Abstract
CUB-domain-containing protein 1 (CDCP1) is a type-I transmembrane protein that is highly expressed in colon, breast, and lung cancers. We recently revealed that CDCP1 is associated with and phosphorylated by Src family kinases and is involved in the regulation of anchorage independence of certain lung cancer cell lines. In this study, we examined whether CDCP1 is involved in the regulation of tumor progression of scirrhous gastric cancer, which is a diffusely infiltrative carcinoma with high invasion potential. Expression and phosphorylation levels of CDCP1 correlated with the invasive potential of scirrhous gastric cancers. Reduction of CDCP1 expression by siRNA suppressed migration, invasion, and anchorage independence without affecting the proliferation of highly invasive scirrhous gastric cancer cells. However, CDCP1 overexpression promoted gastric cancer cell migration with low potential of invasion. Loss of CDCP1 suppressed invasion and dissemination of cancer cells that were orthotopically implanted in the gastric wall of nude mice. Expression and phosphorylation of CDCP1 were also detected in cancer cells of surgically resected tissues of human scirrhous gastric cancer by immunohistochemical analysis. Our results suggest that CDCP1 promotes invasion and peritoneal dissemination of cancer cells through the regulation of cell migration and anchorage independence. Therefore, it is both a potential prognostic and therapeutic target in certain types of gastrointestinal cancers, and suppression of its phosphorylation might be a useful strategy for modulating cancer metastasis.
CUB-domain-containing protein 1 (CDCP1) was first identified as the product of a gene preferentially expressed in colon cancer cells compared to normal tissue.1 The CDCP1 gene contains nine exons, and the first mRNA transcript to be described is ∼6.4 kb in length. Further studies report that CDCP1 mRNA is expressed on hematopoietic stem cells, mesenchymal stem cells, neuronal progenitor cells,2,3 human epidermoid carcinoma cell lines,4,5 lung, breast, and prostate carcinoma cell lines.1,6
The CDCP1 protein, also described as SIMA135 and Trask,4,6 is a type I transmembrane protein containing three putative CUB (complement protein subcomponents Clr/Cls, urchin embryonic growth factor, and bone morphogenic protein 1) domains that are characterized by immunoglobulin-like folds and are involved in protein-protein interaction.7,8 CDCP1 is phosphorylated by Src family kinases (SFKs) and previous studies have suggested possible roles of CDCP1 in cellular adhesion and cell-cycle regulation.5,6,9 We recently purified a major phosphoprotein detected in the suspension culture of anchorage-independent lung cancer cells and identified it as the CDCP1 protein by mass spectrometry. We revealed that tyrosine phosphorylated CDCP1 has a novel role in regulating the anchorage independence of lung cancer cells by linking cell signaling from SFKs to protein kinase Cδ.10
Scirrhous gastric carcinoma diffusely infiltrates a broad region of the stomach and frequently associates with metastasis to lymph nodes and peritoneal dissemination. The process of peritoneal dissemination involves several steps, including invasion, migration, anchorage-independent growth, and proliferation in the peritoneum. Signaling mediated by tyrosine phosphorylation of some transmembrane proteins, including ephrin-B1 and fibroblast growth factor receptor II is reported to be involved in the progression of scirrhous gastric cancer cells.11,12
CDCP1 is frequently expressed in gastric cancer cells, and expression and tyrosine phosphorylation levels of CDCP1 are associated with high invasiveness of tumors. These observations led us to examine in this study whether disruption of CDCP1-mediated signaling suppresses invasion and peritoneal dissemination of scirrhous gastric carcinoma. We show in this study that reduction of CDCP1 expression suppresses the migration and anchorage independence of highly invasive gastric cancer cell lines, and inhibits the peritoneal dissemination of these cells. Our results suggest that CDCP1 represents a rational therapeutic target and suppression of its phosphorylation is a strategy for modulating the metastasis of some types of cancers.
Materials and Methods
Plasmids, Antibodies, and Reagents
Plasmids encoding full-length cDNAs of human CDCP1, and the cytoplasmic domain mutant of CDCP1 Y734F (Tyr734 to Phe) have already been described.10 The pan-Src antibody (Src2) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-Src family (pY416) antibody was purchased from Cell Signaling (Beverly, MA). The FLAG M2 antibody and the α-tubulin antibody were purchased from Sigma (St. Louis, MO). The monoclonal antibody for phosphotyrosine (4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). Polyclonal antibody against CDCP1 and tyrosine-phosphorylated CDCP1 [p-CDCP1 (Tyr734)] were obtained from MBL Co., Ltd. (Woburn, MA) as described previously.10 Fibronectin (bovine), collagen type I, and Matrigel basement membrane matrix were purchased from Sigma, Nitta Gelatin Inc. (Osaka, Japan), and BD Biosciences (San Jose, CA), respectively.
Cell Culture and Transfection
Gastric carcinoma cell lines (HSC-59, HSC-60, 44PE, 44As3, 58, 58As1, 58As9) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum at 37°C with 5% CO2. In suspension culture, cells were seeded on a 2-methacryloyloxyethyl phosphorylcholine-coated plate (Nunc, Roskilde, Denmark). For transfection, cells were seeded on a cell culture plate at 1.5 × 105 cells per six-well plate and transfection was performed after 14 hours. Expression plasmids were transfected by Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Short Interfering RNA (siRNA) Treatment
Two sets of siRNAs of CDCP1 were synthesized as follows (Invitrogen). CDCP1 sense no. 1: 5′-UAAUGUUGCUUUCUCGUGGCAGAGC-3′; CDCP1 antisense no. 1: 5′-GCUCUGCCACGAGAAAGCAACAUUA-3′; CDCP1 sense no. 2: 5′-AUAGAUGAGCGGUUUGCAAUGCUGA-3′; CDCP1 antisense no. 2: 5′-UCAGCAUUGCAAACCGCUCAUCUAU-3′. The control siRNA (scramble II duplex: 5′-GCGCGCUUUGUAGGAUUCGdTdT-3′) was purchased from Dharmacon (Lafayette, CO). siRNAs were incorporated into cells using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). Assays were performed 72 hours after treatment.
To generate a stably expressing siRNA system, the BLOCK-iT Pol II miR RNAi expression vector kit (Invitrogen) was used. The target sequence of CDCP1 has been described previously.10 Cells stably expressing the CDCP1 siRNA (miCDCP1) and the control LacZ siRNA (miLacZ) were established through transfection of miR RNAi vectors that holds these sequence and selection in medium containing blasticidin S (Invivogen) at a concentration of 10 μg/ml.
Western Blotting
Cell lysates were prepared with protease inhibitors in PLC buffer [10 mmol/L Tris-HCl (pH 7.5), 5 mmol/L EGTA, 150 mmol/L NaCl, 1% Triton X-100, 10% glycerol, 10 μg/ml aprotinin, 1 mmol/L sodium orthovanadate (Na3VO4), and 100 μg/ml leupeptin]. Protein concentration was measured by BCA protein assay (Pierce, Rockford, IL). Samples were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA). After the blocking of the membrane with blocking buffer (Blocking One; Nakarai Tesque, Kyoto, Japan), the membrane was probed with antibodies for detection. The membrane was further probed with horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG (1:4000) to visualize the antibody. The images were captured by molecular imager GS-800 (Bio-Rad, Hercules, CA).
Cell Attachment Assay
Cancer cells were detached by phosphate-buffered saline (−) [PBS(-)] containing EDTA (2 mmol/L) and replated onto the chamber slides coated with either collagen type I (100 μg/ml; Nitta Gelatin, Inc.), fibronectin (50 μg/ml, Sigma), or Matrigel (85 μg/ml, BD Biosciences). After incubation for 30 minutes, unattached cells were removed by washing the slides in PBS(−) several times, and the remaining cells were stained with Giemsa’s solution. The number of attached cells on each substrate was counted.
Cell Migration Assay
Migration assay was performed using modified transwell chambers with a polycarbonate nucleopore membrane (BD Falcon, Franklin Lakes, NJ). Precoated filters (6.5 mm in diameter, 8-μm pore size, fibronectin 10 μg/ml) were rehydrated with 100 μl of medium. Then, 4 × 104 cells (HSC-59, 44As3, and 58As9) and 8 × 104 cells (HSC-60) in 100 μl of serum-free RPMI 1640 were seeded onto the upper part of each chamber, whereas the lower compartments were filled with 600 μl of the same medium with 10% fetal bovine serum (FBS). After incubation for 14 hours at 37°C, nonmigrated cells on the upper surface of the filter were wiped out with a cotton swab, and the migrated cells on the lower surface of the filter were fixed and stained with Giemsa’s stain solution (azur-eosin-methylene blue solution; Muto Pure Chemical, Co., Tokyo, Japan). The totals of migrated cells were determined by counting cells in five microscopic fields per well at a magnification of ]times]100, and the extent of migration was expressed as the average number of cells per microscopic field. Cell migration assay were performed three times.
Matrigel Invasion Assay
Invasion of tumor cells into the Matrigel was monitored as described previously.13 Gastric cancer cells treated with CDCP1 siRNA or control siRNA for high invasion potential were detached with Hanks’ balanced salt solutions (HBSS−) containing 2 mmol/L of EDTA and seeded on the Matrigel (100 μg/cm2). After being cultured in the RPIM 1640 medium with 10% FBS for 17 hours, the cells were fixed with 4% paraformaldehyde in PBS and stained with Giemsa’s stain solution. The number of invaded cells were counted in five microscopic fields per well at a magnification of ×100, and the extent of invasion was expressed as the average number of cells per microscopic field. Matrigel invasion assays were performed three times.
Soft Agar Colony Assay
Six-well culture plates were coated with a layer of RPMI 1640 and 10% FBS containing 0.5% UltraPure agarose (Invitrogen). Subconfluent 44As3 cells transfected the miR RNAi vector expressing clones were treated with EDTA, washed in PBS twice, and resuspended in RPMI 1640, 10% FBS at 6 × 103 cells/ml. Then, a 500-μl cell sample was added to 1 ml of RPMI 1640, 10% FBS containing 0.5% UltraPure agarose (final 0.33%). The cells were plated onto the coated tissue culture plates, allowed to solidify, and then placed in a 37°C incubator. After 30 days, colonies were scanned using GS-800 calibrated densitometer (Bio-Rad) and the numbers of colonies/well were counted. Soft agar assays were performed three times.
In Vivo Tumor Cell Invasion Assay
The animal experimental protocols were approved by the Committee for Ethics of Animal Experimentation, and the experiments were conducted in accordance with the guidelines for animal experiments in the National Cancer Center. Peritoneal dissemination of tumors was examined by intraperitoneal injection of 5 × 106 gastric cancer cells suspended in 0.3 ml of RPMI 1640 medium into 6-week-old BALB/c nude mice (Clea Japan, Inc., Tokyo, Japan). The mice (n = 6) were sacrificed 2 weeks after injection, and peritoneal dissemination was evaluated. Orthotopic implantation of gastric cancer cells into BALB/c nude mice has been described previously.11 Briefly, 1 × 106 cells were inoculated into the middle wall of the greater curvature of the glandular stomach using a 30-gauge needle. The mice (n = 12) were sacrificed at 15 to 16 days after the orthotopic transplantation of the cancer cells, and the tumors were examined macroscopically. To determine the effect on the tumor growth in nude mice, 44As3 clones (3 × 106 cells/0.3 ml of serum-free medium) were subcutaneously injected into the right flank of mice. Mice were euthanized at 30 days. The results are expressed as the mean weight of tumors (g) from three mice ± SE.
Tissue Samples and Immunohistochemical Analysis
We obtained 10 paraffin-embedded tumor tissues of gastric scirrhous carcinoma in 2006 at the National Cancer Center Hospital. The study population consisted of five men (50%) and five women (50%). Paraffin blocks were sectioned in slices and subjected for immunohistochemical stains using the indirect polymer method with Envision reagent (DAKO, Carpinteria, CA). Antigen retrieval was performed by placing sections in the citrate buffer and heating in a microwave pressure cooker according to the manufacturer’s instructions. All sections were incubated with specific antibodies against CDCP1 (rabbit polyclonal antibody; dilution, 1:100) and tyrosine-phosphorylated CDCP1 [p-CDCP1 (Tyr734): rabbit polyclonal antibody; dilution, 1:400].
Results
CDCP1 Affects Migration and Anchorage Independence of Gastric Cancer Cells
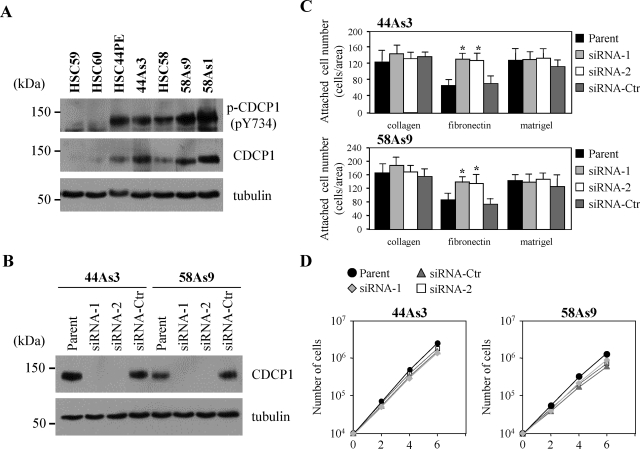
To examine the involvement of CDCP1 for progression of tumors, we analyzed cell lines of scirrhous gastric carcinoma. HSC-59, HSC-60, HSC-44PE, and HSC-58 were originally established from the patients of scirrhous gastric carcinoma, and highly invasive sublines were further selected from these parent cells (44As3 from HSC-44PE; 58As1 and 58As9 from HSC-58).14,15 Expression of CDCP1 was remarkably elevated in highly invasive HSC-44PE, HSC-58 cells, whereas higher expression and phosphorylation levels of CDCP1 was observed in invasive sublines compared with less invasive HSC-59 and HSC-60 cells (Figure 1A).14
Figure 1.
Expression and tyrosine phosphorylation of CDCP1 is higher in invasive gastric cancer cell lines. A: Lysates from cells as indicated were subjected to immunoblotting with anti-CDCP1 and anti-phospho-CDCP1 (Tyr734) in each cell lysate. HSC-59 and HSC-60 of noninvasive cell lines and HSC-44PE, 44As3, HSC-58, 58As9, and 58As1 of invasive cell lines were seeded onto each cell culture plate. B: Cellular levels of CDCP1 were analyzed 72 hours after treatment with siRNAs by Western blotting using α-tubulin as a loading control. Expression of CDCP1 was reduced in cells treated with CDCP1 siRNA (siRNA-1, -2) compared with siRNA control (siRNA-Ctr) and parental cell (parent). C: 44As3 and 58As9 cells treated with CDCP1 siRNA (siRNA-1, -2) or control siRNA (siRNA-Ctr) were detached by EDTA, and replated on the chamber slides coated with either collagen type I (100 μg/ml), fibronectin (50 μg/ml), or Matrigel (85 μg/ml). After incubation for 30 minutes, unattached cells were removed by washing the slides in PBS(−) several times, and the remaining cells were stained with Giemsa’s solution. The number of attached cells on each substrate was counted, and the results from three independent experiments, each in duplicate, are shown as the mean ± SD. The asterisks indicate differences from the control cells. *P < 0.01. D: Cell growth in 44As3 and 58As9 cells were shown by the number of cells. Approximately 1 × 104 cells were seeded onto cell culture plates with medium. The growth medium was changed every 2 days and cell numbers were counted by Coulter particle counter z1 (Beckman).
Cell proliferation, migration, invasion, loss of cell adhesion, and anchorage-independent growth are major functions of the cancer cells required during dissemination. We next examined whether the reduction of CDCP1 affects these functions of gastric cancer cells. The treatment of cells with two independent siRNA of CDCP1 effectively reduced the CDCP1 expression level in 44As3 cells and 58As9 cells (Figure 1B). Proliferation of both cell lines was not significantly affected (Figure 1D). To check whether the expression of CDCP1 affects the cell-extracellular matrix adhesion, the degree of cell attachment to type I collagen, fibronectin, and Matrigel was analyzed. The number of attached cells was increased in 44As3 and 58As9 cells treated by siRNA of CDCP1 on fibronectin, but reduction of CDCP1 expression did not cause significant change in either cell line on type I collagen and Matrigel (Figure 1C).
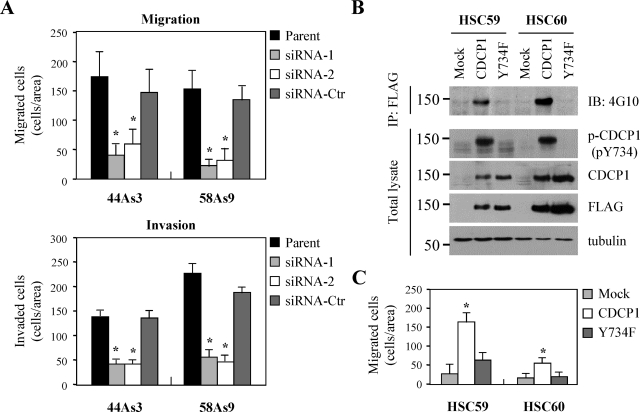
Interestingly, reducing the amount of CDCP1 protein by siRNA inhibited migration and invasion of 44As3 and 58As9 cells by in vitro transwell assay (Figure 2A). On the other hand, overexpression of CDCP1 in less invasive HSC-59 and HSC-60 cells increased cell migration (Figure 2C). In this case, Y734F mutant of CDCP1, which lacks the phosphorylation site by SFKs5,10 did not affect cell migration (Figure 2, B and C). These results suggest that signaling mediated by tyrosine phosphorylation of CDCP1 regulates the invasion of gastric cancer cells via cell migration.
Figure 2.
Tyrosine-phosphorylated CDCP1 regulates the invasiveness of gastric cancer cells via cell migration. A: 44As3 and 58As9 cells treated with either CDCP1 siRNA (siRNA-1, -2), control siRNA (siRNA-Ctr), or untreated parent cells (parent) were plated onto a Transwell membrane in serum-free medium. In the lower chamber, medium containing 10% FBS was added as a chemoattractant. After 14 hours of incubation for migration and 17 hours for invasion, the wells were harvested and cells that migrated to the lower surface of the membrane were counted. The results from three independent experiments, each in duplicate, are shown as the mean values ± SD. The asterisks indicate differences from the cells treated with control siRNA. *P < 0.01. B: CDCP1 or CDCP1 mutant (Y734F) tagged with FLAG were transiently transfected in HSC-59 and HSC-60 cells of noninvasive potential. After 24 hours, cells were treated EDTA and collected. The lysates were subjected to immunoblotting with the indicated antibody or immunoprecipitation of total CDCP1 and Y734F using FLAG M2 antibody and immunoblotting with anti-phosphotyrosine antibody (4G10). C: The transiently transfected cells as indicated were seeded onto a Transwell membrane in serum-free medium. In the lower chamber, medium containing 10% FBS was added. After 12 hours of incubation, the wells were harvested, and cells that migrated to the lower surface of the membrane were counted. The results from three independent experiments, each in duplicate, are shown as the mean values ± SD. The asterisks indicate differences from the cells transfected with mock. *P < 0.01.
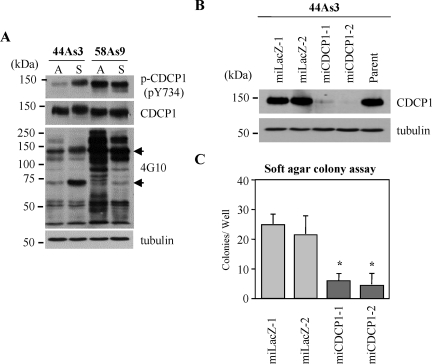
We recently found that CDCP1 protein regulates anchorage-independent growth of lung adenocarcinoma in a tyrosine phosphorylation-dependent manner.10 Tyrosine phosphorylation of CDCP1 was also maintained in the suspension culture of gastric cancer cell lines (Figure 3A), therefore we checked whether CDCP1 can regulate the anchorage-independent growth of gastric cancer cells. We established stable clones of 44As3 cells, miCDCP1-1 and miCDCP1-2, in which the expression of CDCP1 protein was suppressed by siRNA (Figure 3B). Both of these two clones formed significantly fewer colonies in soft agar compared with control miLacZ clones (Figure 3C), suggesting that CDCP1 is also required for the anchorage independence of highly invasive gastric cancer cells.
Figure 3.
CDCP1 confers anchorage independence in highly invasive gastric cancer cells. A: 44As3 and 58As9 cells of highly invasive potential cultured for 24 hours in both adhesion and suspension condition were collected and subjected to immunoblotting with anti-CDCP1, anti-phospho-CDCP1 (Tyr734), or anti-phosphotyrosine (4G10) antibody. A, adhesion; S, suspension. The black arrow indicates CDCP1. B: CDCP1-defective 44As3 clones (miCDCP-1 and miCDCP1-2) were generated by miR RNAi expression vector kit (Invitrogen). miLacZ-1 and miLacZ-2 were control clones. The expression of CDCP1 in each clone cultured for 24 hours in 2-methacryloyloxyethyl phosphorylcholine-coated plates was examined by Western blotting using CDCP1 antibody. The concentration of total protein in each clone was confirmed by the same membrane rehybridized with anti-α-tubulin antibody. C: Each CDCP1-defective clone and control clone was seeded onto soft agar plates (3 × 103 cells). Colonies equal to and larger than 0.5 mm in diameter were counted after 30 days. The results from three independent experiments, each in duplicate, are shown as the mean values ± SD. The asterisks indicate differences from the cells with control miLacZ-1. *P < 0.01.
Reduction of CDCP1 Expression Attenuates Dissemination of Gastric Cancer Cells
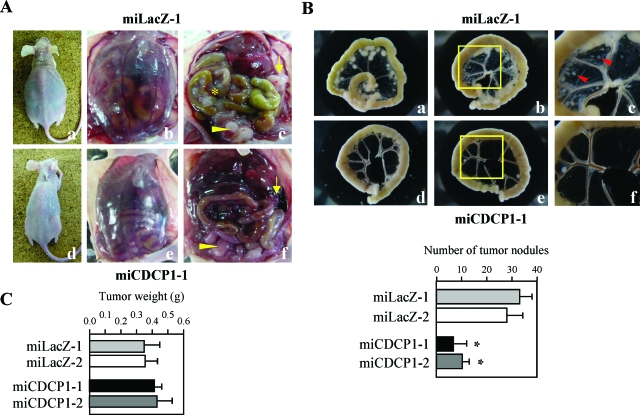
Gastric scirrhous carcinoma frequently associates with peritoneal dissemination through a process by which cancer cells perforate the gastric serosa and become exfoliated and free, then attach on the surface of the peritoneum and start to invade. We further examined the effect of CDCP1 on tumor progression in vivo using a model system of peritoneal dissemination.14,15 When control clones (miLacZ-1, -2) were injected intraperitoneally into nude mice, severe carcinomatous peritonitis was observed in miLacZ clones as previously described (Figure 4A; a–c).15 Innumerable whitish nodules were observed in the mesentery of almost all mice injected with miLacZ clones (Figure 4Ac). In addition, many tumor nodules of miLacZ clone were observed in the peritoneal cavity, including the omentum (Figure 4Ac). On the other hand, when the miCDCP1 clones (miCDCP1-1, -2) in which the expression of CDCP1 is stably suppressed by siRNA, was injected, dissemination of cancer cells was apparently modest (Figure 4A; d–f). Large numbers of tumor nodules of control clones were observed in the mesentery, whereas, miCDCP1-1 and miCDCP1-2 clones formed smaller numbers of colonies in the mesentery (Figure 4B). On the contrary, no significant change in the tumor growth was observed in any 44As3 clone subcutaneously implanted in nude mice (Figure 4C).
Figure 4.
Disruption of CDCP1 expression suppressed the peritoneal dissemination of 44As3 cells. Each CDCP1-defective clone (miCDCP1) and control clone (miLacZ) was injected intraperitoneally into nude mice (5 × 106 cells/mice). A: Representative appearance of peritoneal dissemination at 21 days after the injection of miLacZ-1 clone (a–c) or miCDCP1-1 clone (d–f) is shown. Carcinomatus peritonitis and abdominal distension because of bloody ascites was observed in miLacZ clones (a and b). c and f: The asterisk indicates dissemination of cancer nodules in the mesentery and arrows indicate the tumor mass, including omentum. Arrowheads indicate the tumor nodules of cancer cells disseminated around the rectouterine region. B: Representative dissected intestinal loops from two mice injected with miCDCP1-1 and miLacZ-1 clones, respectively. Top: Representative appearance of peritoneal dissemination 2 weeks after the injection of miLacZ-1 clone (a–c) or miCDCP1-1 clone (d–f) is shown. c and f show high magnification of the mesentery in the middle panels (yellow box). The red arrow indicates local invasion of tumor nodules in the mesentery. Bottom: Tumor nodules equal to and larger than 1.0 mm diameter were counted after 16 days (n = 6). Error bars indicate the SD. The asterisks indicate differences from the cells with control miLacZ-1. *P < 0.01. C: The effect of CDCP1 on the tumor growth in nude mice was determined as described under Materials and Methods. The data represent the weight (g) of tumors from miCDCP1 clones and miLacZ clones (n = 3). Error bars indicate the SD.
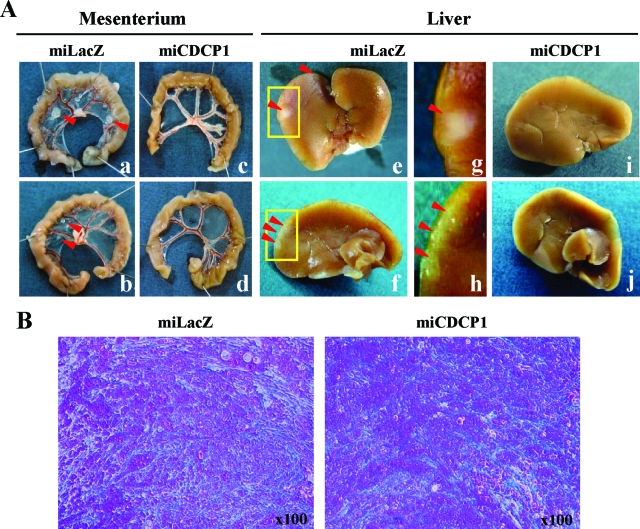
To further evaluate the effect of CDCP1 on the process of tumor invasion and dissemination, we orthotopically implanted gastric cancer cells in the gastric submucosa of nude mice. At 15 to 16 days after implantation, 67% of mice injected with miLacZ clones formed a tumor mass involving the omentum. On the other hand, such local invasion into the omentum was less frequently observed (17%) in mice implanted with miCDCP1 clones (Table 1). The dissemination and invasion of miLacZ clones was observed in several tissues including mesenteric sheets and liver surface (67% and 58%). However, we rarely observed cancer dissemination in mesenteric sheets and invasion in liver surface in mice implanted with miCDCP1 clones (17% and 17%) (Figure 5A, Table 1). Additionally, gastric cancer cells in mice hepatic stroma were checked by histological analysis. None of the cancer cells was detected in hepatic stroma when there were no nodules in the liver surface (see Supplemental Figure S1 at http://ajp.amjpathol.org). These results suggest that CDCP1 is required for peritoneal dissemination and invasion of scirrhous gastric carcinomas. Stromal fibrosis is an important character of scirrhous gastric carcinoma. We examined whether CDCP1 affects stromal fibrosis of scirrhous carcinoma by azan staining, however no significant change of stromal fibrosis was observed in orthotopically implanted gastric cancer cells with or without CDCP1 (Figure 5B).
Table 1.
Dissemination at 15 to 16 Days after Orthotopic Implantation of 44As3 Gastric Cancer Cells
| Cell | Omentum | Mesenterium | Liver |
|---|---|---|---|
| miLacZ (15 days) | 8/12 (67) | 8/12 (67) | 7/12 (58) |
| miCDCP1 (16 days) | 2/12 (17) | 2/12 (17) | 2/12 (17) |
Number of mice bearing tumor at the site per total number of mice bearing tumor (%).
Figure 5.
Disruption of CDCP1 suppressed the peritoneal dissemination of orthotopically implanted 44As3 cells. A: Each CDCP1-defective clone (miCDCP1) and control clone (miLacZ) was orthotopically implanted in submucosa of the gastric wall (n = 12) as described in the Materials and Methods. The representative macroscopic views of dissected organs 15 days (miLacZ) and 16 days (miCDCP1) after implantation including mesenterium (a–d) and liver (e–j) are shown. g and h show high magnification of the liver in the top panels (yellow box). Red arrow indicates tumor nodules. B: Stromal fibrosis of each miCDCP1 and miLacZ clone orthotopically implanted in mice submucosa was detected by azan staining. The region with stromal fibrosis was observed as blue stain.
Tyrosine Phosphorylation of CDCP1 Associates with Invasion of Human Gastric Cancer Cells
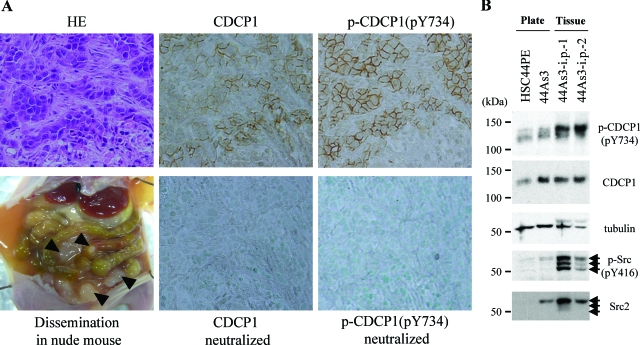
When 44As3 cells were intraperitoneally injected, many tumor nodules were observed not only in the mesentery sheets, but also in the parietal and visceral peritoneum, which leads to carcinomatous peritonitis (Figure 6A, dissemination).11 To further evaluate the involvement of CDCP1 in the formation of peritoneal dissemination of cancer cells, the expression and phosphorylation level of CDCP1 in the disseminated tumor nodules was monitored through immunohistochemical analysis. Antibodies against CDCP1 and against phospho-Tyr734 of CDCP1 revealed that CDCP1 was exclusively expressed and phosphorylated at Tyr734 in the invaded cancer cells, but not in stromal cells derived from mice including fibroblasts (Figure 6A). We and other researchers previously observed that CDCP1 is tyrosine phosphorylated at Tyr734 by SFKs.5,10 Compared to 44As3 cells cultured in vitro under normal tissue plate culture, elevation in the activity of SFK was detected in tumor nodules formed by the same 44As3 cells in nude mice, as judged by the antibody recognizing the phosphorylation of Tyr416 of SFK [Figure 6B; p-Src (pY416)]. Moreover, an outstanding elevation of the phosphorylation level of CDCP1 was observed in 44As3 tumor nodules in nude mice (Figure 6B; 44As3-i.p.−1, −2), suggesting a possible role of CDCP1 phosphorylation in scirrhous carcinoma cells with the peritoneal dissemination.
Figure 6.
Tyrosine phosphorylation of CDCP1 is up-regulated in the tumor nodules of peritoneal dissemination. A: Histology of the tumor nodules 14 days after peritoneal dissemination of 44As3 cells. Left: Representative macroscopic views of organs 14 days after implantation are shown (dissemination). Black arrowheads indicate the tumor of dissemination in nude mouse. The left panels show H&E staining. Middle: Staining of tumor nodules using anti-CDCP1 antibody and neutralized with CDCP1 peptide. Right: Staining of tumor nodules using anti-phospho-CDCP1 (pY734) antibody and neutralized with phospho-CDCP1 peptide. B: 44As3 cells were cultured onto cell culture plate (plate) or injected intraperitoneally into nude mice (tissue). Each lysate was collected and subjected to immunoblotting with anti-CDCP1, anti-phospho-CDCP1 (Tyr734), anti-pan-Src (Src2), anti-phospho-Src family (pY416), or anti-phosphotyrosine (4G10) antibody. α-Tubulin as a loading control. Black arrows indicate Src family kinases.
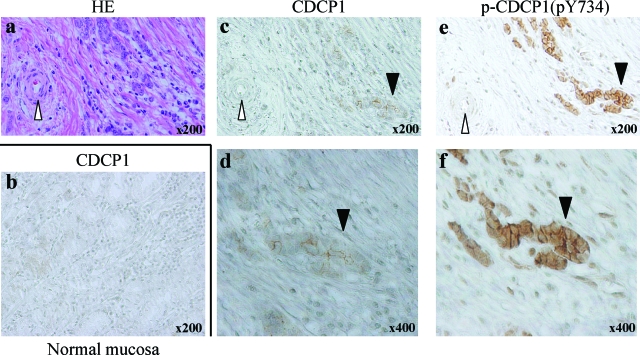
We further examined the expression and phosphorylation of CDCP1 in human scirrhous gastric cancer tissues by immunohistostaining. Although the expression of CDCP1 was very faintly observed in normal gastric mucosa, it was clearly detected in gastric cancer cells invading the gastric wall (Figure 7). Intense staining of tyrosine-phosphorylated CDCP1 was also observed in cancer cells (black arrowhead), but not in stromal tissues including endothelial cells of veins and proprial muscle (white arrowhead). No detectable signal was observed in the immunohistostaining of cancer cells using the second antibody alone (data not shown). Elevated expression and tyrosine phosphorylation of CDCP1 was observed in ∼30% of the scirrhous-type gastric cancer tissues (n = 10) as compared with normal tissue. In addition, the expression of CDCP1 was also observed in some of human nonscirrhous gastric cancer tissue (see Supplemental Figure S2 at http://ajp.amjpathol.org). Further analysis of the expression and phosphorylation of CDCP1 is required to elucidate the role of CDCP1 in the nonscirrhous gastric cancer.
Figure 7.
Expression and tyrosine phosphorylation of CDCP1 is detected in the invasive site of human scirrhous tissue. Human scirrhous tissue was stained with H&E (a) and either CDCP1 (c and d) or phospho-CDCP1 [p-CDCP1(pY734)] (e and f). Normal mucosa was stained with CDCP1 (b). Black arrowheads indicate the area of invasive tumor and white arrowheads indicate blood vessels (c and e). High magnification of the invasive site of the tumor is shown in d and f, respectively. Note that expression and phosphorylation of CDCP1 was detected in invasive sites of human scirrhous tissue but not detected in normal tissue including blood vessel (c–f). Original magnifications: ×200 (c, e); ×400 (d, f).
Discussion
Highly invasive potential is one of the major characteristics of scirrhous carcinoma, which is a major cause of the poor prognosis of this type of cancer. Using two scirrhous gastric cancer cell lines with highly invasive character, we show for the first time that CDCP1 modifies cancer invasion and dissemination in vivo. Disruption of CDCP1 suppressed the migration and anchorage-independent growth of these scirrhous cancer cells. In addition, knockdown of CDCP1 suppressed peritoneal dissemination of both ectopically and orthotopically injected scirrhous cancer cells and invasion of orthotopically implanted scirrhous cancer cells toward the gastric wall and into the liver and the mesentery.
From the original identification, CDCP1 was estimated to be important in the progression of human tumors. Using cDNA chip hybridization techniques to search for genes preferentially expressed in solid tumors relative to normal tissues, Scherl-Mostageer and colleagues1 identified Est sequences corresponding to CDCP1 cDNA. In another approach to identify tumor-associated proteins, Hooper and colleagues4 used subtractive immunization techniques to generate antibodies toward cell surface epitopes preferentially expressed by highly metastatic relative to nonmetastatic carcinomas, and identified a cell surface glycoprotein named SIMA135, identical to CDCP1. We recently discovered a novel function of CDCP1 in cancer progression. It was revealed that CDCP1 is a SFK-binding phosphoprotein that regulates the anoikis resistance of human lung adenocarcinoma. In suspension culture, CDCP1-SFK complex is required for the phosphorylation of PKCδ, which was shown to be a key molecule for anoikis resistance downstream of CDCP1 signaling. Moreover, CDCP1 affects the late phase of metastasis of lung adenocarcinoma in vivo, possibly through regulation of anoikis resistance.10
Scirrhous gastric carcinoma diffusely infiltrates a broad region of the stomach and is frequently associated with metastasis to lymph nodes and peritoneal dissemination. In this study, we found that the expression levels of CDCP1 were higher in the cells of invasive scirrhous gastric carcinoma than in the cells of less invasive type (Figure 1A). This expression pattern of CDCP1 prompted us to examine the involvement of CDCP1 in the invasion and dissemination of scirrhous gastric cancer cells.
It was also shown for the first time that CDCP1 regulates cell migration using gastric cancer cells (Figure 2, A and C). Cell migration mediates some of the functions in the process of cancer cell invasion and is generally presumed to be regulated by cell adhesion molecules. For example, integrins bind extracellular matrix and link to the signaling pathway inside the cells16 and the turnover of integrin contacts may promote cell migration.17 CDCP1 contains three CUB domains, which are thought to play biological roles by mediating protein-protein interaction, in various molecules such as galectins, DMBT1,18 sperm adhesine,19 neuropilin,20 and proteases including Bmp1/C-proteinase, Tolloid, and MASP.7 Recently, it is reported that some type of CUB domain binds to fibronectin and modulates interaction of the other matrix components.21 Moreover, CDCP1 interacts with a number of adhesion and matrix proteins including cadherins and syndecans.6 It is also proposed that special subsets of integrin affect phosphorylation of CDCP1.22 Thus, CDCP1 may modulate cell adhesion. Actually, overexpressions of CDCP1 in MDA-468 cells develop a rounded shape and grow in a loose cell adhesion6 and we show that the loss of CDCP1 increases the attachment of 44As3 and 58As9 cells on fibronectin (Figure 1C). Therefore, CDCP1 might regulate cell migration by affecting the cell adhesion to extracellular matrix in the cell invasion assay in vitro and in the process of stromal invasion of tumors in vivo.
In this study, the requirement of tyrosine phosphorylation site, Y734, in cell migration, is strongly suggested because wild-type CDCP1, but not Y734 mutant, promoted cell migration when expressed in less invasive gastric cells (Figure 2C). This result may be consistent with the observation that the phosphorylation levels of CDCP1 correlated with the levels of cell motility in gastric cancer cells between 44As3 and 58As9 of invasive type and HSC-59 and HSC-60 of less invasive type (Figure 1A; and Figure 2, A and C). Moreover, phosphorylated CDCP1 was identified in a screen for adhesion-dependent phosphorylation in an epithelial wound model.22 Based on these findings, tyrosine phosphorylation of CDCP1 might have a critical role in cell migration, although the precise molecular mechanism of regulation needs to be clarified.
We recently reported that tumor cells expressing CDCP1 regulate anchorage-independent growth in lung cancer cells and the phosphorylation of CDCP1 is important for the signal of resistance to detachment-induced apoptosis.10 CDCP1 is one of the major phosphotyrosine-containing proteins in suspension condition as well in the adherent condition in both gastric cancer cells and in lung cancer cells. Anchorage-independent growth was also regulated by CDCP1 in the invasive gastric cancer cells 44As3 and 58As9 by siRNA experiments (Figure 3, B and C). Several theories have been proposed to explain the mechanism of peritoneal dissemination in human gastric cancer.23,24,25 It has been suggested that cancer cells are detached from primary lesions and freed into the peritoneal cavity, to colonize the peritoneum and induce cancerous peritoneum. Anchorage-independent growth is a form of resistance to apoptosis induced by the loss of cell signals generated from interaction with extracellular matrix. Therefore, CDCP1 might be required for cell detachment from primary lesions and survival in the peritoneal cavity of scirrhous gastric cancer cells.
Loss of CDCP1 attenuates invasion and dissemination of highly invasive gastric cancer cells in vivo (Figure 4, A and B; and Figure 5A). Moreover, we found that the phosphorylation level of CDCP1 in tumor nodules disseminated to mesentery is much higher than in cells growing in cell culture plate (Figure 6B). These results indicate that cancer cells expressing phosphorylated CDCP1 might be involved in the process of peritoneal dissemination of cancer cells via cell migration and anchorage-independent growth. It might be important to examine the phosphorylation state of CDCP1 in vivo in a wide range of cancers other than gastric cancers and lung cancers to determine the organ-specific roles of CDCP1 in cancer progression.
Because early clinical diagnosis of scirrhous gastric carcinoma is difficult, peritoneal dissemination or invasion to lymph nodes has frequently occurred before diagnosis. Histological analysis revealed that expression and phosphorylation of CDCP1 was detected in the invasive site of human scirrhous tissues (Figure 7). It might be useful to examine the expression and phosphorylation level of CDCP1 in a surgical specimen of scirrhous gastric carcinomas to predict the prognosis of the tumors. Dissemination is a frequent form of the recurrence of scirrhous gastric carcinoma, which serves as a major factor determining the prognosis. In addition, our study shows that disruption of CDCP1 suppresses the infiltration of cancer cells into liver and the peritoneal dissemination in animal models. CDCP1 is considered to be a prognosis factor of gastric scirrhous carcinoma, and the inhibition of a specific cellular signal originating from CDCP1 phosphorylation might be a good candidate for regulating its invasion and dissemination.
Supplementary Material
Footnotes
Address reprint requests to Ryuichi Sakai, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan. E-mail: rsakai@gan2.res.ncc.go.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant-in aid for cancer research and grant-in-aid for young scientists); and the Ministry of Health, Labor, and Welfare of Japan (grant-in-aid for the third-term comprehensive 10-year strategy for cancer control).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20:4402–4408. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- Bühring HJ, Kuçi S, Conze T, Rathke G, Bartolović K, Grünebach F, Scherl-Mostageer M, Brümmendorf TH, Schweifer N, Lammers R. CDCP1 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells. 2004;22:334–343. doi: 10.1634/stemcells.22-3-334. [DOI] [PubMed] [Google Scholar]
- Conze T, Lammers R, Kuci S, Scherl-Mostageer M, Schweifer N, Kanz L, Buhring HJ. CDCP1 is a novel marker for hematopoietic stem cells. Ann NY Acad Sci. 2004;996:222–226. doi: 10.1111/j.1749-6632.2003.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, Testa JE, Quigley JP. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products from five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Brown TA, Yang TM, Zaitsevskaia T, Xia Y, Dunn CA, Sigle RO, Knudsen B, Carter WG. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J Biol Chem. 2004;279:14772–14783. doi: 10.1074/jbc.M309678200. [DOI] [PubMed] [Google Scholar]
- Uekita T, Lin J, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CDCP1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kamata R, Takigahira M, Yanagihara K, Sakai R. Phosphorylation of ephrin-B1 regulates dissemination of gastric scirrhous carcinoma. Am J Pathol. 2007;171:68–78. doi: 10.2353/ajpath.2007.070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yashiro M, Matsuoka T, Tendo M, Shimizu T, Miwa A, Hirakawa K. A novel molecular targeting compound as K-samII/FGF-R2 phosphorylation inhibitor. Ki23057, for scirrhous gastric cancer. Gastroenterology. 2006;131:1530–1541. doi: 10.1053/j.gastro.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Uekita T, Gotoh I, Kinoshita T, Itoh Y, Sato H, Shiomi T, Okada Y, Seiki M. Membrane-type 1 matrix metalloproteinase cytoplasmic tail-binding protein-1 is a new member of the cupin superfamily. J Biol Chem. 2004;279:12734–12743. doi: 10.1074/jbc.M309957200. [DOI] [PubMed] [Google Scholar]
- Yanagihara K, Tanaka H, Takigahira M, Ino Y, Yamaguchi Y, Toge T, Sugano K, Hirohashi S. Establishment of two cell lines from human gastric scirrhous carcinoma that possess the potential to metastasize spontaneously in nude mice. Cancer Sci. 2004;95:575–582. doi: 10.1111/j.1349-7006.2004.tb02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K, Takigahira M, Tanaka H, Komatsu T, Fukumoto H, Koizumi F, Nishio K, Ochiya T, Ino Y, Hirohashi S. Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci. 2005;96:323–332. doi: 10.1111/j.1349-7006.2005.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrin: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Moissoglu K, Schwartz MA. Integrin signaling in directed cell migration. Biol Cell. 2006;98:547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J, Deichmann M, Helmke B, Müller H, Kollender G, Holmskov U, Ligtenberg T, Krebs I, Wiemann S, Bantel-Schaal U, Madsen J, Bikker F, Klauck SM, Otto HF, Moldenhauer G, Poustka A. Frequent downregulation of DMBT1 and galectin-3 in epithelial skin cancer. Int J Cancer. 2003;105:149–157. doi: 10.1002/ijc.11072. [DOI] [PubMed] [Google Scholar]
- Romero A, Romão MJ, Varela PF, Kölln I, Dias JM, Carvalho AL, Sanz L, Töpfer-Petersen E, Calvete JJ. The crystal structures of two spermadhesins reveal the CUB domain fold. Nat Struct Biol. 1997;4:783–788. doi: 10.1038/nsb1097-783. [DOI] [PubMed] [Google Scholar]
- Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069–18076. doi: 10.1074/jbc.M201681200. [DOI] [PubMed] [Google Scholar]
- Kuznetsova SA, Mahoney DJ, Martin-Manso G, Ali T, Nentwich HA, Sipes JM, Zeng B, Vogel T, Day AJ, Roberts DD. TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biol. 2008;27:201–210. doi: 10.1016/j.matbio.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Gil SG, Carter EG. Anchorage mediated by integrin alpha6beta4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kD protein. J Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis. 1996;14:43–54. doi: 10.1007/BF00157685. [DOI] [PubMed] [Google Scholar]
- Fujita S, Suzuki H, Kinoshita M, Hirohashi S. Inhibition of cell attachment, invasion and metastasis of human carcinoma cells by anti-integrin β1 subunit antibody. Jpn J Cancer Res. 1992;83:1317–1326. doi: 10.1111/j.1349-7006.1992.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Ochiai A, Yamada T, Akimoto S, Yanagihara K, Kitajima M, Hirohashi S. Integrin α6β4 as a suppressor and a predictive marker for peritoneal dissemination in human gastric cancer. Gastroenterology. 2000;118:497–506. doi: 10.1016/s0016-5085(00)70255-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.