 days (50 mg/kg/day), and morphometric measurements and hydroxyproline content were determined at the end of the experimental period. No statistical difference was found between ADA-WT (n = 5) and ZM-treated ADA-WT mice (n = 3).
Interestingly, the fibrogenic cytokines IL-13 and TGF-β1 were reduced by 34 ± 8% (P < 0.05) and by 36 ± 8% (P < 0.05) (Figure 3), respectively, as a result of the A2AR antagonist treatment, suggesting that the overexpression of these cytokines in the skin of ADA-KO mice is mediated in part through the A2AR.
When we investigated the effects of A2AR blockade on CTGF expression by immunohistochemistry, we found a significant decrease (52 ± 7% decrease, n = 5, P < 0.01) compared to ADA-KO mice (Figure 4,A–B) that was further confirmed by Western blot analysis (38.6 ± 7.2% decrease, n = 4, P < 0.05) (Figure 4C). A similar pattern was observed regarding α-SMA expressing myofibroblasts, since A2AR antagonist treatment reduced positive-stained cells by 31 ± 4% compared to nontreated ADA-KO mice (n = 5, P < 0.01) (Figure 5,A–B). Western blot analysis also confirmed this finding (39.7 ± 6.1% decrease, n = 4, P < 0.05) (Figure 5C) previously assessed by immunohistochemistry.
Finally, A2AR antagonist treatment of ADA-KO mice reduced PDGF-A expression (26.3 ± 5.1%, n = 4, NS) without affecting basal levels on ADA-WT mice.
Taken together these findings confirm an important role for the A2ARs in the development of dermal fibrotic conditions.
Discussion
The experiments reported here were undertaken to investigate further the role of adenosine and adenosine A2ARs in skin fibrosis. We demonstrated that elevated tissue adenosine levels lead to fibrosis of the skin, as was spontaneously manifested in ADA-deficient mice. Increases in collagen, myofibroblasts, as well as the production of profibrotic mediators, characterize the skin phenotype of the ADA-deficient mice. Furthermore, treatment with an A2AR antagonist effectively reduced collagen accumulation and fibrogenic biochemical and cellular markers in this adenosine-mediated fibrotic model.
The profibrogenic role for adenosine shown here is consistent with previous reports by our group and others, however, the mechanisms that lead to adenosine-mediated fibrosis remain to be completely dissected. Montesinos and colleagues have demonstrated that occupancy of adenosine A2AR promotes wound healing in a murine model, in part by accelerating matrix deposition.24,25 A direct stimulation of collagen synthesis has been also demonstrated in human dermal fibroblasts through an A2AR/mitogen-activated protein kinase kinase-1/mitogen-activated protein kinase-mediated pathway.5 Furthermore, Chunn and colleagues have reported that elevated lung adenosine levels are associated with spontaneous development of pulmonary fibrosis in ADA-deficient mice.6,7
Interestingly, a correlation between plasma ADA activity in scleroderma patients and stage of the disease has previously been demonstrated.26 However, direct effects of elevated tissue adenosine have not been studied in the skin. It is possible that the increase in adenosine deaminase activity is an adaptive mechanism to counteract the sclerosing properties of circulating and tissue adenosine in this disease. Alternatively, the inflammatory environment is conducive to the up-regulation of anti-inflammatory influences such as adenosine, thus providing a potential link between the control of inflammation and fibrous tissue deposition in the skin.
IL-13 is a pleiotropic cytokine implicated in many fibrotic disorders. Whether IL-13 induces fibrosis by a TGF-β-dependent or -independent mechanism remains controversial.27,28,29 In our studies, we found that elevations in skin adenosine concentration stimulate secretion of both cytokines. Interestingly, ADA-deficient mice have been shown to secrete elevated levels of IL-13 in lung, and IL-13 transgenic mice have elevated levels of lung adenosine.30 In addition, adenosine has been reported to directly induce IL-13 expression on mast cells through activation of A2BR.31 However, when we treated ADA-deficient mice with an A2AR antagonist, levels of IL-13 were significantly reduced, indicating a role for A2AR in regulating IL-13 production, either directly or indirectly.
With regard to TGF-β1, it is worth mentioning that adenosine has been shown to up-regulate TGF-β1 mRNA in hepatic stellate cells32 and both protein and message levels of TGF-β are overexpressed in the lungs of ADA-deficient mice,6,7 which are reduced with A2BR antagonist treatment.33
Although the roles of different adenosine receptors in fibrosis remain controversial, it is likely that adenosine-mediated mechanisms of fibrosis are tissue specific and although common mechanisms exist, patterns of expression of adenosine receptors may differ from one organ to the other, influencing the overall effects of adenosine. In fact, deletion of A2AR in ADA-deficient mice provokes enhanced pulmonary inflammation,34 whereas we have demonstrated that loss of A2AR prevents bleomycin-induced dermal fibrosis5 and CCl4- and thioacetamide-mediated liver fibrosis.35 Thus, different tissues may respond differently to chronic elevations in adenosine levels.
CTGF is not generally expressed in normal fibroblasts or tissues and its secretion has been associated with pathogenic fibrotic processes such as scleroderma, where it functions in maintaining and enhancing the profibrotic actions of TGF-β1.20 In accordance with this phenomenon, CTGF expression is greatly increased in skin of ADA-deficient mice and almost undetectable in healthy skin of ADA-WT mice. A2AR antagonist treatment reduced these levels, further suggesting adenosine receptor blockade’s potential as therapy for ameliorating fibrotic disorders. In addition, the association between adenosine elevation and CTGF overexpression, as well as the protection mediated by A2AR blockade, is novel. However it remains to be elucidated whether decreased CTGF is a consequence of reduced TGF-β1 expression, since CTGF is potently induced by TGFβ1 and has been described as a downstream effector of TGF-β1,20 but it has also been shown that constitutive overexpression of CTGF in scleroderma fibroblasts might be independent of TGF-β1 signaling36 and different mediators, such as endothelin-1, have been implicated in the induction of CTGF independently from the TGF-β1 ligand.37
We found an increased number of myofibroblasts associated with elevated skin adenosine concentration, a phenomenon that was prevented by pharmacological blockade of A2AR. The origin of the myofibroblast population in fibrotic lesions is still unclear, although TGF-β1, CTGF and even IL-13 have been reported to activate differentiation of fibroblasts into α-SMA expressing myofibroblasts.38,39,40 In addition, epithelial cells and fibrocytes have been suggested as precursors of myofibroblasts.41 Previous studies with ADA-deficient mice have also related adenosine and myofibroblast increments in lung.7
The present results suggest that adenosine, and moreover, A2ARs play important roles in the development of dermal fibrosis, enhancing collagen deposition and dermal thickening directly and indirectly through stimulation of involved key profibrotic mediators such as TGF-β1, CTGF, and IL-13 and profibrotic cell mediators such as myofibroblasts. Our results suggest that adenosine is an orchestrator of the fibrotic process and thus pharmacological blockade of A2ARs may treat or prevent the chronic fibrotic state.
Acknowledgments
We thank Dr. Herman Yee and Dr. Janci Chunn for their expert advice and helpful technical assistance.
Footnotes
Address reprint requests to Edwin S.L. Chan., M.D., NYU School of Medicine, 550 First Ave., NBV16N1, New York, NY 10016. E-mail: chane01@nyu.edu.
Supported by grants from the Arthritis National Research Foundation (to E.S.L.C.), the Scleroderma Foundation (to E.S.L.C.), the National Institutes of Health (grants AA13336, AR41911, GM56268, and HL70952 to B.N.C.), King Pharmaceuticals (to B.N.C.), the Spanish Ministry of Education and Science, the General Clinical Research Center (M01RR00096), and the Kaplan Cancer Center of New York University School of Medicine.
Disclosures: E.S.L.C. and B.N.C. hold a patent on the use of adenosine A2A receptor antagonists in the treatment of fibrotic diseases. B.N.C. is a consultant for King Pharmaceuticals, Can-Fite Biopharma, Inc., Bristol-Myers, Squibb, and Tap Pharmaceuticals. He is the recipient of honoraria for speaking from Merck Pharmaceuticals, Tap Pharmaceuticals, and Amgen. All authors concur with the submission and the material submitted for publication has not been previously reported and is not under consideration for publication elsewhere. There are no conflicting financial interests.
References
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, Tung CF, Khoa DN, Pillinger MH, Reiss AB, Tomic-Canic M, Chen JF, Schwarzschild MA, Cronstein BN. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, Blackburn MR. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L579–L587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MR, Datta SK, Kellems RE. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem. 1998;273:5093–5100. doi: 10.1074/jbc.273.9.5093. [DOI] [PubMed] [Google Scholar]
- Markova MS, Zeskand J, McEntee B, Rothstein J, Jimenez SA, Siracusa LD. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol. 2004;123:1052–1056. doi: 10.1111/j.0022-202X.2004.23494.x. [DOI] [PubMed] [Google Scholar]
- Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140–146. doi: 10.1016/s0923-1811(01)00128-1. [DOI] [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto M, Takehara K, Sato S. Pathogenesis of systemic sclerosis: altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. J Dermatol Sci. 2005;39:1–7. doi: 10.1016/j.jdermsci.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujimoto M, Hasegawa M, Takehara K, Tedder TF. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol. 2004;41:1123–1133. doi: 10.1016/j.molimm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]
- Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Rice AB, Geisenhoffer K, Madtes DK, Bonner JC. IL-13 and IL-1beta promote lung fibroblast growth through coordinated up-regulation of PDGF-AA and PDGF-Ralpha. FASEB J. 2004;18:1132–1134. doi: 10.1096/fj.03-1492fje. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Antao-Menezes A, Mangum JB, Lyght O, Lee PJ, Elias JA, Bonner JC. Opposing actions of Stat1 and Stat6 on IL-13-induced up-regulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J Immunol. 2006;177:4141–4148. doi: 10.4049/jimmunol.177.6.4141. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P, Filipe P, Emerit I, Freitas J, Guerra Rodrigo F, Manso C. Adenosine deaminase in progressive systemic sclerosis. Acta Derm Venereol. 1995;75:297–299. doi: 10.2340/0001555575297299. [DOI] [PubMed] [Google Scholar]
- Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest. 2006;116:163–173. doi: 10.1172/JCI25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- Hashmi AZ, Hakim W, Kruglov EA, Watanabe A, Watkins W, Dranoff JA, Mehal WZ. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G395–G401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production and angiogenesis in adenosine deaminase deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–L761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, Leask A. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem. 2003;278:41728–41733. doi: 10.1074/jbc.M305019200. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, Pearson JD, Bou-Gharios G, Dashwood MR, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol. 2007;26:625–632. doi: 10.1016/j.matbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107:1001–1008. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]