Abstract
The role of microglia in neurodegeneration is controversial, although microglial activation in the retina has been shown to provide an early response against infection, injury, ischemia, and degeneration. Here we show that endogenous bone marrow (BM)-derived microglia play a protective role in vascular and neural degeneration in the retinitis pigmentosa model of inherited retinal degeneration. BM-derived cells were recruited to the degenerating retina where they differentiated into microglia and subsequently localized to the degenerating vessels and neurons. Inhibition of stromal-derived factor-1 in the retina reduced the number of BM-derived microglia and accelerated the rate of neurovascular degeneration. Systemic depletion of myeloid progenitors also accelerated the degenerative process. Conversely, activation of BM-derived myeloid progenitors by systemic administration of both granulocyte colony-stimulating factor and erythropoietin resulted in the deceleration of retinal degeneration and the promotion of cone cell survival. These data indicate that BM-derived microglia may play a protective role in retinitis pigmentosa. Functional activation of BM-derived myeloid progenitors by cytokine therapy may provide a novel strategy for the treatment of inherited retinal degeneration and other neurodegenerative diseases, regardless of the underlying genetic defect.
Inherited degeneration of the retina affects as many as 1 in 3500 individuals, for a total of more than 1 million throughout the world, and is characterized by progressive night blindness, visual field loss, optic nerve atrophy, vessel attenuation, altered vascular hyperpermeability, and loss of central vision, often progressing to complete blindness.1 Molecular genetic analysis has identified mutations involving more than 110 different genes, accounting for only a relatively small percentage of the known affected individuals.2 Recent advances in gene therapy have led to successful reversal of retinal degeneration, slow (rds)3 and retinal degeneration (rd)4 phenotypes in mice, and the RPE65 phenotype in dogs.5 The development of other neuroprotective approaches that affect secondary biochemical pathways would be advantageous because they would not depend on the underlying disease-causing mutation. The potential use of calcium channel blockers,6 trophic factors,7 and dietary supplements8 has been explored.
Recently, intravitreally injected, lineage-negative (Lin−) hematopoietic stem cells (HSCs) have been reported to rescue retinal degeneration in rd1 and rd10 mice.9 In the study, exogenous Lin− HSCs prevented retinal vascular degeneration and this vascular rescue correlated with neuronal rescue.9 Although this approach showed a dramatic rescue effect, there was a limitation in that intravitreally injected bone marrow (BM)-derived stem cells were effectively incorporated into the retina only during an early, postnatal developmental stage, but not in adult mice. Directly injected exogenous Lin− HSCs only target activated astrocytes that are observed in neonatal mice or an injury-induced model in the adult.10
In the current study, we have investigated the role of endogenous circulating BM-derived stem cells in inherited retinal degeneration and explored a new therapeutic potential. Recruited endogenous BM-derived stem cells in the retina contain a variety of progenitor cells, including those capable of becoming vascular endothelial cells,10,11 mural cells,12 epithelial cells (including retinal pigment epithelium),13 and microglia/macrophages.14 The role of BM-derived stem cells has recently been suggested in several neural degenerative diseases,15,16 including Alzheimer’s disease17 and amyotrophic lateral sclerosis.18 However, the relationship between BM-derived stem cells and inherited retinal degeneration remains elusive.
Materials and Methods
Mouse Strains
All animals were handled in accordance with the standards of the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and vision research. All experimental procedures were approved by the Institutional Review Board at Kyoto University Graduate School of Medicine. C57BL/6, C3H/HeJ (rd1/rd1), and C57BL/6-Tg (CAG-EGFP) mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). B6.CXB1-Pde6brd10/J (rd10) and B6(A)-Rpe65rd12/J (rd12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C3H/HeJ is homozygous for the rd1 mutation, which causes rapid early onset retinal degeneration. This mutation is located in exon 7 of the Pde6b gene that encodes the rod photoreceptor cGMP phosphodiesterase β subunit. Mutations in this gene have been found in human patients with autosomal recessive retinitis pigmentosa (RP).19 Retinal degeneration in rd10 mice is caused by a mutation in exon 13 of Pde6b gene. This is also a clinically relevant RP model with later onset and milder retinal degeneration than rd1/rd1.20 Retinal degeneration in rd12 mice is caused by a mutation in exon 3 of the Rpe65 gene, and this strain is a model for human Leber congenital amaurosis. This mutation causes slower onset and slower progression of retinal degeneration than rd1/rd1 or rd10.21
BM Transplantation
The tibias and femurs were dissected and the BM was extracted by slowly flushing medium (RPMI 1640 containing 2.5% HEPES and 1% gentamicin) inside the diaphyseal channel with a 27-gauge needle. The BM that was obtained was homogenized, filtered (70 μm nylon filter; BD Biosciences, Tokyo, Japan), centrifuged, and resuspended in the medium, as described earlier. Recipient mice were lethally irradiated (950 cGy) using a Gammacell 40 exactor (Nordion, Vancouver, Canada) and 1 × 107 nonpurified BM cells were administered intravenously.
Counting the Number of Infiltrated GFP+ Cells into the Retina
Eyes were nucleated after fixation with 4% paraformaldehyde and the retinas were laid flat with radial relaxing incisions to obtain a whole-mount preparation. For quantification of GFP+ cells in the retina, fluorescent microscopy (AxioVision; Zeiss, Oberkochen, Germany) was used and four independent fields (×40 objective lens) were chosen randomly from the mid-peripheral area between the optic disk and the retina-ciliary body border. GFP+ cells in all of the retinal layers were summed up in each field and the mean number of the four fields was calculated for each eye.
Immunohistochemistry
Retinas were harvested at various time points and fixed with 4% paraformaldehyde and methanol, followed by blocking in 50% fetal bovine serum/20% normal goat serum for 1 hour at room temperature. To stain retinal vasculature, anti-CD31 (BD Biosciences Pharmingen, San Diego, CA) and anti-collagen type IV (Chemicon, Temecula, CA) were used. To confirm microglial phenotype, anti-Iba1 (Wako, Osaka, Japan), anti-F4/80 (Serotec, Oxford, UK), anti-CD11b (BD Biosciences), and CD68 (Serotec) were used. To confirm other cellular surface markers of astrocytes, mural cells, and neurons, we used anti-GFAP Cy3-conjugated (Sigma, St. Louis, MO), anti-α smooth muscle actin Cy3-conjugated (Sigma), and anti-NeuN antibodies (Chemicon), respectively. For secondary antibodies, Alexa 488- or 594-conjugated antibodies (Invitrogen, Eugene, OR) were used. The retinas were laid flat with radial relaxing incisions to obtain a whole-mount preparation and the images were obtained by confocal microscopy (LSM 5 Pascal, Zeiss). For a sectional view, cryostat sections (10 μm) were counterstained by DAPI (Sigma) and the images were taken by fluorescence microscopy (AxioVision, Zeiss).
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total mRNA was prepared from freshly dissected whole retinas of mice, using a RNeasy kit (Qiagen, Tokyo, Japan). The mRNA was transcribed using a First-Strand cDNA synthesis kit (Applied Biosystems, Tokyo, Japan), and real-time PCR was performed using TaqMan PreAmp master mix (Applied Biosystems). Primers for stromal-derived factor (SDF)-1 were purchased from Applied Biosystems (assay ID: Mm00445552_m1). All samples were normalized to β-actin (Applied Biosystems). Real-time PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems) for 40 cycles.
Intravitreal Injection
Mice were anesthetized, and an anti-SDF-1 antibody (MAB310; R&D Systems, Minneapolis, MN) or saline control was injected intravitreally using 33-Gauge needle (Hamilton, Reno, NV). A total volume of 0.5 μl was administered to achieve a final effective concentration of 100 μg/ml of antibody in the vitreous.
Quantification of Retinal Vascular Length
We quantified the retinal vessel length following a previously described method.9 Briefly, images of vasculature in intermediate and deep vascular plexuses were obtained using a confocal microscope (LSM 5 Pascal, Zeiss) with an attached software. For quantification of the vasculature, four independent fields (461 μm × 461 μm) were chosen randomly from the midportion of the intermediate and deep vascular layer, and the total length of the vasculature was measured using Angiogenesis Image Analyzer software (Kurabo, Osaka, Japan).
Counting the Number of Neurons in the Outer Nuclear Layer
Mice were transcardially perfused with 4% paraformaldehyde, and the eyes were enucleated, fixed, embedded in paraffin, and processed for standard hematoxylin and eosin (H&E) staining. A single section (4 μm) containing the superior pole, inferior pole, optic nerve head, and the entire peripheral retina was visualized with a light microscope (AxioVision, Zeiss) with a ×20 objective lens. The number of nuclei located in the outer nuclear layer in the mid-peripheral area of two different fields (1000 pixels in Photoshop) within one section were counted and averaged for analysis.
Measurement of Cone Cell Density
We measured cone cell density following a previously described method,22 with a slight modification. Briefly, eyecups were fixed in 4% paraformaldehyde for 30 minutes and then the entire retina was carefully dissected away from the retinal pigment epithelium. Retinas were placed in Alexa Fluor 594-conjugated lectin PNA (Molecular Probes, Tokyo, Japan) at a concentration of 5 μg/ml in phosphate-buffered saline (PBS) with 20% normal goat serum for 2 hours at 4°C. The flat-mounted retinas were examined using a confocal microscope (LSM 5 Pascal, Zeiss) with a ×20 objective lens. The number of cones present within four 230 × 230 μm (512 × 512 pixels) squares located 500 μm away from the center of the optic disk was counted and each of four independent fields was averaged.
Colony Forming Unit-Granulocyte Macrophage (CFU-GM) Assay
BM cells obtained from tibias and femurs were homogenized, filtered, and corrected by light-density gradient centrifugation (Histopaque 1083; Amersham Biosciences, Buckinghamshire, UK). A total of 2 × 103 cells per well was seeded into MethoCult medium (GF M3534; StemCell Technologies, UK) and after 7 days in culture, colonies (>50 cells) in three wells were scored by two independent investigators.
Systemic Depletion of Circulating Microglial Progenitors
Clodronate-loaded liposomes were provided by N. van Rooijen (Vrije Universiteit, Amsterdam, The Netherlands) and were injected intraperitoneally, with appropriate dilution for each experiment, at 0.1 ml volume per day.
Systemic Administration of Granulocyte Colony-Stimulating Factor (G-CSF) and Erythropoietin (Epo)
Human recombinant G-CSF (300 μg/kg) and Epo (1000 IU/kg) (Kirin Pharma, Tokyo, Japan) were co-administered once daily in 0.1 ml of saline.
Recording of Electroretinograms (ERGs)
After overnight dark adaptation, mice were anesthetized by intraperitoneal injection of 50 μg/kg of ketamine and 10 μg/kg of xylazine. ERGs were recorded from the corneal surface of each eye after pupil dilation (0.4% tropicamide and 0.5% phenylephrine hydrochloride) using a gold loop corneal electrode with a light-emitting diode (Mayo Corp., Inazawa, Japan). A reference electrode was placed in the mouth and a ground electrode was inserted into the tail. Stimuli were produced with a light-emitting diode stimulator (Mayo Corp.). The ERG response signals were amplified, digitized at 10kHz with a band-pass filter of 0.3 to 500 Hz, and computer-analyzed (PowerLab 2/25; AD Instruments, New South Wales, Australia). Rod-cone maximal combined responses were recorded by a white flash at an intensity of 30 cd′s/m2. After 10 minutes of light adaptation at an intensity of 25.1 cd/m2, cone responses were estimated by a white stimulus of 30 cd′s/m2. The stimulus frequency was 1 Hz and 32 photopic measurements were averaged.
Statistical Analysis
All results for continuous variables are expressed as the mean ± SEM. Comparisons between two groups were analyzed by Student’s t-test for paired and unpaired analyses. A P value less than 0.05 was considered statistically significant. All analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL).
Results
BM-Derived Cells Are Recruited into the Degenerating Retina Where They Differentiate into Microglia, and Localize to the Site of Degenerating Vessels and Neurons
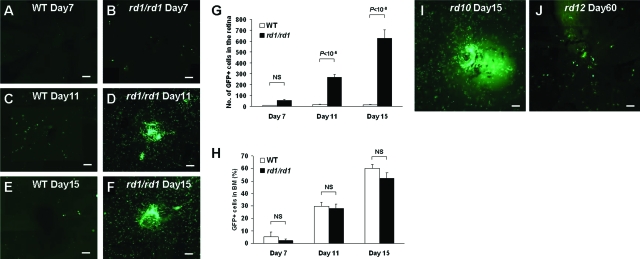
The relationship between BM-derived cells and inherited retinal degeneration is unknown. To clarify this, we first developed a bone marrow transplantation (BMT) model. BM obtained from donor C57BL/6-Tg (CAG-EGFP) mice was transplanted into recipient rd1 mice or wild-type (WT) C57BL/6 mice on postnatal (P) day 15. Seven days after BMT, both rd1 and controls showed few GFP+ cells in the retina (Figure 1, A and B). On day 11, however, numerous GFP+ cells had been recruited in the retina of rd1 but not WT mice (Figure 1, C and D). By day 15, the number of GFP+ cells detected in the rd1 retina was 43-fold greater than in controls (Figure 1, E–G). The number of GFP+ cells detected in the BM of transplanted mice was not different between the two groups (Figure 1H). This recruitment of BM-derived cells into the degenerating retina was also seen in the other strains of mice with retinal degeneration [ie, rd10 (B6.CXB1-Pde6brd10/J) and rd12 (B6(A)-Rpe65rd12/J); Figure 1, I and J]. This suggests that the recruitment of BM-derived cells into the degenerating retina does not depend on the background of the recipient strain or to the specific type of gene mutation, but rather to retinal degeneration itself.
Figure 1.
BM-derived cells were recruited into the degenerating retina. A–F: Retinal flatmount after BMT with GFP donor mice. Seven days after BMT in wild-type (WT) mice (A) and rd1/rd1 mice (B). Eleven days after BMT in WT mice (C) and rd1/rd1 mice (D). Fifteen days after BMT in WT mice (E) and rd1/rd1 mice (F). G: GFP+ cell counts in the retina of WT mice and rd1/rd1 mice (n = 6). H: GFP+ cell percentage of BM in WT mice and rd1/rd1 mice (n = 6). NS, not significant. Retinal flatmount after GFP labeling and BMT in other degeneration models, rd10 (I) and rd12 (J). BMT was performed at P15 in rd10, and at P30 in rd12. The images were collected 15 days after BMT in rd10 and 60 days after BMT in rd12. Scale bars = 100 μm.
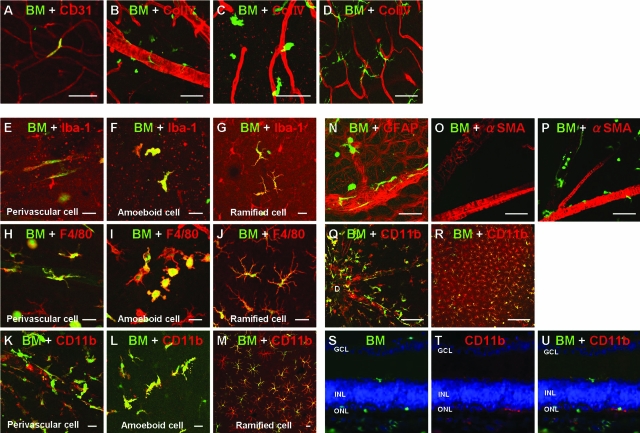
Next, we wanted to determine the localization of transplanted BM-derived cells in the degenerating retina and whether they differentiated (Figure 2). We noted that few GFP+ cells were incorporated into the capillary vessels, indicating that the cells originated from circulating endothelial progenitor cells (Figure 2A). However, the vast majority of the GFP+ cells were classified into three types by morphology (Figure 2, B–D), as follows: 1) the spindle shape along the retinal vessels (Figure 2B), 2) the amorphous/amoeboid shape around the relatively superficial retinal vessels (Figure 2C), and 3) the ramified morphology in the deeper layer of the retina (Figure 2D). Subsequently, we performed immunohistochemistry using several cellular surface markers, including vascular endothelium, mural cells, astrocytes, microglia, and neurons, and found that all three types of cells expressed microglial markers (ie, Iba-1, F4/80, and CD11b; Figure 2, E–M). We found very few CD31+ endothelial phenotype (Figure 2A) and no GFAP+ astrocytes (Figure 2N), α-smooth muscle actin+ mural cells (Figure 2, O and P), or Neu-N+ neurons (data not shown). These BM-derived microglia were especially condensed around the optic disk and retinal vascular trunk (Figure 2Q). In addition, 4 months after BMT, most of the retinal microglia (more than 80%) were shown to be of BM origin and the remainder were tissue resident microglia (Figure 2R). These results may indicate that microglia in the degenerating retina may give rise to a turnover by infiltration of BM-derived microglia. These recruited cells were demonstrated, both in rd1 and rd10 mice, to migrate toward the outer nuclear layer or subretinal spaces where no microglia exist in physiological conditions (Figure 2, S–U).23 In summary, the degenerating retina recruits the BM-derived cells and most of these BM-derived cells differentiate into cells with a microglial phenotype. In addition, BM-derived microglia migrate toward the regressing vessels and dying neurons in the retina probably respond to some signals originating from degenerating vessels and neurons, and this phenomenon may suggest their roles in neurovascular degeneration.
Figure 2.
Retinal images after BMT. A: Incorporated BM-derived cells (green) in the retinal vasculature (CD31, red) in rd1 mice. B–D: Three types of morphologies of BM-derived cells (green) and association with retinal vasculature (collagen IV, red). B: Spindle-shaped BM-derived cells were around the superficial retinal vasculature. C: Amoeboid BM-derived cells were in the intermediate retinal vasculature. D: Ramified BM-derived cells were in the deep retinal vasculature. E–M: Morphological and immunohistochemical analysis of BM-derived cells (green) in the degenerating retina for Iba-1 (E–G), F4/80 (H–J), and CD11b (K–M) (red). N: BM-derived cells (green) are found in the astrocyte network (GFAP, red). O: No transplanted BM-derived cells (green) were found around the retinal vessels (α-smooth muscle actin, SMA; red) in WT retina. P: Many transplanted BM-derived cells were found around retinal vessels (α-SMA, red) in rd1 retina. Q: Numerous BM-derived cells (green) were found around the optic disk (D) expressing CD11b (red). R: Four months after transplantation in rd1 mice, >80% of CD11b+ microglia (red) are BM-derived (green). S–U: Sectional view of the retina of rd1 mice at P30 (15 days after BMT). S: BM-derived cells (green) and DAPI (blue). T: CD11b (red). U: Merged image. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 50 μm (A–D, N–P); 20 μm (E–M); 100 μm (Q); 200 μm (R).
SDF-1 Plays a Role in the Recruitment of Circulating Microglial Progenitors into the Degenerating Retina and Inhibition of SDF-1 Accelerates Neurovascular Degeneration
We next investigated the mechanism underlying the recruitment of BM-derived stem cells into the degenerating retina. We focused on SDF-1, which is known as an entrapment factor of BM-derived stem cells to the lesion sites.24 Quantitative real-time polymerase chain reaction showed that the expression patterns of SDF-1 mRNA were similar in both WT C57BL/6 and rd1 mice (Figure 3A). The expression of SDF-1 mRNA was stable from P6 to P12, elevated at P14, and highest on P18. SDF-1 mRNA was significantly higher in rd10 mice than the controls at P16 and P18. This peak of SDF-1 mRNA expression was later than the reported peak of rod photoreceptor cell degeneration (approximately P14 to P16)23 and the peak of expression of other degeneration-related cytokines, such as MCP-1, RANTES, and tumor necrosis factor-α (approximately P12).23
Figure 3.
SDF-1 plays a crucial role for the retention of BM-derived cells in the degenerating retina and inhibition of SDF-1 accelerates retinal degeneration. A: Real-time PCR analysis for SDF-1 in the retina of WT mice and rd1/rd1 mice (n = 6 per group). B and C: Retinal flatmount of saline-injected eye and anti-SDF-1 neutralization antibody-injected eye (injected at P8) 15 days after GFP donor BMT. D: Marked reduction of BM-derived cells in the anti-SDF-1-treated eyes (n = 8). E–H: Deep vasculature (P25) of saline- and anti-SDF-1-injected eyes in WT (E, F) and rd10 mice (G, H). No change in anti-SDF-1-injected eyes of WT mice (F), in contrast, loss of microvasculature occurred in anti-SDF-1-injected eyes of rd10 mice (H), compared to saline-injected eyes (G). I–L: Sectional images (P25) of saline- and anti-SDF-1-injected eyes in WT (I, J) and rd10 mice (K, L). No change was observed in WT mice (I, J); however, there was a marked loss of photoreceptor cells in the outer nuclear layer (ONL) in anti-SDF-1-injected eyes of rd10 mice (K, L). GCL, ganglion cell layer; INL, inner nuclear layer. M: Vascular length of deep vasculature (P25) was reduced in anti-SDF-1-injected eyes of rd10 mice (n = 8). N: Number of photoreceptor cells in ONL (P25) was reduced in anti-SDF-1-injected eyes of rd10 mice (n = 8). *P < 0.05, **P < 0.01. Scale bars = 100 μm.
To investigate whether SDF-1 plays a role in the recruitment of BM-derived cells into the degenerating retina, we injected an anti-SDF-1 neutralizing antibody into the vitreous of rd1 mice (EGFP+ BM cell-transplanted). We found a marked decrease in the number of BM-derived cells (GFP+ cells) in the anti-SDF-1-injected retinas compared to the saline-injected retinas (Figure 3, B–D), suggesting that SDF-1 plays a role in the recruitment of BM-derived cells into the degenerating retina.
The retinas of anti-SDF-1-injected rd1 eyes were significantly thinner than those of control rd1 eyes (data not shown). Because it is possible that anti-SDF-1 influences normal vascular development of the retina,25 we used rd10 mice to investigate whether the inhibition of SDF-1 truly affected retinal degeneration, including both neurons and vessels (Figure 3, E–N). We injected the anti-SDF-1 antibody on P20, when normal vascular development is nearly complete and when degeneration begins in rd10 mice. In WT mice (C57BL/6), injection of anti-SDF-1 antibody did not change the vasculature or neurons (Figure 3, E, F, I, and J). In contrast, anti-SDF-1 injection in rd10 mice accelerated vascular and neural degenerations (Figure 3, G, H, and K–N), similar to rd1 mice.
In addition, we investigated whether BM-derived microglia play a role in cone photoreceptor cell degeneration. In rd1 mice, rod photoreceptor degeneration begins on P10 and is almost complete by P21, whereas cone cell numbers are essentially normal at P21 but are reduced to undetectable levels by 2 to 4 months of age.26 We selectively stained cone cells with PNA lectin in a flatmount retina26 and found a marked decrease in the number of cone cells in anti-SDF-1-injected eyes at P21 in rd1 mice (Figure 4, A–C). We also found a strong positive correlation between the number of microglia and the density of cone cells (Figure 4D). Thus, depletion of BM-derived microglial cells by SDF-1, resulting in an accelerated degeneration of the retina suggested a protective role of these cells in inherited neurovascular degeneration, especially for secondary cone cell loss after primary rod cell degeneration.
Figure 4.
Inhibition of SDF-1 accelerates cone photoreceptor degeneration. A and B: Cone density and number of microglia were (P21) markedly decreased in anti-SDF-1-injected eyes of rd1 mice (injected on P8). Inner and outer segments of cone photoreceptor were stained by lectin PNA (red). Microglia were stained by CD11b (green). C: Quantitative analysis of cone density in rd1 mice (n = 6). D: Cone density and the number of microglia have a strong positive correlation (n = 12). **P < 0.01. Scale bars = 50 μm.
Systemic Depletion of Circulating Microglial Progenitors Accelerates Inherited Neurovascular Degeneration
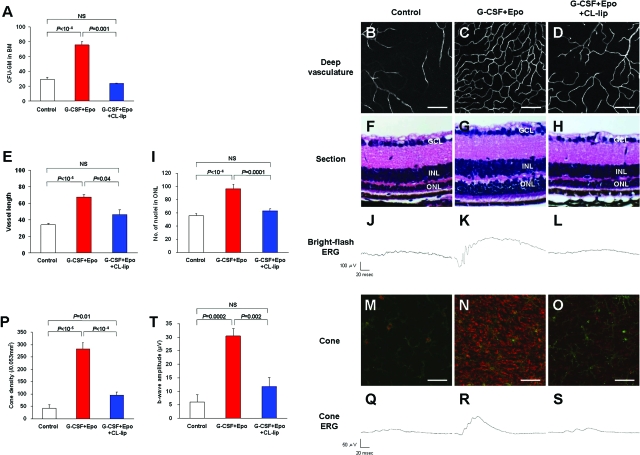
To further confirm the role of BM-derived microglia in retinal degeneration, clodronate liposome (CL-lip), which is well known to have the powerful effect of microglia/macrophage depletion27 using its phagocytosis capability, was used. Inhibition of the BM-derived GFP+ cells recruited to the retina was observed in the CL-lip-injected group [BMT on P15 and CL-lip injection from P20 to P24 (25 mg per day)] compared to PBS-lip-injected controls in rd1 mice (Figure 5A). In addition, although CL-lip did not influence the number of tissue resident microglia in control mice (Figure 5B), the colony-forming unit of granulocyte-macrophage (CFU-GM) was markedly reduced in the CL-lip-treated mice (Figure 5C). These data indicated that CL-lip treatment can reduce the recruitment of BM-derived microglia into the degenerating retina and this treatment did not influence the number of tissue-resident microglia. CL-lip was shown to have a significant power for systemic reduction of circulating microglial precursors.
Figure 5.
Systemic depletion of microglial progenitors accelerates inherited retinal degeneration. A: GFP+ cells recruited into the degenerating rd1 retina (P25) were significantly reduced in the clodronate liposome (CL-lip)-injected group compared to the vehicle (PBS-lip)-injected group (n = 6). B: Retina resident microglia positive for Iba-1 was almost at the same level between the groups (n = 6). C: Microglial progenitors in BM reduced to almost zero in the CL-lip-treated group (n = 6). CFU-GM, colony forming unit-granulocyte macrophage. D–G: Deep vasculature of PBS-lip- or CL-lip-treated eyes (P25) in WT (D, E) and rd10 mice (F, G). No change was seen in WT mice. In contrast, in rd10 mice, a marked loss of vasculature was observed in the CL-lip-treated group. H–K: Sectional images of the PBS-lip- and CL-lip-treated eyes (P25) in WT (H, I) and rd10 mice (J, K). No change was observed in WT mice, however, in rd10 mice, slightly reduced photoreceptor cells were seen in the CL-lip-treated group. GCL, ganglion cell layer; INL; inner nuclear layer; ONL, outer nuclear layer. L: The vascular length of the deep vasculature was significantly reduced in the CL-lip-treated group of rd10 mice (n = 8). M: The number of photoreceptor cells in ONL was significantly reduced in the CL-lip-treated group of rd10 mice (n = 8). *P < 0.05, **P < 0.01. NS, not significant. Scale bars = 100 μm.
We then determined whether this reduction of circulating microglial precursors could influence retinal neurovascular degeneration (Figure 5, D–M). CL-lip was systemically injected into rd10 mice from P20 to P24 (25 mg per day) to avoid the effect of CL-lip on retinal vascular development.25,28 In WT mice (C57BL/6), no effect on the vasculature or neurons was shown, either in the CL-lip- or in the PBS-lip-injected groups (Figure 5, D, E, H, and I). However, in rd10 mice, severe vascular damage and regression were seen in the CL-lip-injected group. In intermediate and deep vascular plexuses, vessels were significantly regressed in the CL-lip-injected group (Figure 5, F, G, and L). Moreover, in the superficial vascular layer, marked attenuation and bending of the vessels were seen in the CL-lip-injected group (data not shown). In addition, as in the SDF-1 experiments, the number of photoreceptor cells was significantly reduced in the CL-lip-injected group compared to the PBS-lip-injected controls (Figure 5, J, K, and M). These data strengthen the possibility that BM-derived microglia are recruited into the retina and have a protective role for inherited retinal neurovascular degeneration.
Activation of Circulating BM-Derived Stem Cells Decelerates the Progression of Inherited Neurovascular Degeneration
Based on the results that showed blocking BM-derived microglia resulted in an acceleration of retinal photoreceptor degeneration, it follows that an increase in the number or activation of those cells into the retina could rescue the photoreceptors. To investigate this hypothesis, the systemic administration of G-CSF and Epo was tested. G-CSF is a well known mobilization factor of circulating HSCs and Epo has been reported to have a mobilizing capacity of HSCs in BM into the circulation.29 We found that although systemic administration of G-CSF (300 μg/kg) or Epo (1000 IU/kg) for 5 days exhibited a mild increase of CFU-GM, simultaneous injection of both G-CSF and Epo showed an approximate threefold increase of CFU-GM compared to saline-injected controls (Figure 6A). We also found that additional CL-lip injections (5 mg/day) could neutralize this CFU-GM increase back to the normal level (Figure 6A), indicating this increase in CFU-GM is mediated by macrophages/microglial cells. Subsequently, we injected G-CSF and Epo systemically into rd10 mice from P20 to P29 and observed vascular and neuronal degeneration on P30. Effectively, both vascular and neural degeneration clearly delayed the G-CSF- and Epo-injected groups compared to the controls (Figure 6, B, C, E, F, G, and I). Because both G-CSF and Epo have been reported to have a direct protective effect on neural cells,30,31 we additionally administered CL-lip (5 mg/day) to neutralize the CFU-GM activation effect by G-CSF and Epo (Figure 6A) to clarify that this rescue effect is derived from direct or indirect processes. Under these conditions, vascular and neural rescue were also reduced, but not to the levels of controls, suggesting that the combined systemic administration of G-CSF and Epo may have both direct and indirect rescuing effects for retinal degeneration (Figure 6, D, E, H, and I). A bright-flash ERG showed functional rescue of G-CSF and Epo treatment, and this rescue effect was negated by plus CL-lip treatment (Figure 6, J–L). Judging from these data, indirect rescue via BM-derived microglia may be a major mechanism for this vascular and neuronal rescue.
Figure 6.
Functional activation of microglial progenitors using systemic injection of G-CSF and Epo decelerates inherited retinal degeneration. A: CFU-GM in BM was markedly increased by systemic G-CSF and Epo combined treatment compared to saline-injected controls, and this activation was negated by the additional intraperitoneal injection of clodronate liposome (CL-lip). B–D: Deep vasculature of each group on P30 in rd10 mice. E: Vessel loss was significantly protected by G-CSF and Epo treatment, and this protection was neutralized by additional CL-lip treatment (n = 10). F–H: Sectional image of the retina on P30 in rd10 mice. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. I: The number of residual photoreceptor cells in the ONL was significantly increased in the G-CSF- and Epo-treated group, and this protective effect was negated by additional CL-lip treatment (n = 10). J–L: Bright-flash ERG was recorded in each group on P30 in rd10 mice. Highly preserved response in G-CSF- and Epo-treated group. M–O: Cone stained by lectin PNA (red) and CD11b+ microglia (green) on the same planes were photographed on P40 of rd10 mice. Cone inner segments were well-preserved in G-CSF and Epo treatment and this protective effect was completely negated by additional CL-lip treatment. P: Cone density was counted on P40 (n = 6). Q–S: Cone ERG was recorded on P30. T: B-wave amplitude of cone ERG was markedly preserved in G-CSF and Epo treatment eyes and this protective effect was negated by additional CL-lip treatment (n = 6). NS, not significant. Scale bars: 100 μm (A–E); 50 μm (M–O).
Furthermore, we investigated how this rescue effect contributed to cone photoreceptor degeneration (Figure 6, M–T). We found that cone density was markedly preserved in the G-CSF- and Epo-treated group (Figure 6, M, N, and P) and that this rescue effect was almost abrogated by CL-lip treatment (Figure 6, O and P). Similarly, this combined treatment with G-CSF and Epo dramatically rescued the cone photoreceptor function (Figure 6, Q–T). We also observed cone density preservation in rd1 mice, but the effect was not as marked as in rd10, probably because the degeneration speed is higher in rd1 (data not shown). These results suggest that activation of BM-derived microglial cells has a significant rescue effect against the inherited retinal degeneration and the cone rescue effect was characteristic in this strategy. In addition, the possibility of a new cytokine therapy against inherited retinal degeneration, including RP, was suggested.
Discussion
The physiological and pathological roles of microglia in the retina have been under investigation.14,23,25,28,32,33 Microglial activation in the retina provides an early response against infection, injury, ischemia, and degeneration.33,34 In retinal degeneration, activated microglia migrate into the deeper retina with the expression of tumor necrosis factor-α before the onset of photoreceptor cell death,23 suggesting that microglial activation may trigger neuronal cell death. On the other hand, microglia secrete neurotrophic factors and promote photoreceptor survival in a light-induced retinal degeneration model,32 and promote vascular repair in an oxygen-induced retinopathy model.28 Although contributions of both tissue resident and BM-derived microglia have been suggested in previous reports, such roles were ambiguous. In the current study, we demonstrated the protective functions of BM-derived microglia in both vascular and neural degeneration. We also proposed that activating the protective functions of BM-derived microglia by systemic cytokine therapy may provide a novel strategy for delaying retinal degeneration. On the other hand, the role of BM-derived microglia in ocular inflammation remains unclear. We did not perform mRNA quantification or immunohistochemistry of proinflammatory or inflammatory cytokines produced by BM-derived microglia in this study. Furthermore, the role in inflammation of tissue resident microglia and the difference of the roles between tissue-resident and BM-derived are also unknown.
Many cytokines are probably involved in the recruitment of BM-derived cells into the degenerating retina. In this study, we investigated the role of SDF-1, which is known as a recruitment factor in several retinal diseases such as diabetic retinopathy35 and age-related macular degeneration.36 We measured mRNA expression level of SDF-1 in rd1 mice and found a significantly higher expression level on P16 and P18 compared to WT mice. Intravitreal injection of anti-SDF-1 neutralizing antibodies reduced the number of BM-derived cells in the retina. In this study, BMT was performed on P15, so we could not determine whether the higher expression of SDF-1 on P16 to P18 is really related to the recruitment of BM-derived cells to the retina. The reconstitution of BM by transplantation needs time as shown in Figure 1. BMT in newborn mice may overcome this problem.
The precise mechanisms of retinal protection by BM-derived microglia remain elusive. One hypothesis is that microglia phagocytose cellular debris and clear the degenerative environment. We observed phagocytosed photoreceptor cells inside CD11b+ microglia (data not shown). A recent report on Alzheimer’s disease demonstrated that BM-derived microglia can eliminate amyloid deposits by a cell-specific phagocytic mechanism.17 Another possible mechanism is that microglia secrete neurotrophic factors to promote residual cell survival. In the light-induced retinal degeneration model, microglia secrete nerve growth factor or ciliary neurotrophic factor and modulate secondary neurotrophic factor expression in Muller glia, contributing to the protection of photoreceptor cells.32 Muller glia are known to be activated and to express GFAP in retinal degeneration models. In a laser-induced choroidal neovascularization model, BM-derived cells activate Muller cells to express pERK.14 Activated Muller cells together with BM-derived microglia may possibly play a role in the degenerative process. In addition, the endogenous BM-derived cells also condensed around the optic disk (Figure 2Q) and retinal vessels (Figure 2, B, N, and P), where activated astrocytes exist. It is possible that in addition to the direct effect, those glia-glia interactions may provide a novel protective system for retinal neurovascular degeneration.
It is interesting that a marked protective effect occurred by BM-derived microglia to cone photoreceptor cells in the models of inherited rod photoreceptor cell degeneration (Figure 4, A–D; and Figure 6, M–T). The mechanisms underlying secondary cone degeneration are still not well understood, and some researchers have suggested oxidative stress as causal.22 Our observation has offered new insight for the function of BM-derived microglia and the mechanism underlying secondary cone loss. Interestingly, a similar cone rescue effect through vascular rescue achieved by direct injection of Lin− HSCs into the eye was reported.9
G-CSF and Epo are commonly used drugs in hematopoietic diseases because they induce the BM stem cells into the circulation, including HSCs. In addition to the hematopoietic effects, both drugs recently reported having neuroprotective effects.30,31 Several possible mechanisms of G-CSF-mediated neuroprotection have been suggested, including a direct effect for the neurons and a BM-derived cell-mediated indirect effect. In the current study, we have found that the combined administration of G-CSF and Epo induced a synergistic effect in myeloid progenitor activation (ie, an increase of CFU-GM) and promoted a dramatic rescue of retinal degeneration. Although the single use of G-CSF or Epo also had a retinal protective effect (data not shown), combined therapy showed a synergetic rescue effect, especially for cones. Our data suggested that an indirect effect via activation of microglial progenitors is more potent on the rescue event.
There are several possible advantages for possible cytokine therapy, the first of which would be wide-use regardless of the underlining genetic defect. Also, because the neuroprotective effect of activated BM-derived microglia may be a physiological response to neural degeneration, it is possibly applicable for all types of retinal degeneration. This is a great advantage because there are so many mutations known in human patients. A powerful cone photoreceptor rescue effect is another potential benefit. Cone photoreceptor survival is key to maintain the patient’s visual acuity and quality of life. We are hoping this approach will be clinically applicable in the near future because both cytokines are already widely used in human diseases. Of course, there are problems including the dose and undesired side effects that must be addressed for both drugs; systemic activation of microglial progenitors can be a novel therapy for delaying the progression of the disease and maybe other neurodegenerative diseases in the central nervous system. Other drugs that can activate BM-derived microglia are also candidates that should be tested.
Acknowledgments
We thank our colleagues and laboratory members, Kayo Nishida, Hiroko Hizaki, Norimoto Gotoh, He Zou, Tetsushi Kimura, Masafumi Kurimoto, Naoaki Kawagoe, Kaori Asamoto, Junko Nakamura, Megumi Sugimoto, Mayumi Yoshida, and Chiharu Sengoku, for their helpful advice and invaluable technical support; and Yoshinobu Toda and Hisami Taniguchi for their technical support in the histological sectioning of the mouse eyes.
Footnotes
Address reprint requests to Atsushi Otani, M.D., Ph.D., Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, 54 Shogoin-Kawahara-cho, Sakyo-ku, Kyoto 606-8386, Japan. E-mail: otan@kuhp.kyoto-u.ac.jp.
Supported by the Ministry of Education, Science, Sports, and Culture, Japan (grant-in-aid no. 17689045).
References
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Humphries P, Kenna P, Farrar GJ. On the molecular genetics of retinitis pigmentosa. Science. 1992;256:804–808. doi: 10.1126/science.1589761. [DOI] [PubMed] [Google Scholar]
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, Fauser S, Reichel MB, Kinnon C, Hunt DM, Bhattacharya SS, Thrasher AJ. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Frasson M, Sahel JA, Fabre M, Simonutti M, Dreyfus H, Picaud S. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med. 1999;5:1183–1187. doi: 10.1038/13508. [DOI] [PubMed] [Google Scholar]
- Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Reinoso MA, Pina Y, Csaky KG, Caicedo A, Cousins SW. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp Eye Res. 2005;80:369–378. doi: 10.1016/j.exer.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Baxter L, Afzal A, Sengupta N, Caballero S, Rosinova E, Grant MB. Hematopoietic stem cells provide repair functions after laser-induced Bruch’s membrane rupture model of choroidal neovascularization. Am J Pathol. 2006;168:1031–1044. doi: 10.2353/ajpath.2006.050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Espinosa-Heidmann DG, Pina Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, Del Bo R, Comi GP. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain. 2004;127:2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vis Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, Hauswirth WW, Nusinowitz S, Thompson DA, Heckenlively JR. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci. 2005;46:2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116:3266–3276. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Schuetz E, Thanos S. Microglia-targeted pharmacotherapy in retinal neurodegenerative diseases. Curr Drug Targets. 2004;5:619–627. doi: 10.2174/1389450043345164. [DOI] [PubMed] [Google Scholar]
- Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, McLeod DS, Merges C, Hasegawa T, Lutty GA. Localisation of SDF-1 and its receptor CXCR4 in retina and choroid of aged human eyes and in eyes with age related macular degeneration. Br J Ophthalmol. 2006;90:906–910. doi: 10.1136/bjo.2006.090357. [DOI] [PMC free article] [PubMed] [Google Scholar]